Abstract
Genetic improvement for drought stress tolerance in rice involves the quantitative nature of the trait, which reflects the additive effects of several genetic loci throughout the genome. Yield components and related traits under stressed and well-water conditions were assayed in mapping populations derived from crosses of Azucena×IR64 and Azucena×Bala. To find the candidate rice genes underlying Quantitative Trait Loci (QTL) in these populations, we conducted in silico analysis of a candidate region flanked by the genetic markers RM212 and RM319 on chromosome 1, proximal to the semi-dwarf (sd1) locus. A total of 175 annotated genes were identified from this region. These included 48 genes annotated by functional homology to known genes, 23 pseudogenes, 24 ab initio predicted genes supported by an alignment match to an EST (Expressed sequence tag) of unknown function, and 80 hypothetical genes predicted solely by ab initio means. Among these, 16 candidate genes could potentially be involved in drought stress response.
Keywords: Rice genome sequence, Candidate genes, Drought stress, Quantitative Trait Loci (QTL)
INTRODUCTION
Drought stress is a major constraint to rice (Oryza sativa) production and yield stability in rained ecosystems (Dey and Upadhyaya, 1996). Rice must be made more drought tolerant, but this is a somewhat contradictory objective considering that rice is most commonly grown under flooded conditions. Achieving drought tolerance in rice will require a deeper understanding of the possible physiological mechanisms available for water stress tolerance and the identification of favorable alleles for introgression into rice varieties that otherwise suit specific environments. Quantitative Trait Loci (QTL) are useful starting points for identifying such alleles. QTL relating to drought tolerance have been identified in rice, barley, and grain sorghum (Price et al., 2002a).
However, positionally cloning a QTL gene locus normally requires fine scale mapping with large mapping populations across many seasons. Availability of the whole rice genome sequences (Goff et al., 2002; Yu et al., 2002; Sasaki et al., 2002; Feng et al., 2002) provides a new tool for this task, along with a means of characterizing their associated molecular functions. In this study, we exploited this new source of data by anchoring drought stress tolerance QTL maps reported by other researchers (Lafitte et al., 2002; Price et al., 2002a; 2002b; 2002c; Causse et al., 1994; Harushima et al., 1998) to a rice physical and sequence map using sequence based markers. We then identified genes as candidates with respect to position.
Drought stress most severely impacts yield when applied during the reproductive stage of the rice plant. The sd1 is one of the most important genes in rice, whose recessive character results in a shortened culm with improved lodging resistance and a harvest index (Jennings, 1964). The rice height-related QTL was shown to be tightly linked to restricted fragment length polymorphism (RFLP) markers RZ730 and RG810 on the long arm of chromosome 1 (Li et al., 2003), consisting of the QTL region of drought resistance (Lafitte et al., 2002). As further evidence for drought tolerance gene candidacy, we aligned EST sequences from an available drought stressed panicle library onto our candidate gene structures. We also assessed gene candidacy based on literature support.
MATERIALS AND METHODS
Genetic map for IR64×Azucena and Bala× Azucena QTL mapping populations
Published genetic mapping data from a double haploid mapping population, IR64×Azucena (Temnykh et al., 2001) and Bala×Azucena (Price et al., 2002b) were used as starting points for this study. IR64×Azucena map included 93 amplified fragment length polymorphism (AFLP), 481 simple sequence repeat (SSR), 15 ‘known’ genes, 1 isozyme, a restriction fragment length polymorphism (RFLP) and 13 random amplification of polymorphic DNA (RAPD) markers adapted from oats. The Bala×Azucena population includes 7 SSR, 105 RFLP and 34 AFLP markers, as well as one marker derived from oats.
Consensus map construction
To identify genetic map regions containing multiple overlapping QTL across populations, we computationally merged the maps of the two aforementioned mapping populations with common anchor markers using the NCGR ISYS comparative mapping tool (NCGR; http://www.ncgr.org/isys; Siepel et al., 2001).
Rice genome sequence map
Identification of candidate genes corresponding to a QTL interval requires a contiguous genome sequence map. Although chromosome 1 and chromosome 4 were essentially completed by the International Rice Genome Sequencing Project (IRGSP) during the latter part of this project, early in the project we needed to assemble our own sequence map using available public data. This assembly work focussed on rice chromosome 1. The input data for map assembly included the list of clone names, GenBank accession numbers and the public rice physical map (Chen et al., 2002). We assembled the bacterial artificial chromosome (BAC) clone sequences into a pseudo-molecule using a suite of Perl scripts graciously provided by Dr. David Beare of the Sanger Centre, United Kingdom.
