Abstract
Recent progress in mouse genetics has led to an increased interest in developing procedures for assessing mouse behavior, but relatively few of the behavioral procedures developed involve positively reinforced operant behavior. When operant methods are used, nose poking, not lever pressing, is the target response. In the current study differential acquisition of milk-reinforced lever pressing was observed in five inbred strains (C57BL/6J, DBA/2J, 129X1/SvJ, C3H/HeJ, and BALB/cJ) and one outbred stock (CD-1) of mice. Regardless of whether one or two levers (an “operative” and “inoperative” lever) were in the operant chamber, a concomitant variable-time fixed-ratio schedule of milk reinforcement established lever pressing in the majority of mice within two 120-min sessions. Substantial differences in lever pressing were observed across mice and between procedures. Adding an inoperative lever retarded acquisition in C57BL/6J, DBA/2J, 129X1/SvJ, and C3H/HeJ mice, but not in CD-1 and BALB/cJ mice. Locomotor activity was positively correlated with number of lever presses in both procedures. Analyses of durations of the subcomponents (e.g., time to move from hopper to lever) of operant behavior revealed further differences among the six types of mice. Together, the data suggest that appetitively reinforced lever pressing can be acquired rapidly in mice and that a combination of procedural, behavioral, and genetic variables contributes to this acquisition.
Keywords: behavioral genetics, operant acquisition, one-lever, two-lever, concomitant schedule, lever press, mice
Advances in genetic research now allow investigators to manipulate the DNA of nonhuman animals with great precision and reliability. Research in nonhuman mammalian genetics primarily has been focused on the laboratory mouse (see Knight & Abbott, 2002). Its robust reproductive physiology has made it the species of choice in the production of genetically mutated animals. Therefore, it is not surprising that there has been a renewed interest in mouse models of behavior among biologists, geneticists, neuroscientists, and psychologists (see e.g., Benson, 2004; Bucan & Abel, 2002).
Understanding how genes influence behavior is the conceptual basis for work in mouse behavioral genetics, the study of the behavioral effects of manipulating genes and describing their influence on psychological constructs and disorders (i.e., behavioral phenotypes of mouse genotypes; see Crawley, 2000). Genes can be manipulated experimentally, as is done with transgenic and knockout mice in which a gene is added or disabled, respectively. Genes also can be manipulated through formalized techniques of inbreeding that produce genetically “restricted” strains1 of mice (i.e., inbred or isogenic strains). Various inbred strains frequently serve as the “background” for mice with targeted mutations; that is, they are the mice in which the gene manipulation is expressed. Behavior of mutant mice is a function of the gene mutation as it interacts with the environment and its interaction with the background genotype (Gerlai, 1996). Potential effects of the background genotype on behavior make it essential to phenotype the background mouse—usually an inbred strain—in the absence of experimentally induced mutations.
A variety of behavioral procedures and methods—many adopted from the field of psychopharmacology (see Willner, 1991)—are used for assessing the influence of gene manipulations on behavior. Popular procedures include the object-recognition task, Morris water maze, elevated plus maze, rotarod, forced-swim test, prepulse inhibition of startle, and conditioned place preference. The use of operant methods with positive reinforcement contingencies has been relatively neglected in mouse behavioral genetics and neuroscience (Mihalick, Langlois, Krienke, & Dube, 2000). One factor hindering the widespread adoption of these methods is the presumed difficulty of establishing such behavior (Baron & Meltzer, 2001; see, e.g., Crawley, 2000, pp. 114–119). Recently, Baron and Meltzer showed that an automated operant procedure could be used to establish milk-reinforced nose poking relatively rapidly. They modified a two-session procedure previously conducted with rats (Byrne, Lesage, & Poling, 1997) for use in various types of mice. In the first session (120 min of “dipper-training”) mice were exposed to a variable-time (VT) 60-s schedule in which 0.01 ml of evaporated milk was presented noncontingently via a dipper; the dipper remained raised until a mouse entered the hopper and 10 s had elapsed. In the second 120-min session, conducted the next day, two response holes (i.e., nose-poke operanda) were introduced and food (10-s access) was delivered for each (fixed-ratio [FR] 1) poke in the “operative” hole; nose pokes in the “inoperative” hole did not result in food presentation. This session terminated at 120 min or after 120 operative nose pokes, whichever occurred first. Mice acquired operant nose poking within two sessions or approximately 240 min. There were differences across mouse types in various measures of acquisition, such as mean number of operative, inoperative, and total responses and the number of mice per group that emitted 50 or more operative responses. Baron and Meltzer also reported large and significant differences in locomotor activity—assessed with photobeam detectors—across types of mice, but suggested these differences were not correlated with acquisition of reinforced nose pokes.
