Abstract
Maintaining genetic diversity is a major issue in conservation biology. In this study, we demonstrate the differences of genetic diversity levels between wild and captive individuals of Elliot’s Pheasant Syrmaticus ellioti. Wild individuals showed a higher genetic diversity level than that of the captive individuals. Nucleotide diversity and haplotype diversity of wild individuals were 0.00628 and 0.993, while those of captive individuals were 0.00150 and 0.584 respectively. Only 3 haplotypes of mtDNA control region sequence were identified among 36 captive individuals, while 16 unique haplotypes were identified among the 17 wild individuals in this study. One captive haplotype was shared by a wild individual from Anhui Province. It is concluded that a low number of founders was the likely reason for the lower level genetic diversity of the captive group. Careful genetic management is suggested for captive populations, particularly of such an endangered species, to maintain genetic variability levels.
Keywords: Control region, Haplotype, Genetic diversity, Mitochondrial DNA, Syrmaticus ellioti
INTRODUCTION
Genetic diversity is a major issue of conservation biology recognized by the IUCN (Frankham et al., 2002). Within a population it reflects the evolutionary potential to adapt to novel environmental changes. Therefore, during the past few years, the genetic diversity of many threatened mammals, birds, fish, insects and plants have been investigated (Frankham et al., 2002). As a direct and indirect consequence of human actions, more and more species or populations are facing changing environments and are thus experiencing a corresponding reduction in population sizes. Therefore the loss of genetic diversity is becoming an increasingly central topic in conservation genetics (Avise, 1994), as effective population sizes are continuing to diminish, inbreeding continues to increase, populations continue to fragment and other detrimental factors persist. On the other hand, in order to enhance the population size and save the threatened or endangered species from extinction, many captive breeding projects have been carried out. Since most of the captive populations are small, it is not surprising the evidence suggests that captive populations generally have a lower genetic variability than that of wild populations.
Elliot’s Pheasant (Syrmaticus ellioti), regarded as “Vulnerable” in the 2003 IUCN Red List of Threatened Species (http://www.redlist.org), is endemic to China. Its population size is thought to be rapidly declining because of ongoing habitat loss and hunting (Ding and Jiang, 2000). The captive history of this species can be traced back to as early as 1873 when Père David obtained individual specimens from Fujian Province and initiated captive breeding of the species in Paris (Knoder, 1983). In the later part of the 20th century, the species was captive bred in several zoos in China, such as Shanghai Zoo and Ningbo Zoo (Zheng and Wang, 1998). Currently, the captive population abroad is estimated to be 500–600 individuals as noted by the American Zoo and Aquarium Association (AZA) Regional Studbook (Fuller and Garson, 2000). Although a wide range of projects concerning ecological adaptation and conservation strategy has been instigated, very little information regarding the genetic diversity of wild or captive populations of the species has been presented.
Recently, genetic diversity has been measured using many different types of data, including quantitative characters, chromosomes, proteins, nuclear DNA loci, chloroplast DNA, and mtDNA. Thus an increasing body of data is being generated for study at the DNA level. Sequencing of mtDNA provides a marker of maternal inheritance with high mutation rates and high variation observed in vertebrates. In addition, mtDNA can be sequenced using non-invasive sampling, thus is becoming more suitable for threatened and endangered species.
In the present study, we investigated whether the captive individuals of Elliot’s Pheasants display low genetic diversity, as is the case in observations of many other small captive populations of threatened species. In this paper, we assessed the genetic diversity among wild and captive individuals of Elliot’s Pheasant, based on haplotypic variation of mtDNA control region sequences. The reasons for the significant loss of genetic diversity of captive individuals, especially in Ningbo Zoo, are discussed. We then make some recommendations for genetic management of captive populations of Elliot’s Pheasant.
MATERIALS AND METHODS
Sample collection
The blood samples were obtained from 36 captive Elliot’s Pheasants in Ningbo Zoo, Zhejiang Province. All captive individuals originated from five ancestors (2 males and 3 females) introduced in 1988. A total of 17 individuals of wild Elliot’s Pheasants were obtained from Zhejiang Province and two other adjoining provinces, Anhui Province and Fujian Province, from which the pad and blood samples were collected for the examination (Table 1).