Anchoring sequence-based genetic markers to the sequence map
We used a local implementation of the “electronic polymerase chain reaction” approach (“ePCR”; Schuler, 1997) using Perl regular expression alignment of primer sequences with orientation and threshold distance constraints to identify putative PCR amplicons in target sequences. The primers for the analysis were obtained retrieved from either the Gramene database (Ware et al., 2002; www.gramene.org) or the Japan Rice Genome Project (RGP) database (Sasaki, 2001). Markers were correlated with the physical map by ePCR, run against the rice genome BAC sequences, retrieval from the Gramene database and by BLAST (Altschul et al., 1990) alignment searches of the rice BAC sequences.
Database storage and visualization of maps
The resulting genetic (QTL), physical and sequence maps compiled for the study were stored in the International Rice Information System (IRIS; www.iris.irri.org; Bruskiewich et al., 2003) and visualized using a comparative mapping tool developed at the National Center for Genome Resources. To facilitate identification of candidate genes, we also have developed QTL2Gene (http://ibi.zju.edu.cn/qtl2gene/qtl2gene.htm) as a flexible database using the public rice genome sequence and RFLP and SSR markers. The database provides an interface for searching the genes underlying one QTL using two flanking markers’ name. Other information related with this paper has been published on website (http://ibi.zju.edu.cn/publish/QTL2gene/drought.htm).
Choice of candidate QTL region for detailed analysis
The choice of candidate QTL region on rice chromosome 1 was chosen based on a survey of drought QTL (Price et al., 2002c; Lafitte et al., 2002) and International Rice Research Institute (IRRI) fine mapping work (1Zhang Li, personal communications). This region is flanked by RM212 and RM319, which were shown to be located on 148.7 cM (CentiMorgan) and 150.5 cM of IR64×Azucena genetic map respectively, and spans a map distance of approximately 1.8 cM across the sd1 gene locus.
Gene and functional annotation
We downloaded gene annotation for the RM212-RM319 candidate region from the Japan Rice Genome Project (RGP) sequence data found in GenBank (www.ncbi.nlm.nih.gov) and from the rice databases of The Institute for Genomic Research (TIGR; www.tigr.org). These annotations were assessed using the gbrowse sequence browser from the Generic Model Organism Database project (http://www.gmod.org; Stein et al., 2002; Lewis et al., 2002).
Drought panicle cDNA library
An IR64-based drought stressed rice panicle cDNA library was constructed by researchers at the International Rice Research Institute (Arumugam et al., 2005). Briefly, the normalized library was constructed from pooled mRNA obtained from the rice panicles collected from control (well watered) and water stressed plants at 2 d before heading, at heading, 50% flowering and 4 d after 50% flowering. Water stress was applied by not watering for several consecutive days. Expressed sequence tags (EST) obtained by 5′ and/or 3′ end sequencing of the clones were clustered and annotated using standard in silico approaches (Altschul et al., 1990).
RESULTS
Alignment of the rice genetic map onto the physical map and gene identification
The chromosome 1 consensus map for IR64×Azucena and Bala×Azucena was anchored using sequence-based markers to the assembled rice physical map. The candidate sequence interval spanning the candidate QTL flanked by RM212 to RM319 was then identified. The assembly of seven BAC clones in this interval yielded a contiguous sequence region of 855008 base pairs within which we identified 175 predicted gene structures. Fig.1 shows a synopsis of gene annotation categories. The RGP annotation included 48 (27%) genes annotated by functional homology to known genes, 23 (13%) pseudogenes, 24 (14%) ab initio predicted genes supported by an alignment match to an EST of unknown function, and 80 (46%) hypothetical genes predicted by ab initio algorithms.
Fig. 1.