The purpose of the present study was to establish operant lever pressing (as distinct from nose poking) in mice using a modified version of the automated procedure described by Baron and Meltzer (2001). To our knowledge, there have been no published reports demonstrating that operant lever pressing can be established in various types of inbred mice within two 120-min training sessions. Like Baron and Meltzer, we also assessed the relation between locomotor activity and operant acquisition. We altered their procedure in several ways. First, the VT 60-s (first session) and FR 1 (second session) schedules were combined into a concomitant reinforcement schedule (i.e., simultaneous combination of response-independent and response-dependent food delivery), so that mice could experience the reinforcement contingency in the first session. Second, response acquisition of naive mice was assessed under separate one- and two-lever procedures. This allowed us to examine the effects of an inoperative lever on acquisition of operative lever pressing. Finally, we used a “force-plate actometer,” a device with high temporal and spatial resolution (see below; Fowler, Birkestrand, et al., 2001), to measure locomotor activity in mice before exposure to the operant acquisition procedure (i.e., within-subject comparison). Although Baron and Meltzer reported that general locomotor activity could be dissociated from response acquisition, they used a photobeam-based system for measuring locomotion in separate mice groups (i.e., between-subjects comparison), but of the same type, from those assessed during nose-poke training. Their relatively low-resolution activity recording device likely was incapable of detecting much of the animal's movements (see Fowler, Birkestrand, et al., 2001). Thus their results concerning the noncorrelation of activity with response acquisition should be interpreted with caution.
Method
Subjects
Subjects were 71 male mice from five inbred strains (C57BL/6J [stock no. 000664], DBA/2J [no. 00671], 129X1/SvJ [no. 000691], C3H/HeJ [no. 000659], and BALB/cJ [no. 000651]: Jackson Laboratory, Bar Harbor, ME) and one outbred stock (CD-1: Charles River, Wilmington, MA).2 There were 35 mice (n = 6 of each type, except C57BL/6J [n = 5]) in the one-lever procedure and 36 mice (n = 6 of each type) in the two-lever procedure. Before the one-lever procedure began, a C57BL/6J mouse died of an unknown cause.
These mice are some of the most widely used in the neural, genetic, and behavioral sciences (see Festing & Fisher, 2000); thus a relatively broad base of information is available on these mice. For example, the C57BL/6J mouse is the most widely used inbred strain; it is considered an “alcohol-preferring” strain (i.e., readily consumes 10% ethanol solutions; see Phillips & Crabbe, 1991) and is, so far, the only mouse to have its genome sequenced (Mouse Genome Sequencing Consortium, 2002). The DBA strain is believed to be the first inbred mouse, created in 1909 by Clarence Little (Beck et al., 2000). The DBA/2J substrain was reported to be less sensitive to cocaine's disruptive effects on operant behavior (FR 15) than C57BL/6J, BALB/cByJ, and CD-1 mice (Heyser, McDonald, Beauchamp, Koob, & Gold, 1997). Mice of the 129/SvJ strain have been characterized as “poor” learners in the Morris water maze (see Crawley et al., 1997). In addition, 129 mouse substrains frequently provide the embryonic stem cells needed in creating knockout mice (Simpson et al., 1997). Several C3H substrains, including C3H/HeJ, are homozygous for a mutation that leads to blindness by approximately 20 days of age (Sidman & Green, 1965), although their locomotor activity may be discriminative of light versus dark conditions through 100 days of age (Nagy & Misanin, 1970). In various animal models of “anxiety,” BALB/c mice have been characterized as substantially more anxious than several other inbred strains (Belzung & Griebel, 2001). CD-1 mice are a common outbred albino stock, and the outbred mating strategy makes it likely that these mice will have more variable genes (alleles) and, possibly, more variable behavior than the inbred strains (Festing, 1999).
