Abstract
To determine the pharmacokinetics of gemcitabine (2′,2′-difluorodeoxycytidine) in Chinese non-small-cell lung cancer (NSCLC) patients. Six study subjects were administered gemcitabine at a fixed dose rate of 10 mg/m2 per min (1200 mg/m2, two hours infusion) and carboplatin, and plasma gemcitabine concentrations were measured by ion-pair reversed-phase high-performance liquid chromatography (HPLC). 3P97 Pharmaceutical Kinetics Software was used for the calculation of pharmacokinetic parameters. The obtained mean parameters, elimnation half life (t 1/2) (10.67±3.38 min), area under the curve (AUC) (7.55±1.53 (µg·h)/ml), and clearance (CL) (3940.05±672.08 ml/min), were consistent with those reported in literature. The hematologic toxicology result showed that the regimen was effective on and tolerated by the patients.
Keywords: Gemcitabine, Non-small-cell lung cancer, Pharmacokinetics
INTRODUCTION
Gemcitabine (2′,2′-difluorodeoxycytidine) is a synthetic pyrimidine nucleoside analogue with cytotoxic activity in non-small-cell lung cancer (NSCLC) (Anderson et al., 1994; Abratt et al., 1994; Lund et al., 1994). Gemcitabine is a prodrug which, through deoxycytidine kinase and other nucleotide kinases, exerts its cytotoxic effects through its active intracellular metabolites, gemcitabine diphosphate and triphosphate (Guchelaar et al., 1996; Storniolo et al., 1997).
Gemcitabine’s antitumor activity targets both DNA replication and DNA repair (Huang et al., 1991; Ruiz van Haperen et al., 1993), and is, thus, cell cycle-specific. This suggests that the infusion rate may be very important in determining gemcitabine efficacy. Many in vitro and in vivo studies showed that the gemcitabine plasma concentration affects the accumulation of the active triphosphate metabolite (Abbruzzese et al., 1991; Gandhi and Plunkett, 1990; Grunewald et al., 1990; 1991). These studies revealed that gemcitabine plasma concentrations ranging from 10 to 20 μmol/L (2.99–5.99 μg/ml) produce maximum intracellular triphosphate concentrations. Higher plasma concentrations fail to significantly increase intracellular triphosphate concentrations (Abbruzzese et al., 1991; Grunewald et al., 1990; 1991). A dose rate of 10 mg/m2 per min had been reported to provide gemcitabine plasma concentrations of 10 to 20 μmol/L (Shord et al., 2003).
Prolonged infusion of gemcitabine had been shown to increase levels of the active triphosphate metabolite that theoretically could elevate the survival rate (Tempero et al., 2003). Furthermore, gemcitabine administered at a fixed rate dose of 10 mg/m2 per minute infusion for two hours in combination with carboplatin has satisfactory therapeutic effect and excellent profile of toxicity for the treatment of NSCLC (Manuel et al., 2002).
This study aimed at analyzing the efficacy and safety of this combination using gemcitabine infusion rate of 10 mg/m2 per min (1200 mg/m2 two hours infusion day 1 and 8) with carboplatin (AUC 5, day 1) in advanced Chinese NSCLC patients.
PATIENTS AND METHODS
Patients
Six adult patients with historically or cytologically proven stage IIIB or IV NSCLC, not amenable to surgery or radiotherapy, were enrolled into this study. The inclusion criteria included: no previous chemotherapy or chemotherapy and radiation therapy≥1 month before enrollment; Karnofsky performance status≥70; estimated life expectancy≥3 months; 18 to 75 years of age; body weight of 50 to 60 kg; serum transaminase≤2 times normal value; adequate bone marrow function (white blood cell (WBC) count≥4.0×109 L−1, platelet count≥100×109 L−1); adequate renal function (serum creatinine≤1.5 times normal value). The exclusion criteria included: pregnant or lactating women; serious infection or impairments of organ function; central nervous system (CNS) metastasis or more than two metastasis. Written informed consent to undergo pharmacokinetic studies was obtained from each patient.
Study design
The study was approved by the Ethical Committee of the First Affiliated Hospital, Zhejiang University and was carried out in accordance with the Declaration of Helsinki. Six patients with advanced NSCLC received the following in a 3-week schedule: gemcitabine infusion at a rate of 10 mg/m2 per min (1200 mg/m2, two hours infusion day 1 and 8) with carboplatin (AUC 5, day 1).
Drug and other chemicals
For clinical use, gemcitabine (trade name Gemzar®) was obtained from Eli Lilly Company (USA). Carboplatin (trade name Paraplatin®) was provided by Bristol-Myers Squibb Company (USA). Acetonitrile was HPLC grade and other chemicals were analytical grade.