Table 1.
Summary information of samples in this study
| Groups | Site | N | Resource | Collected year | Code |
| Wild | Anhui | 6 | Pad | 2000 | A1–6 |
| Zhejiang | 2 | Pad | 1985 | Z1–5 | |
| Zhejiang | 3 | Blood | 2002 | Z1–5 | |
| Fujian | 6 | Pad | 1985 | F1–6 | |
| Captive | Ningbo zoo | 36 | Blood | 2002 | C1–36 |
PCR amplification, cloning and sequencing
Genomic DNA was extracted using standard proteinase K digestion and phenol/chloroform procedures (Sambrook et al., 1989). A DNA fragment of approximately 1153 bp was amplified from all the specimens. PCR amplification was carried out on a PTC-200 Peltier Thermal Cycler in 50 μl reaction (DNA primers: Randi and Lucchini, 1998). The thermal cycling profile was as follows: an initial hot-start at 95 °C for 4 min; 30 amplification cycles of denaturizing at 94 °C for 1 min, annealing at 59.5 °C for 1 min and extension at 72 °C for 1 min; and a final incubation at 72 °C for 10 min. The PCR amplification products were separated and eluted by agarose gel electrophoresis and UNIQ-5 Column DNA Gel extraction kit (Sangon, China), and then were ligated into pMD 18-T vector (TaKaRa, China). The products were sequenced in both directions following the extension-dideoxy-chain termination method with universal primers (M13+/M13−) and the BigDye terminator cycle sequencing kit (Perkin Elmer) according to the manufacturer’s instructions.
Statistical analysis
Multiple sequence alignments were obtained using the CLUSTAL X (Thompson et al., 1997). Initial sequence comparisons and identification of haplotypes were performed using MEGA version 2.1 (Kumar et al., 2001). The values of sequence distance, haplotype diversity (h), nucleotide diversity (π) and Tajima’s D test of selective neutrality were carried out by DnaSP version 3.51 (Rozas and Rozas, 1999).
RESULTS
Approximately 1153 bp of sequence was obtained. The base composition included 13.9% G, 26.6% A, 32.7% T and 26.8% C, in agreement with the characteristics of other avian control region sequences (Baker and Marshall, 1997). This confirmed that the sequence data was originally from mtDNA control region.
For all the individuals examined, 53 nucleotide positions were variable among the sequences which defined 18 haplotypes of CR sequences over 53 individuals. A summary of the haplotypes is provided in Table 2. The mean haplotype diversity (h) was estimated to be 0.795, though differences in genetic diversity between wild and captive individuals were apparent (h=0.993, 0.584 respectively). The differences in genetic diversity were also reflected in the estimation of nucleotide diversity (π) (Table 3). Tajima’s D test detected that the wild group departed from the standard neutral model (P<0.01).
Table 2.
Polymorphic nucleotide sites defining the 18 mitochondrial haplotypes resolved among the wild and the captive individuals of Elliot’s Pheasants examined
| Haplotypes | Nucleotide position |
|||||
| 111 | ||||||
| 11122 | 2222222223 | 3334444455 | 5555666677 | 8888888999 | 000 | |
| 3557837912 | 2233446991 | 3770266801 | 3457146979 | 2456669236 | 066 | |
| 2043722822 | 4814492781 | 9256446468 | 2923285493 | 2625671181 | 427 | |
| A1 | ACCCTACTCC | TATCACTCTT | AAACTCCGAT | TTCCCAGCCC | AGTTTTCTTC | TTT |
| A2 | G . . . . . . . . . | ....C.T.... | .....TA.. | ........T. | .......... | ... |
| A3* | ....C.T.T. | .......... | ......TA.. | ........T. | .......... | ... |
| A4 | ......T.T. | ........C. | ......TA.. | ....T...T. | .......... | ... |
| Z1 | ...T..T... | .......... | ......TA.. | ........T. | .......... | ... |
| Z2 | ......T... | .......... | GG....TAG. | ..T.....T. | ......TC.. | ... |
| Z3 | ......T... | ....G..... | ......TA.. | ......A.T | .......... | ... |
| Z4 | C.C ..T.T.C | G.....TCTT | ......TA.. | ........T. | G......... | ... |
| Z5 | ......T.T. | .......... | ...AC.TA.. | ........T. | .......... | .C. |
| F1 | ......T.T. | .......... | ......TA.. | ........T. | ........CT | ... |
| F2 | .....GT.T. | .....T.... | ......TA.. | .......TT. | .A........ | ... |
| F3 | .T....T... | .......... | ..G...TA.. | .A......T | ...C...... | ... |
| F4 | ....C.T.T. | .......... | .....TTA.. | ...T....T. | .......... | ... |
| F5 | ......T.T. | .......... | ......TA.C | .....T..T. | ....CC.... | C.. |
| F6 | C.C..T.T.C | ......T.TT | ......TA.. | C.......T. | .......... | ..C |
| C1# | ......T.T. | ...T...... | ......TA.. | ........T. | .......... | ... |
| C2 | ......T.T. | ......C... | ......TA.. | ........T. | .......... | ... |
| C3 | ..T...T.T. | ..CT...... | ..G...TA.. | ........T. | ..C....... | ... |
: Haplotype A3 shared by the individuals A5
: Haplotype C1 shared by wild individuals A4
Table 3.