Upon arrival, mice (approximately 6 weeks of age) were group housed (3 to 4 per cage) for 1 week; thereafter, they were individually housed. Food-restriction (Purina® Rat Chow #5001) began when mice were approximately 9 weeks of age. After a 1-week period of gradual food restriction, their mean body weights ranged from 82% to 90% of their free-feeding weights. Mean body weights (and standard errors of the mean) are shown in Table 1. An individually adjusted daily ration of 2.2 to 3.0 g of chow maintained these weights. Water was available in their home cages ad libitum. Sessions were conducted in the morning and early afternoon during the light period of a 12:12 hr light/dark cycle (lights on at 6:00 a.m.). Experimental sessions began when the mice were approximately 10 weeks of age. The University of Kansas Animal Care and Use Committee approved all procedures.
Table 1. Descriptive statistics of body weights (in grams) and percentage of their free-feeding weight for all mouse types before experimental procedures began.
| Strain or stock | One-lever procedure |
Two-lever procedure |
||||
| M | SEM | % of free feed | M | SEM | % of free feed | |
| C57BL/6J | 20.8 | 0.4 | 85.2 | 20.9 | 0.5 | 83.8 |
| DBA/2J | 21.3 | 0.7 | 87.1 | 19.1 | 0.5 | 88.4 |
| 129X1/SvJ | 21.2 | 0.4 | 87.0 | 21.9 | 0.5 | 90.2 |
| C3H/HeJ | 22.1 | 0.6 | 84.7 | 22.0 | 0.6 | 88.7 |
| BALB/cJ | 21.1 | 0.5 | 82.3 | 21.5 | 0.5 | 86.8 |
| CD-1 | 29.5 | 0.9 | 86.5 | 30.3 | 1.1 | 83.9 |
Apparatus
Force-plate Actometer
Locomotor activity was assessed with a “force-plate actometer,” an automated apparatus that has been used in our laboratory to measure various rodent behaviors (e.g., Fowler, Zarcone, Vorontsova, & Chen, 2002; Wang & Fowler, 2001). The actometer and associated peripherals, as well as its method of data collection and analysis, have been described in detail elsewhere (see Fowler, Birkestrand, et al., 2001). Briefly, the force-plate actometer consists of a low-mass and highly stiff sensing surface (28 cm by 28 cm) made of “sandwiched” aluminum honeycomb and supported by four Sensotec (Columbus, OH) Model 31 load cells (0 to 250 g range). Each force plate rested below a Plexiglas enclosure, which was suspended 2 mm above the plate and applied no load onto the plate. A 5-V houselight, mounted on top of the Plexiglas enclosure, illuminated the actometer. A sound-attenuating cubicle (ENV-018, Med Associates Inc., St. Alban, VT) minimized outside noise while providing a support surface for the base of the actometer. Four force-plate actometers operated simultaneously and independently. The mouse's horizontal and vertical movements were recorded along the sensing surface with a spatial resolution of 1 mm and a temporal resolution of 0.02 s.
Operant Chamber
Six operant rat chambers (#E10-18-TC) from Coulbourn Instruments (Allentown, PA) were used. A Plexiglas inset (25 cm wide by 25 cm high) inside each chamber reduced the cage arena to a size (10 cm long by 25 cm wide by 33 cm high) consistent with many mouse chambers described in the literature. Each chamber was enclosed in a sound-attenuating box with an exhaust fan. A grid floor was positioned 3 cm above the base of the chamber and was made of stainless steel rods (0.5 cm diameter), with centers located 1 cm apart. A hopper (#E14-05; 3 cm wide by 6.5 cm high) recessed 3.5 cm behind the center of the intelligence panel (30 cm long by 25 cm wide by 33 cm high), 2 cm above the grid floor. A hole (0.8 cm diameter), located 1.5 cm deep inside the hopper provided access to 0.01 ml of a milk mixture (tap water ∶ Borden's Eagle® sweetened condensed milk was 2∶1) via an electromechanical dipper mounted behind the hopper. Above the access hole (1 cm above the hopper bottom and 1.5 cm inside the hopper), a photobeam detected hopper entries and withdrawals; a hopper light (28 V) was affixed to the top of the hopper. The operative and inoperative levers (mouse lever #E21-04) were as physically identical as the manufacturing method allowed. Both levers were positioned 2 cm above the grid floor and protruded 1 cm into the chamber; the response criterion was a minimum force of approximately 0.059 N for 0.02 s. In both the one- and two-lever procedures, an operative (reinforcement) lever was located 7.5 cm to the right of the hopper. In the two-lever procedure, a second (inoperative) lever was located 7.5 cm to the left of the hopper. Presses on the inoperative lever did not result in milk presentations. A “triple cue lamp” (#E11-02) made of three light-emitting diodes (LEDs; red, yellow, and green) was located 5 cm above each lever. These LEDs were in the “off” state while a lever was pressed with sufficient force to close its microswitch, but were “on” otherwise. A 28-V houselight was mounted on the top center of the intelligence panel. Digital input/output was obtained with a BaseBoard® PCI system (Scientific Solutions, Mentor, OH) connected to a Pentium I computer running MS-DOS 6.22. The controlling software was written by the second author in Free Pascal 1.06.