Measurement of gemcitabine in plasma
A stock solution of gemcitabine was diluted with acetonitrile to a series of concentrations of working solutions. Calibration curves were prepared by analysis of 1 ml plasma samples spiked with 100 μl each of the gemcitabine working solutions to obtain the concentration range of 0.1–100 (0.1, 0.5, 1.0, 5.0, 10.0, 25.0, 50.0, 100.0) μg/ml. Then 0.5 ml of these standard calibration samples was vortex-mixed vigorously with 30% trichloracetic acid for 20 s and centrifuged at 10800 rpm for 15 min. The supernatants of the mixtures were applied to Millex™ and the filtrate (10 μl) was injected into the chromaography column.
Analysis of the serum concentration of gemcitabine was carried out using a Waters 2690 high-performance liquid chromatography (HPLC) system with a Waters 996 diode array UV detector. A Waters Symmetry C18 cartridge (4.6 mm×250 mm, 5 μm) fitted with a Security guard cartridge was used and maintained at a temperature of 25 °C. The mobile phase consisted of 0.52% phosphate buffer (pH 2.66) and acetonitrile (containing 0.202% sodium heptanesulfonate) at ratio of 85:15 (v:v) on a flow rate of 1.0 ml/min. Compounds were quantified by UV absorbance at a wave length of 273 nm. The chromatography data were collected and processed on Millennium32 software.
Pharmacokinetics
Plasma concentrations versus time data were analyzed using established noncompartmental methods with computer program 3P97 Pharmaceutical Kinetics Software to determine a number of pharmacokinetic parameters and to simulate expected gemcitabine plasma concentrations. Venous blood samples were withdrawn into EDTA-2Na anticoagulated collection tubes at 30, 120, 130, 140, 150, 165, 180, 210 min after the start of the infusion. All blood samples were placed in an ice water bath until they were disposed as described above. Plasma samples were then stored in polypropylene tubes at −20 °C until analyzed. The maximum concentration (C max) was observed values. The area under the curve (AUCtn) was estimated by trapezoidal rule with extrapolation to infinity using the ratio Cn/K e where Cn was the last measurable concentration. The elimination rate constant (K e) was estimated from the terminal linear segment of the log serum concentration/time data. The elimination half life (t 1/2) was calculated from ln2/K e. Clearance (CL) of the plasma drug was calculated by dividing the dose (D) of gemcitabine by the formula AUCtn: CL=D/AUC tn (ml/min). Mean residence time (MRT) was calculated by the formula AUMC tn (area under the first moment curve)/AUC tn.
Safety
Physical and clinical laboratory examination involving hematology, serum chemistries, urinalysis and vital sign measurements were done for each subject before dosing, during the dosing period, and at the end of the study to assess the tolerability of gemcitabine. Due to myelosuppression being the main adverse effect of gemcitatine, percentage decrease in WBC (white blood cell), NE (neutrophil), PLT (platelet), and Hb (hemoglobin) was calculated using the following equation: percentage decrease=(pretreatment value−value of the final dose)/(pretreatment value)×100% was evaluated in the safety analysis.
RESULTS
Determination of gemcitabine in plasma
The calibration curve of gemcitabine in plasma was linear in the range of 0.1 to 100 μg/ml. The concentrations (C) were calculated by peak area (A) values. The calibration curve’s regression was C=20037A−21.873, r=0.9999. The minimum detectable concentration of gemcitabine (signal-to-noise ratio of 3) in plasma was determined to be approximately 0.05 μg/ml. The overall precision, expressed as %RSD (Relative Standard Deviation) (n=6), was less than 1.94% and 7.34% for intra-day and inter-day assay, respectively. The method recovery (n=6) of 0.5, 10.0, and 100.0 μg/ml was within 97.39% to 103.11%.
Pharmacokinetic studies
Pharmacokinetic parameters describing gemcitabine disposition in the 6 Chinese NSCLC patients are presented in Table 1. The results indicated that the data from the individual subjects (Fig.1) fitted two compartments.
Table 1.
Pharmacokinetic parameters for gemcitabine summarized from published data (Abbruzzese et al., 1991; Bhargava et al., 2001; Kroep et al., 1999), infusion at a rate of 10 mg/(m2·min) (1000 mg/m2, 30-min infusion) and calculated for 6 Chinese NSCLC subjects after infusion at a rate of 10 mg/(m2·min) (1200 mg/m2, two hours infusion)
| Pharmacokinetic parameters | x¯±SD | Reported data |
| Ke (min−1) | 0.07±0.02 | |
| t1/2 (min) | 10.67±3.38 | 9–22.3 |
| Cmax (μg/ml) | 4.92±1.79 | 10.0–18.3 |
| AUCtn ((μg·h)/ml) | 7.55±1.53 | 7.5–11.4 |
| CL (ml/min) | 3940.05±672.08 | 2550–4080 |
| MRT (min) | 88.99±9.86 |
Fig. 1.