Measures of mtDNA diversity observed in wild and captive individuals
| Groups | N | Nhap | D (%) | h | π* (%) | Tajima’s D |
| Captive | 36 | 3 | 0.20 | 0.584±0.054 | 0.150±0.028 | −2.13598# |
| Wild | 17 | 16 | 0.60 | 0.993±0.023 | 0.628±0.085 | 0.52891 |
| Total | 53 | 18 | 0.30 | 0.795±0.045 | 0.330±0.049 |
N: Number of individuals; N hap: Number of haplotype; D: Distance of sequence (overall mean); h: Haplotype diversity; π: Nucleotide diversity
Estimated using Kimura 2-parameter distance (Kimura, 1980)
Significant departure from neutrality (P<0.01)
The distribution and relative frequency of all eighteen unique haplotypes is illustrated in Fig.1. Sixteen haplotypes were identified in seventeen wild individuals, while only three haplotypes were identified in thirty-six captive individuals. Wild individuals, A3 and A5, shared the same haplotype. Furthermore, there was a haplotype shared by a wild and a captive individual. However in the captive population, three haplotypes (C1, C2 and C3) were shared widely among individuals. Haplotype C1, with the highest relative frequency (55.56%), was shared by twenty individuals, twelve individuals shared haplotype C2 (33.33%) and four individuals shared haplotype C3 (11.11%).
Fig. 1.
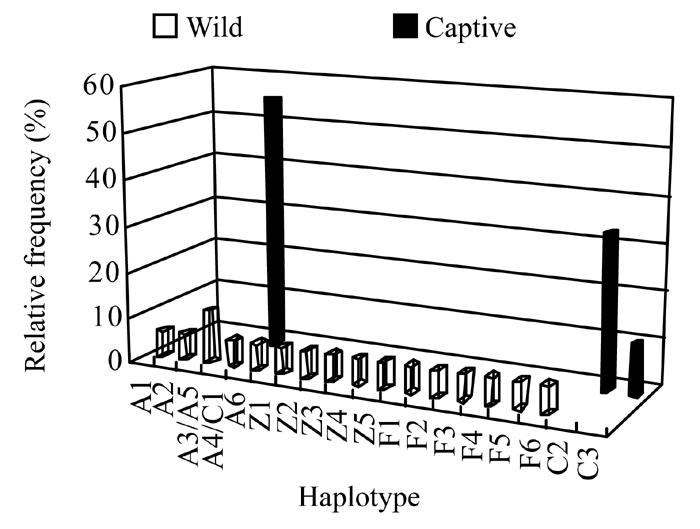
The distribution and relative frequency histograms of mtDNA haplotypes of wild and captive individuals
DISCUSSION
Differences of genetic diversity in wild and captive individuals
From the analysis conducted in this study, it is clear that the wild individuals have higher genetic diversity than the captive individuals. The distribution of haplotypes was significantly different between the two groups. Only three haplotypes were widely distributed among captive individuals. As the mtDNA is a maternally inherited marker in vertebrates, theoretically, three mtDNA haplotypes may be contributed by captive offspring since only three female founders are recorded in Ningbo Zoo. In the present study three haplotypes were identified among captive individuals accorded with the three female founders. It is certain that the low number of female founders is the overwhelming factor resulting in less number of haplotypes leading to lower level of haplotypic diversity in the captive population. Moreover the captive population was biased towards captive haplotype I. The disproportionate haplotypic distribution in the captive population is presumably linked to human interference in captive breeding such as ignoring genetic data and focusing attention on few individuals possessing good reproductive ability.