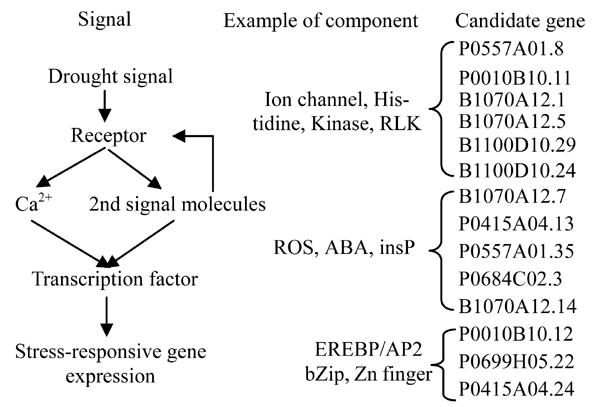
Possible assignment of candidate genes identified in candidate interval as components in a generic pathway for the transduction of drought stress signals in plants
Mapping drought stressed panicle library EST into the region
To collect further supporting evidence for the function of the predicted genes in the region, EST sequences obtained from the IRRI drought stressed panicle library were aligned by BLAST (Altschul et al., 1990) against candidate gene sequences. This analysis revealed that 30 candidate gene predictions match EST sequences in the library (Table 1).
Table 1.
EST alignments to candidate genes
| Gene name | Gene |
BLAST score | Percent identity | EST GenBank accession ID | EST |
Annotation | ||
| Start | Stop | Start | Stop | |||||
| B1011A07.16 | 607 | 852 | 690 | 75.61 | CA762217 | 2 | 247 | Putative pectinesterase 2.1 precursor |
| B1070A12.1 | 2131 | 2424 | 1380 | 96.6 | CA765415 | 1 | 294 | Putative serine/threonine kinase |
| B1070A12.17 | 1305 | 973 | 1655 | 99.7 | CA760294 | 352 | 685 | Hypothetical protein |
| P0010B10.6 | 1221 | 1024 | 990 | 100 | CA760259 | 488 | 685 | Hypothetical protein |
| P0010B10.7 | 511 | 344 | 831 | 99.4 | CA763140 | 493 | 660 | Zinc protease |
| P0010B10.12 | 1 | 249 | 1245 | 100 | CA762979 | 439 | 687 | Helicase-like transcription factor |
| P0010B10.21 | 458 | 570 | 330 | 77.19 | CB096315 | 212 | 325 | Hypothetical protein |
| P0010B10.21 | 458 | 570 | 321 | 76.32 | CB096974 | 175 | 288 | Hypothetical protein |
| P0010B10.22 | 861 | 443 | 1663 | 88.54 | CA759417 | 267 | 685 | Secretory carrier membrane protein |
| B1100D10.21 | 582 | 1 | 2868 | 99.31 | CA762908 | 135 | 715 | Acyl-CoA:1-acylglycerol-3-ph-osphate acyltransferase |
| B1100D10.25 | 223 | 38 | 513 | 76.72 | CB096516 | 86 | 271 | Hypothetical protein |
| B1100D10.33 | 1790 | 1918 | 304 | 71.76 | CA764883 | 1 | 130 | Serine/threonine kinase receptor precursor (EC 2.7.1.37) (S-receptor kinase) |
| P0458E05.33 | 354 | 47 | 720 | 74.14 | CA759325 | 282 | 592 | Hypothetical protein |
| P0482C06.17 | 483 | 227 | 638 | 74.81 | CA760143 | 176 | 429 | Hypothetical protein |
| P0682C06.15 | 330 | 1 | 1533 | 96.06 | CA766823 | 326 | 655 | Putative prolyl endopeptidase |
| P0682C06.16 | 360 | 2 | 1375 | 87.19 | CA763742 | 143 | 500 | Unknown protein |
| P0699H05.4 | 1520 | 2043 | 2593 | 99.43 | CA766000 | 2 | 525 | Putative subtilase |
| P0699H05.7 | 2136 | 1602 | 2524 | 97.01 | CB096421 | 317 | 851 | Putative subtilase |
| P0699H05.7 | 1678 | 2136 | 2286 | 99.78 | CA766686 | 1 | 459 | Putative subtilase |
| P0699H05.7 | 378 | 926 | 2414 | 93.88 | CA763105 | 162 | 714 | Putative subtilase |
| P0699H05.9 | 120 | 44 | 162 | 74.07 | CA766969 | 204 | 279 | Hypothetical protein |
| P0699H05.17 | 292 | 1 | 1436 | 99.32 | CB096487 | 1 | 291 | Hypothetical protein |
| P0699H05.17 | 323 | 1 | 1606 | 99.69 | CA766130 | 35 | 357 | Hypothetical protein |
| P0699H05.22 | 670 | 708 | 195 | 100 | CA767024 | 2 | 40 | Ethylene-responsive element binding factor 3 |
| P0699H05.22 | 708 | 194 | 1977 | 87.4 | CA759952 | 183 | 696 | Ethylene-responsive element binding factor 3 |
Candidate genes placed into drought signal pathways
In our study, we found 16 candidate genes in the region with some literatures support (Shinozaki and Yamaguchi-Shinozaki, 1996; Ingram and Bartels, 1996; Yamaguchi-Shinozaki et al., 2002) potentially involved in drought stress response (Table 2).