Procedure
Assessment of Locomotor Activity
Two to 5 days before operant training, locomotor activity (distance traveled in mm) was assessed in all mice on the force-plate actometer in a single 120-min session. A 5-V houselight illuminated the actometer throughout the session.
Operant Acquisition
The one- and two-lever procedures were conducted approximately 7 months apart in a between-subject design. In both lever procedures, mice were exposed to two 120-min sessions—one session a day for 2 consecutive days. The programmed contingencies were identical for each session. The houselight was illuminated throughout the session. The hopper light was illuminated when the dipper was raised and remained on until the dipper was lowered. At the start of each session, the milk dipper was raised once for 2 min, delivering a “free” reinforcer. The free reinforcer at the start of each session was programmed to increase the probability that a mouse would contact milk early in a session. Following the free milk presentation, a concomitant variable-time (VT) 60-s fixed-ratio (FR) 1 schedule of reinforcement was in effect. VT values consisted of 12 intervals and were computer generated using the Fleshler and Hoffman (1962) formula. Under the VT 60-s component, the dipper was raised until the mouse entered and withdrew from the hopper, or until the mouse entered the hopper and 5 s had elapsed, whichever occurred first. Under the FR 1 component, the dipper was raised for 10 s following a lever press, or until the mouse entered and withdrew from the hopper, whichever occurred first. If VT milk was scheduled during an FR reinforcer, the VT schedule was delayed until the dipper was lowered. Unpublished data from our laboratory indicated that while mice were in the hopper, they occasionally made superfluous movements that caused the photobeam to rapidly change state. This usually was detected as short (< 0.20 s) interhopper intervals. To prevent the dipper from lowering because of these rapid interruptions of the photobeam, program code did not allow the dipper to lower until an interhopper interval was greater than 0.20 s.
Data Analysis
The average number of operative and inoperative lever presses was determined for each strain or stock in each session and procedure (one or two levers). A three-way analysis of variance (ANOVA) was computed for the number of operative lever presses on log10-transformed data with two between-group factors (lever procedure [two levels] and mouse type [six levels]) and one repeated measures factor (session [two levels]). Pearson correlations were computed to determine whether a relation existed between operative and inoperative lever presses in each session of the two-lever procedure. Cumulative records allowed us to examine operative lever pressing in individual subjects on a within- and between-group (i.e., strain and stock and procedure) basis.
One method of characterizing response acquisition has been to count the number of animals that emitted some criterion number of target responses in a session, such as 50 operative nose pokes (e.g., Baron & Meltzer, 2001); however, because presses on the operative lever alone do not ensure reinforcer consumption, we examined response acquisition in terms of the number of response cycles (defined below), rather than the number of operative lever presses. We determined the number of mice in each group that emitted at least 10, 50, and 100 response cycles during the two-session procedure. It was assumed that the acquisition process would be largely completed once 100 response cycles were emitted.
A response cycle consisted of four response components occurring in temporal succession: pressing the operative lever, moving to the hopper, consuming milk in the hopper, and moving back to the operative lever (i.e., lever-press to reinforcement to lever-press). Three components of the first 100 response cycles are presented with respect to their duration: lever-hopper movement duration, hopper duration, and hopper-lever movement duration. These durations were log10-transformed for each mouse at each of the first 100 response positions; the average value in each mouse type was then determined across the first 100 response positions. Note, however, that the number of mice that contributed to the mean varied because not all mice emitted at least 100 response cycles. For example, if 1 of 6 mice from a strain emitted 75 response cycles during acquisition, but the other 5 mice emitted 100 or more response cycles, then beginning at response position 76, 5 mice contributed to the mean. Finally, a LOWESS (locally weighted least squares) smooth, with a tension of 0.20, was applied to the logged durations to gain a graphic appreciation of trends in movement durations during lever-press acquisition. Although lever-press durations were analyzed, these data are not shown because lever-press durations did not change appreciably across the first 100 responses, and they did not differ substantially among mouse type and procedure.