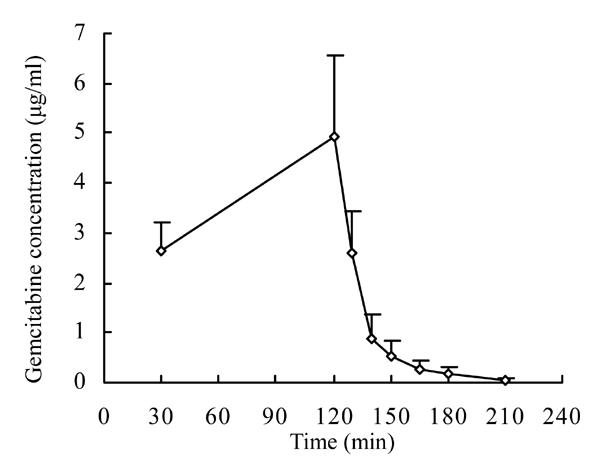
Plasma concentration of gemcitabine-time curve after infusion at a rate of 10 mg/(m2·min) (1200 mg/m2, two hours infusion) into six Chinese NSCLC subjects
Hematologic toxicology in patients
The changes of the hematologic parameters in NSCLC patients after infusion of gemcitabine at a rate of 10 mg/m2 per min for 2 h (1200 mg/m2) are presented in Table 2. WBC, NE, and PLT decreased by more than 50 percent, and Hb decreased by less than 20 percent.
Table 2.
Changes of the hematologic parameters in patients
| Items | Values |
| Number of study patients | 6 |
| Sex (male, female) | 5, 1 |
| Age (years, mean±SD) | 64.17±8.8 |
| Weight (kg, mean±SD) | 54.91±3.8 |
| Body surface area (m2, mean±SD) | 1.57±0.096 |
| WBC percentage of decrease (mean±SD) | 65.6%±0.17 |
| NE percentage of decrease (mean±SD) | 66.45%±0.22 |
| PLT percentage of decrease (mean±SD) | 55.5%±0.17 |
| Hb percentage of decrease (mean±SD) | 18.9%±0.14 |
DISCUSSION
This paper describes an ion-pair reversed-phase HPLC quantitative determination of gemcitabine in human plasma. We describe here an alternative procedure by precipitation by trichloroacetic acid in which the extraction procedure is comparatively simpler, faster and cheaper than solid-phase extraction. The method is suitable for determination of the drug plasma levels in patients undergoing clinical investigations and proved to be useful for evaluating pharmacokinetic properties of gemcitabine on the aspects of its specificity and reproducibility.
Numerous phase I studies of gemcitabine as a single agent led to the dose recommendation of 1000 mg/m2, administered as a 30-min infusion (Guchelaar et al., 1996; Storniolo et al., 1997). Using this treatment schedule, the toxicity profile of gemcitabine is low, with myelosuppression being the major side effect (Green, 1996). Prolonged gemcitabine infusion (fixed dose rate of 10 mg/(m2·min) for 120 min) showed to have efficacy comparable to that schemes using bolus administration of similar dose of gemcitabine (Manuel et al., 2002). However, this regimen has never been characterized in the Chinese advanced NSCLC patients until this study. In this respect, pharmacokinetic study on gemcitabine is considered to be essential.
Analysis of plasma concentrations of gemcitabine in 6 advanced Chinese NSCLC patients showed that the pattern of the concentration-time profile was similar to the results of other studies conducted with a dose of 1000 mg/m2 with 30-min infusion. Compared to pharmacokinetic data from the literature (Abbruzzese et al., 1991; Bhargava et al., 2001; Kroep et al., 1999), no apparent difference was found with respect to t 1/2, AUC, and CL. The mean parameters as t 1/2 (10.67±3.38 min), AUC (7.55±1.53 (µg·h)/ml), and CL (3940.05±672.08 ml/min). The maximum concentration (C max) was 4.92±1.79 μg/ml, which differed significantly from published data that showed C max was 10.0~18.3 μg/ml (a dose of 1000 mg/m2 with 30-min infusion). The discrepancy may be due to the different infusion time and dosage. The prolonged infusion time in our study resulted in gemcitabine plasma concentrations being maintained substantially higher than the effective anti-tumor concentration of 10 μmol/L for longer time than the 30-min infusion and will increase the clinical therapeutic effect. Meanwhile, the hematologic toxicology was moderate. Leucopenia, neutropenia, thrombocytopenia were more frequent, but anemia was less frequent.