Table 2.
Genes encoding proteins of known function possibly that may possibly be responsive to drought stress
| Classes | Gene name | Putative functional description | Protein_id | Reference |
| Receptor | P0557A01.8 | Kinase-like | BAB89770.1 | (Fujimoto et al., 2000) |
| P0010B10.11 | Kinase-like protein receptor | BAB63567.1 | (Zhu, 2002) | |
| B1070A12.1 | Serine/threonine kinase | BAB92578.1 | (Xiong and Zhu, 2001) | |
| B1070A12.2 | Receptor kinase | BAB92579.1 | (Xiong et al., 2002) | |
| B1070A12.5 | Receptor kinase | BAB92581.1 | (Xiong et al., 2002) | |
| B1100D10.29 | Diacylglycerol kinase | BAB92552.1 | (Arisz et al., 2003) | |
| Ca2+ | B1100D10.24 | Cyclic nucleotide and calmodulin-regulated ion channel | (Urao et al., 1994) | |
| Signalling | B1070A12.7 | 1,4-benzoquinone reductase | BAB92583.1 | (Choi et al., 2002) |
| P0415A04.13 | Peroxidase-like protein contains EST AU075654 (E1982) | (Kim et al., 2003) | ||
| P0557A01.35 | Polyphenol oxidase | BAB89784.1 | (Zhang and Kirkham, 1994) | |
| P0684C02.3 | Polyphenol oxidase | BAB89047.1 | (Zhang and Kirkham, 1994) | |
| B1070A12.14 | Auxin-responsive GH3 | BAB92590.1 | (Kovtun et al., 2000) | |
| Transcript | P0010B10.12 | Helicase-like transcription factor | BAB63568.1 | (Zhu, 2002) |
| P0699H05.22 | Ethylene-responsive element binding factor 3 | (Fujimoto et al., 2000) | ||
| P0415A04.24 | Nuclear transport factor 2 | BAB90110.1 | (Seki et al., 2002) | |
| Vacuolar | P0010B10.27 | Vacuolar sorting-associated protein | (Gaxiola et al., 2001) |
DISCUSSION
In this study, we anchored rice QTL maps to the rice physical map to identify drought stress tolerance candidate genes based on candidate QTL position. We chose the sd1 region of chromosome 1 for our attempt to link drought stress tolerance genotype to phenotype using bioinformatics because of strong combined genetic evidence for the existence of a large effect QTL for stress tolerance and because of the relatively complete rice genome sequences in the region. A set of candidate genes of known or inferred function were identified in this region using rice genome annotation. Published literature supports candidacy of some of these genes in drought stress response.
Fig.1 shows one generic drought signal pathway (Zhu, 2002) against which the candidate genes identified in this study could potentially be assigned. This pathway has three classes of genes: (1) signal perception, such as P0557A01.8; (2) generation of second messengers, such as B1070A12.7; and (3) gene expression. Candidate genes potentially implicated in this pathway are listed on the right hand side of the Fig.1.
Another possible pathway for drought signals is the Salt-Overly-Sensitive (SOS) pathway (Xiong et al., 2002), as shown in Fig.2. Hypothetically, a myristoylated calcium-binding protein encoded by SOS3 may sense a drought stress or salt-elicited calcium signal and translate it downstream; SOS3 interacts with and activates SOS2, a serine/threonine protein kinase. SOS2 and SOS3 regulate the expression level of SOS1, a salt tolerance effecter gene encoding a plasma membrane Na+/H+ antiporter. In our study, we found several genes that could act in this pathway: a vacuolar protein, a serine/threonine kinase, three kinds of putative receptor kinase genes and a putative diacylglycerol kinase gene.