Distance traveled on the force-plate actometer was calculated by sampling the mouse's (x, y) coordinates every 0.64 s and adding the distance between samples across the 120-min session. The average distance traveled in each strain and stock was converted from millimeters to meters, for ease of presentation. One-way ANOVA was computed for distance traveled with mouse type (six levels) as a between-groups factor. To assess the relation between general locomotor activity and operant acquisition, we plotted the average number of lever presses in both operant sessions as a function of distance traveled on the force-plate actometer for each mouse type. First-order polynomials (i.e., straight lines) were fit to these data, and the proportion of variance accounted for by the fit (R2) was computed for both the one- and two-lever procedures. ANOVAs and Pearson correlations were computed in SYSTAT® 10.2. LOWESS smoothes and first-order polynomials were calculated in GraphPad Prism® 4.0b.
Results
Group Means of Operative and Inoperative Lever Presses
The left and right panels of Figure 1 show the mean number of operative lever presses for each mouse type in the one- and two-lever procedures. The inset of the right panel depicts the inoperative lever presses. An ANOVA on the number of operative lever presses indicated a significant effect of mouse type, F(5, 59) = 11.63, p < .001, and lever procedure, F(1, 59) = 19.20, p < .001. Although there was no statistically significant interaction between mouse type and lever procedure, F(5, 59) = 1.83, p = .121, Figure 1 shows that substantially fewer operative lever presses were emitted by the C57BL/6J, DBA/2J, 129X1/SvJ, and C3H/HeJ strains in the two- than in the one-lever procedure; such an effect was not observed in the BALB/cJ strain and CD-1 stock. The lack of a significant interaction between mouse type and lever procedure is likely due to the variance produced by the presence of non- or low-responders in some groups of mice.
Figure 1. Mean (± 1 SEM) number of operative lever presses for each session of the one- (left panel) and two-lever (right panel) acquisition procedures in each mouse strain and stock.
Mean number of inoperative lever presses in the two-lever procedure is indicated in the inset of the right panel (note the different y axes). Note that the CD-1 stock exposed to the two-lever procedure has a missing horizontal error line to indicate error boundary of operative presses in the second session. This error boundary is not within the scale; the standard error value is 145.67.
A significant effect of session, F(1, 59) = 18.98, p < .001, statistically confirmed the increase in operative lever presses from the first to second session by most mouse types during both lever procedures. The significant interaction between session and mouse type, F(5, 59) = 3.82, p = .005, suggests that the increase in number of operative lever presses from the first to the second session differed among mouse types. Figure 1 shows that this interaction is attributable primarily to the 129X1/SvJ and C3H/HeJ strains: In the one- and two-lever procedures, the number of operative lever presses by the 129X1/SvJ strain did not differ appreciably between the first and second session; however, the change in number of operative lever presses between the first and second session was greater in the C3H/HeJ strain than in the other mouse types.
Although BALB/cJ and CD-1 mice pressed the inoperative lever more frequently than the other strains (see inset of right panel in Figure 1), they also pressed the operative lever more than the other strains. Similarly, 129X1/SvJ and C3H/HeJ mice pressed the inoperative and operative levers less frequently than other mice. Pearson correlations of the first (r = 0.90, r2 = 0.81) and second (r = 0.92, r2 = 0.85) session of the two-lever procedure statistically confirmed this correlation between operative and inoperative lever presses among mouse types. The greater proportion of responding on the operative lever than on the inoperative lever, however, suggests that lever pressing was discriminative of the contingencies.