Captive haplotype I was also shared by one individual from Anhui Province. It is therefore likely that the three female founders might have been collected from Anhui Province, and not Zhejiang Province, as originally mentioned or have closer relationship to the lineage of individuals from Anhui Province.
As Tajima’s test revealed that the wild group departed from the standard neutral model (P<0.01), a possible causative factor is that there were too few wild individuals in this study in contrast to the large number of wild Elliot’s Pheasant’s individuals in existence.
Implications for conservation
As assessed above, the captive population of Elliot’s Pheasant has lower genetic diversity than that of the wild population. There is no doubt that the reduction of genetic diversity has the tendency to compromise the ability of the populations to evolve to cope with novel environmental changes and reduces their chances of long-term existence. Since the distribution of haplotypes in the captive group was biased towards the haplotypes C1 and C2, special attention should be paid to individuals with the C3 haplotype when considering captive breeding management. This is required to enhance the reproduction of the individuals with the C3 haplotype in order to avoid the loss of the valuable genes of the individuals with the C3 haplotype. It is supposed that pedigree information regarding the genetic background of each ancestor could be usefully applied in the practical management. With the help of it, it may be possible to minimize genetic loss by choosing individuals with the lowest relationship in the population, to be parents of the subsequent generation. This would result in the highest degree of retention of genetic variation.
Acknowledgments
We thank Zhejiang National Museum, the State Forestry Administration of Fujian Province and Ningbo Zoo for supplying samples. Special thanks are due to Dr. Yongmei Xi and Chris Wood for their helpful advice on the manuscript.
Footnotes
Project (No. 30170144) supported by the National Nature Science Foundation of China
References
- 1.Avise J. Molecular Markers, Natural History and Evolution. New York: Chapman and Hall; 1994. [Google Scholar]
- 2.Baker AJ, Marshall HD. Mitochondrial Control Region Sequences as Tools for Understanding Evolution. In: Midell DP, editor. Avian Molecula Evolution and Systematics. San Diego, California: Academic Press; 1997. pp. 51–79. [Google Scholar]
- 3.Ding P, Jiang SR. Fragmentation study of Elliot’s Pheasant in the west of Zhejiang Province. Chinese Zoology Reseach. 2000;21(1):65–69. (in Chinese) [Google Scholar]
- 4.Frankham R, Ballou JD, Briscoe DA. Introduction to Conservation Genetics. UK: Cambrige University Press; 2002. [Google Scholar]
- 5.Fuller RA, Garson PJ. 2000. Pheasants. Status Survey and Conservation Action Plan 2000-04. IUCN, Gland, Switzerland and Cambridge, UK, and the World Pheasant Association, Reading, UK. [Google Scholar]
- 6.Kimura M. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleaotide sequence. J Mol Evd. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 7.Knoder CE. Elliot’s Pheasant conservation. World Pheasant Assoc J. 1983;8:11–28. [Google Scholar]
- 8.Kumar B, Tamura K, Jakobsen IB, et al. MEGA2: Molecular Evolutionary Genetics Analysis Software. Tempe, Arizona, USA: Arizona State University; 2001. [DOI] [PubMed] [Google Scholar]
- 9.Randi E, Lucchini V. Organization and evolution of the mitochondrial DNA control region in the avian Genus Alectoris. J Mol Evol. 1998;47:449–462. doi: 10.1007/pl00006402. [DOI] [PubMed] [Google Scholar]
- 10.Rozas J, Rozas R. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics. 1999;15:174–175. doi: 10.1093/bioinformatics/15.2.174. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 12.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng GM, Wang QS. China Red Data Book of Endangered Animals: Aves. Beijing, China: Science Press; 1998. (in Chinese) [Google Scholar]