Fig. 2.
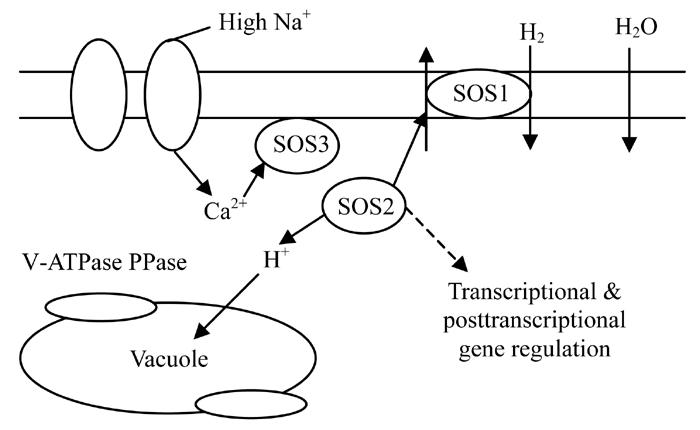
Regulation of ion homeostasis by the SOS pathway
Endogenous abscisic acid (ABA) levels have been reported to increase as a result of water deficit in many physiological studies, and therefore ABA is thought to be involved in the signal transduction. ABA-dependent and ABA-independent signal pathways in the activation of stress-inducible genes under dehydration conditions have been reported (Shinozaki and Yamaguchi-Shinozaki, 1997), as shown in Fig.3. We identified an ethylene-responsive element binding factor (EREBP) gene (P0699H05.22) that could be a candidate related to this pathway. EREBP-like genes are known to be induced by a variety of abiotic stresses, including drought and chilling stresses (Gilmour et al., 1998).
Fig. 3.
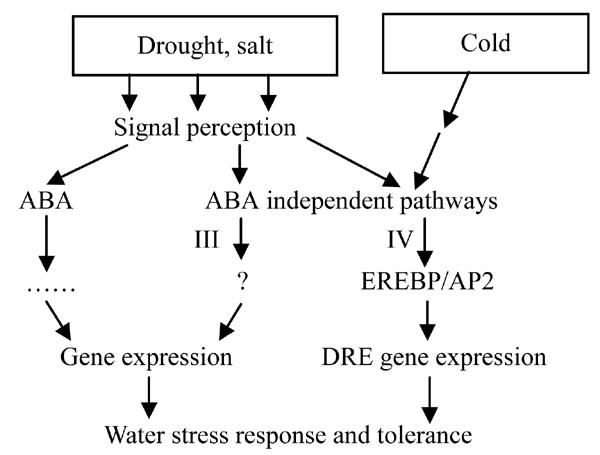
ABA-dependent and ABA-independent pathway
A few of the smaller gene clusters were composed of a single family of closely related genes underlying one QTL, and most of these are within 1–2 cM. Thus, we investigated this region for gene family and found that 4 rust resistance protein clusters on BAC clone (Accession number: B1100D10). These genes have above 89% identity to proteins as shown by ClastalW tool with default parameters. The rust resistance family in this region indicated that it may related to disease resistance.
One major challenge is to discover the drought candidate genes of not only those genes for which functions are already known, but also those with still unknown functions. Clearly, for such genes, fine mapping and functional genomic experimentation will be required to clarify their candidacy.
Acknowledgments
The authors of this paper would like to acknowledge the kind advice of Renee Lafitte, Zhikang Li and John Bennett in this project. We thank Aixia Ren for assistance with the pathway studies. Some of the drought panicle EST sequences and annotation were obtained from the Hans Bonhert laboratory at the University of Illinois. Additional drought panicle EST sequence gene annotation was generated by S. Rudd of the Munich Information on Protein Sequences (MIPS) center.
Footnotes
rted partly by the Rockefeller Foundation thesis dissertation training grant and the National Hi-Tech Research and Development Program (863) of China
Li, Z.K., 2002. Personal communications.