Cumulative Records of Operative Lever Presses
Figures 2 (one-lever procedure) and 3 (two-lever procedure) show the cumulative records of operative lever pressing during each acquisition session. The left and right columns of each figure correspond to the first and second acquisition session, respectively. Both figures show large variability in number of operative lever presses within mouse type, regardless of procedure or session. Nevertheless, differences in operative lever pressing between strains and stock are discernable in the cumulative records. During the first session of the one-lever procedure (Figure 2, left column), most C57BL/6J, DBA/2J, BALB/cJ, and CD-1 mice were lever pressing, relatively rapidly, within the first 80 min; in the second session, most of these mice began lever pressing within the first 5 min. Three mice of the 129X1/SvJ strain also lever pressed in a similar manner; however, the remaining 3 mice emitted relatively few operative lever presses in both sessions. Unlike most of the other mouse types during the first session of the one-lever procedure, lever pressing by the C3H/HeJ strain began relatively late, approximately 80 min into the session. In the second session of the one-lever procedure, lever pressing by C3H/HeJ mice again began relatively late, but by 40 min these mice were lever pressing in a proficient manner. As suggested by Figure 3, operative lever pressing by the C57BL/6J, DBA/2J, 129X1/SvJ, and C3H/HeJ strains was retarded by the presence of an inoperative lever, particularly in the first session. Although several C57BL/6J and DBA/2J mice lever pressed in a proficient manner by the second session, the number of lever presses emitted by these mice was usually fewer, when compared with these same strains in the one-lever procedure (see Figure 2). For 129X1/SvJ and C3H/HeJ mice of the two-lever procedure, operative lever pressing was either low or, in most cases, nonexistent. Operative lever pressing by BALB/cJ and CD-1 mice, however, did not differ appreciably between the one- and two-lever procedures.
Figure 2. Cumulative records of operative lever presses by individual mice during the first (left panels) and second (right panels) session of the one-lever procedure.
Figure 3. Cumulative records of operative lever presses by individual mice during the first (left panels) and second (right panels) session of the two-lever procedure.
In the second session, a CD-1 mouse emitted 1,350 operative presses.
Nonrate Measures of Response Acquisition
As described earlier, a response cycle can be conceptualized as a sequence of four components (i.e., pressing the operative lever, moving to the hopper, consuming the reinforcer while inside the hopper, and moving back to the lever), with the completion of these four components constituting one operant response cycle. Counting response cycles, rather than number of operative lever presses, as a measure of acquisition is possibly a more accurate approach because it ensures that behaviors relevant to the reinforcement contingency also are emitted. That is, lever presses are followed by hopper entries and lever presses follow those hopper entries. Table 2 shows the number of mice for each type that emitted at least 10, 50, and 100 response cycles in the one- and two-lever procedures. In the one-lever procedure, all mice of the C57BL/6J, DBA/2J, C3H/HeJ, BALB/cJ, and CD-1 group emitted at least 10, 50, and 100 response cycles, whereas, 4 mice of the 129X1/SvJ strain emitted at least 10 response cycles, and only 3 mice of this strain emitted at least 50 and 100 response cycles. In the two-lever procedure, all mice of the BALB/cJ and CD-1 groups emitted at least 100 response cycles; however, fewer mice of the remaining strains emitted 10, 50, and 100 response cycles. Whereas the majority of C57BL/6J and DBA/2J mice from the two-lever procedure emitted 10, 50, and 100 response cycles, 3 C3H/HeJ mice emitted 100 response cycles. Two mice of the 129X1/SvJ strain emitted 10 response cycles and only 1 mouse of this strain emitted at least 100. These data show that more mice acquired the lever-press contingency in the one- than in the two-lever procedure. These data also show that the two-lever procedure afforded greater separation among mouse types in terms of their acquisition of lever pressing.
Table 2. Number of mice by strain or stock that emitted at least 10, 50, and 100 response cycles in the one- and two-lever acquisition procedures.
| Strain or stocka | One-lever procedure |
Two-lever procedure |
||||
| 10 | 50 | 100 | 10 | 50 | 100 | |
| C57BL/6J | 5 | 5 | 5 | 5 | 4 | 4 |
| DBA/2J | 6 | 6 | 6 | 4 | 4 | 4 |
| 129X1/SvJ | 4 | 3 | 3 | 2 | 1 | 1 |
| C3H/HeJ | 6 | 6 | 6 | 4 | 3 | 3 |
| BALB/cJ | 6 | 6 | 6 | 6 | 6 | 6 |
| CD-1 | 6 | 6 | 6 | 6 | 6 | 6 |
Six mice of each strain and stock in the one- and two-lever procedure, except C57BL/6J in the one-lever procedure (n = 5).