CONCLUSION
In conclusion, the two-hour infusion (fixed dose rate of 10 mg/m2 per min) appeared to produce the desired gemcitabine effective plasma concentration and moderate hemotologic toxicity in the Chinese NSCLC patients following treatment in combination with carboplatin. Although the present study has some limitations due to the small number of patients and short follow-up, this is the first study to characterize the pharmacokinetic parameters of gemcitabine in Chinese NSCLC patients. Results from this study suggested that the combination of gemcitabine and carboplatin warrants further investigation in larger, randomized clinical trials. Furthermore, prospective studies evaluating pharmacodynamics and toxicology according to the AUC of gemcitabine are essential.
Footnotes
Project (No. 2004A028) supported by the Medical Science Research Foundation of Zhejiang Province, China
References
- 1.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9(3):491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Abratt RP, Bezwoda WR, Falkson G, Goehals L, Hacking D, Rugg TA. Efficacy and safety profile of gemcitabine in non-small cell lung cancer: a phase II study. J Clin Oncol. 1994;12(8):1535–1540. doi: 10.1200/JCO.1994.12.8.1535. [DOI] [PubMed] [Google Scholar]
- 3.Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single agent activity of weekly gemcitabine in advanced non-small cell lung cancer: a phase II study. J Clin Oncol. 1994;12(9):1821–1826. doi: 10.1200/JCO.1994.12.9.1821. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava P, Marshall JL, Fried K, Williams M, Lefebvre P, Dahut W, Hanfelt J, Gehan E, Figuera M, Hawkins MJ, et al. Phase I and pharmacokinetic study of two sequences of gemcitabine and docetaxel administered weekly to patients with advanced cancer. Cancer Chemother Pharmacol. 2001;48(2):95–103. doi: 10.1007/s002800100317. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi V, Plunkett W. Modulatory activity of 2′,2′-difluorodeoxycytidine on the phosphorylation and cytotoxicity of arabinosyl nucleosides. Cancer Res. 1990;50(12):3675–3680. [PubMed] [Google Scholar]
- 6.Green MR. Gemcitabine safety overview. Semin Oncol. 1996;23(5 suppl 10):32–35. [PubMed] [Google Scholar]
- 7.Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarassoff P, Plunkett W. Pharmacologically directed design of the dose rate and schedule of 2′,2′-difluorodeoxycytidine (gemcitabine) administration in leukemia patients. Cancer Res. 1990;50(21):6823–6826. [PubMed] [Google Scholar]
- 8.Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation of 2′,2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27(4):258–262. doi: 10.1007/BF00685109. [DOI] [PubMed] [Google Scholar]
- 9.Guchelaar HJ, Richel DJ, van Knapen A. Clinical, toxicological and pharmacological aspects of gemcitabine. Cancer Treat Rev. 1996;22(1):15–31. doi: 10.1016/s0305-7372(96)90014-6. [DOI] [PubMed] [Google Scholar]
- 10.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51(22):6110–6117. [PubMed] [Google Scholar]
- 11.Kroep JR, Giaccone G, Voorn DA, Smit EF, Beijnen JH, Rosing H, van Moorsel CJ, van Groeningen CJ, Postmus PE, Pinedo HM, et al. Gemcitabine and paclitaxel: pharmacokinetic and pharmacodynamic interactions in patients with non-small-cell lung cancer. J Clin Oncol. 1999;17(7):2190–2197. doi: 10.1200/JCO.1999.17.7.2190. [DOI] [PubMed] [Google Scholar]
- 12.Lund B, Ryberg M, Petersen PM, Anderson H, Thatcher N, Dombernowsky P. Phase II study of gemcitabine (2′,2′-difluorodeoxycytidine) given as a twice weekly schedule to previously untreated patients with non-small cell lung cancer. Ann Oncol. 1994;5(9):852–853. doi: 10.1093/oxfordjournals.annonc.a059018. [DOI] [PubMed] [Google Scholar]
- 13.Manuel D, Laura GE, Ana L. A phase II trial of a two hour infusion of gemcitabine with carboplatin for advanced non-small-cell lung cancer (NSCLC) Ann Oncol. 2002;13(suppl. 5):143. [Google Scholar]
- 14.Ruiz van Haperen VW, Veerman G, Vermorken JB, Peters GJ. 2′,2′-difluorodeoxycytidine (gemcitabine) incorporation into RNA and DNA of tumor cell lines. Biochem Pharmacol. 1993;46(4):762–766. doi: 10.1016/0006-2952(93)90566-f. [DOI] [PubMed] [Google Scholar]
- 15.Shord SS, Faucette SR, Gillenwater HH, Pascatore SL, Hawke RL, Socinski MA, Lindley C. Gemcitabine pharmacokinetics and interaction with paclitaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother pharmacol. 2003;51(4):328–336. doi: 10.1007/s00280-002-0560-1. [DOI] [PubMed] [Google Scholar]
- 16.Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol. 1997;24(2 Suppl 7):2–7. [PubMed] [Google Scholar]
- 17.Tempero M, Plunkett W, Ruiz van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21(18):3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]