References
- 1.Altschul SF, Warren G, Webb M, Eugene WM, David JL. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Arisz SA, Valianpour F, van Gennip AH, Munnik T. Substrate preference of stress-activated phospholipase D in Chlamydomonas and its contribution to PA formation. Pant J. 2003;34:595–604. doi: 10.1046/j.1365-313x.2003.01750.x. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam K, Lafitte R, Chen J, Bennet J. Expression microarrays and their application in drought stress research. Field Crops Research. 2005 in press. [Google Scholar]
- 4.Bruskiewich RM, Cosico AB, Eusebio W, Portugal AM, Ramos LM, Reyes MT, Sallan MA, Ulat VJ, Wang X, McNally KL, et al. Linking genotype to phenotype: the International Rice Information System (IRIS) Bioinformatics. 2003;S1:I63–I65. doi: 10.1093/bioinformatics/btg1006. [DOI] [PubMed] [Google Scholar]
- 5.Causse M, Fulton TM, Cho YG, Ahn SN, Chunwongse J, Wu K, Xiao J, Yu Z, Ronald PC, Harrington SB, et al. Saturated molecular map of the rice genome based on an interspecific backcross population. Genetics. 1994;138:1251–1274. doi: 10.1093/genetics/138.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Presting G, Barbazuk WB, Goicoechea JL, Blackmon B, Fang G, Kim H, Frisch D, Yu Y, Sun S. An integrated physical and genetic map of the rice genome. Plant Cell. 2002;14(3):537–545. doi: 10.1105/tpc.010485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DW, Rodriguez EM, Close TJ. Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol. 2002;129:1781–1787. doi: 10.1104/pp.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dey MM, Upadhyaya HK. Yield Loss Due to Drought, Cold and Submergence Tolerance. In: Evenson RE, Herdt RW, Hossain M, editors. Rice Research in Asia: Progress and Priorities. UK: International Rice Research Institute in Collaboration with CAB International; 1996. [Google Scholar]
- 9.Feng Q, Zhang Y, Hao P, Wang S, Fu G, Huang Y, Li Y, Zhu J, Liu Y, Hu X, et al. Sequence and analysis of rice chromosome 4. Nature. 2002;420:316–320. doi: 10.1038/nature01183. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell. 2000;12:393–404. doi: 10.1105/tpc.12.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA. 2001;98:11444–11449. doi: 10.1073/pnas.191389398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 13.Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, Glazebrook J, Sessions A, Oeller P, Varma H, et al. A draft sequence of the rice Genome (Oryza sativa L. ssp. japonica). Science. 2002;296:92–100. doi: 10.1126/science.1068275. [DOI] [PubMed] [Google Scholar]
- 14.Harushima YM, Yano A, Shomura M, Sato T, Shimano Y, Kuboki T, Yamamoto SY, Lin BA, Antonio A, Parco H, et al. A High-density rice genetic linkage map with 2275 markers using a single F2 population. Genetics. 1998;148:479–494. doi: 10.1093/genetics/148.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- 16.Jennings PR. Plant type as a rice breeding objective. Crop Sci. 1964;4:13–15. [Google Scholar]
- 17.Kim JS, Kim YO, Ryu HJ, Kwak YS, Lee JY, Kang H. Isolation of stress-related genes of rubber particles and latex in fig tree (Ficus carica) and their expressions by abiotic stress or plant hormone treatments. Plant Cell Physiol. 2003;44:412–414. doi: 10.1093/pcp/pcg058. [DOI] [PubMed] [Google Scholar]
- 18.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafitte HR, Courtois B, Arraudeau M. Genetic improvement of rice in aerobic systems: progress from yield to genes. Field Crops Res. 2002;75:171–190. [Google Scholar]
- 20.Lewis SE, Searle SMJ, Harris N, Gibson M, Iyer V, Ricter J, Wiel C, Bayraktaroglu L, Birney E, Crosby MA, et al. Annotation of the Drosophila melanogaster euchromatic genome: a systematic review. Genome Biol. 2002;3:1–22. doi: 10.1186/gb-2002-3-12-research0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li ZK, Yu SB, Lafitte HR, Huang N, Courtois B, Hittalmani S, Vijayakumar CHM, Liu GF, Wang GC, Shashidhar HE, et al. QTL x environment interactions in rice. I. Heading date and plant height. Theor Appl Genet. 2003;108(1):141–153. doi: 10.1007/s00122-003-1401-2. [DOI] [PubMed] [Google Scholar]
- 22.Price AH, Cairns JE, Horton P, Jones HG, Griffiths H. Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot. 2002;53:989–1004. doi: 10.1093/jexbot/53.371.989. [DOI] [PubMed] [Google Scholar]
- 23.Price AH, Steele KA, Moore BJ, Jones RGW. Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes: II. Mapping QTL for root morphology and distribution. Field Crops Res. 2002;76:25–43. [Google Scholar]
- 24.Price AH, Townend J, Jones MP, Audebert A, Courtois B. Mapping QTLs associated with drought avoidance in upland rice grown in the Philippines and West Africa. Plant Mol Biol. 2002;48:683–695. doi: 10.1023/a:1014805625790. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T. The Rice Genome Project in Japan. In: Wilson RF, Hou CT, Hildebrand DF, editors. Dealing with Genetically Modified Crops. Champaign Illinois: AOCS Press; 2001. pp. 102–109. [Google Scholar]
- 26.Sasaki T, Matsumoto T, Yamamoto K, Sakata K, Baba T, Katayose Y, Wu J, Niimura Y, Cheng Z, Nagamura Y, et al. The genome sequence and structure of rice chromosome 1. Nature. 2002;420:312–316. doi: 10.1038/nature01184. [DOI] [PubMed] [Google Scholar]
- 27.Schuler GD. Sequence mapping by electronic PCR. Genome Res. 1997;7:541–550. doi: 10.1101/gr.7.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, et al. Monitoring the expression pattern of around 7000 Arabidopsisgenes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics. 2002;2:282–291. doi: 10.1007/s10142-002-0070-6. [DOI] [PubMed] [Google Scholar]
- 29.Shinozaki K, Yamaguchi-Shinozaki K. Molecular responses to drought and cold stress. Curr Opin Biotech. 1996;7:161–167. doi: 10.1016/s0958-1669(96)80007-3. [DOI] [PubMed] [Google Scholar]
- 30.Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siepel A, Farmer A, Tolopko A, Zhuang M, Mendes P, Beavis W, Sobral B. ISYS: a decentralized, component-based approach to the integration of heterogeneous bioinformatics resources. Bioinformatics. 2001;17:83–94. doi: 10.1093/bioinformatics/17.1.83. [DOI] [PubMed] [Google Scholar]
- 32.Stein LD, Mungall C, Shu S, Caudy M, Mangone M, Day A, Nickerson E, Stajich JE, Harris TW, Arva A, et al. The generic genome browser: a building block for a model organism system database. Genome Res. 2002;12:1599–1610. doi: 10.1101/gr.403602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S. Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res. 2001;11:1441–1452. doi: 10.1101/gr.184001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urao T, Katagiri T, Mizoguchi T, Yamaguchi-Shinozaki K, Hayashida N, Shinozaki K. Two genes that encode Ca2+-dependent protein kinases are induced by drought and high-salt stresses in Arabidopsis thaliana. Mol Gen Genet. 1994;244:331–340. doi: 10.1007/BF00286684. [DOI] [PubMed] [Google Scholar]
- 35.Ware D, Jaiswal P, Ni J, Pan X, Chang K, Clark K, Teytelman L, Schmidt S, Zhao W, Cartinhour S, et al. Gramene: a resource for comparative grass genomics. Nucleic Acids Res. 2002;30:103–105. doi: 10.1093/nar/30.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong L, Zhu JK. Abiotic stress signal transduction in plants: Molecular and genetic perspectives. Physiol Plant. 2001;112:152–166. doi: 10.1034/j.1399-3054.2001.1120202.x. [DOI] [PubMed] [Google Scholar]
- 37.Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. The Plant Cell. 2002;14(Suppl):S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi-Shinozaki K, Kasuga M, Liu Q, Nakashima K, Sakuma Y, Abe H, Shinwari ZK, Seki M, Shinozaki K. Biological mechanisms of drought stress response. JIRCAS Working Report. 2002;23:1–8. [Google Scholar]
- 39.Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica) Science. 2002;296:79–92. doi: 10.1126/science.1068037. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Kirkham MB. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheate species. Plant Cell Physiol. 1994;35:758–791. [Google Scholar]
- 41.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]