Figures 4 through 9 depict, for the first 100 response cycles, the durations of three components: lever-hopper duration, hopper duration, and hopper-lever duration. Each data point represents the mean of the logged values for each mouse type at a particular response position, but as mentioned above, the number of mice that contribute to the mean varies because not all mice from each strain or stock emitted 100 response cycles. Table 2 shows that, with the exception of the 129X1/SvJ strain from the two-lever procedure, at least half of the mice—and in most cases, a majority—from each strain or stock emitted 100 or more response cycles by the end of the 4-hr exposure to operant contingencies. Beginning at response position 24, only 1 mouse of the 129X1/SvJ strain during the two-lever procedure contributed to each point. Thus these points do not depict an average; rather, they depict 1 (129X1/SvJ) mouse. The results obtained from this method of presenting response durations, therefore, should be interpreted carefully. Nevertheless, a descriptive account of the temporal dimensions of operant behavior might show differences in acquisition to be related, at least in part, to differential motor behaviors among the strains and stock.
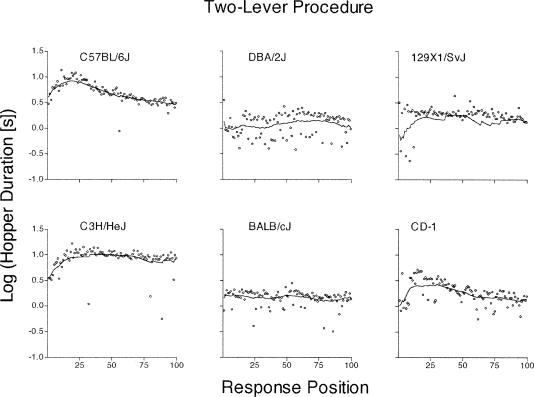
Figure 4. Mean of the log10 lever-hopper duration at each of the first 100 response positions from the one-lever procedure.
Each fitted line represents the LOWESS smooth (tension = 0.20) in a particular strain or stock. Log values of zero and 1 are equal to 1 and 10 s, respectively.
Figure 5. Mean of the log10 lever-hopper duration at each of the first 100 response positions from the two-lever procedure.
See Figure 4 caption for details.
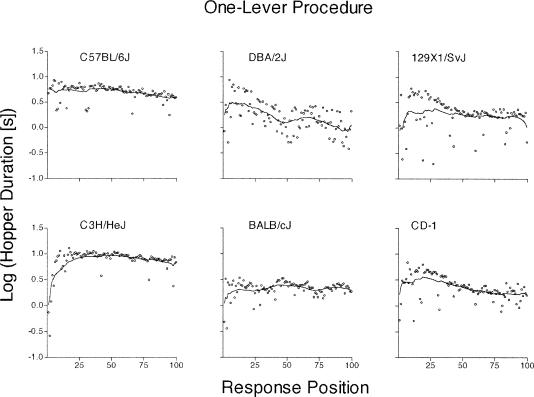
Figure 6. Mean of the log10 hopper duration at each of the first 100 responses from the one-lever procedure.
See Figure 4 caption for details. Four points are below the y-axis scale for the 129X1/SvJ strain.
Figure 7. Mean of the log10 hopper duration at each of the first 100 response positions from the two-lever procedure.
See Figure 4 caption for details.
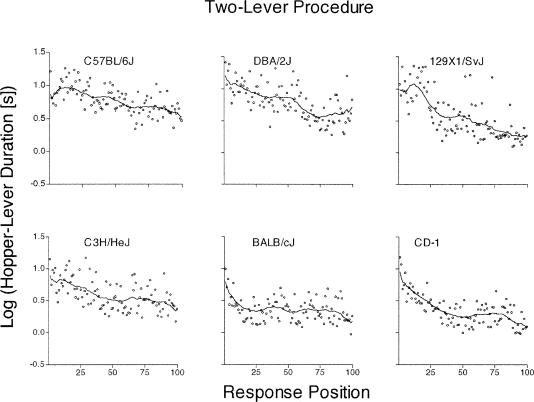
Figure 8. Mean of the log10 hopper-lever duration at each of the first 100 response positions from the one-lever procedure.
See Figure 4 caption for details.
Figure 9. Mean of the log10 hopper-lever duration at each of the first 100 response positions from the two-lever procedure.
See Figure 4 caption for details.
Lever-hopper Movement Durations
Lever-hopper durations during the one- and two-lever procedures are shown in Figures 4 and 5, respectively. Overall, both figures provide evidence of faster movements as the acquisition process continued. Each mouse type emitted increasingly shorter lever-hopper durations that reached asymptote between the 25th and 50th response. The lever-hopper durations for the C3H/HeJ strain of the one-lever procedure, however, did not reach asymptote until approximately the 75th response position. In the one-lever procedure (Figure 4), the first few lever-hopper durations in the 129X1/SvJ and C3H/HeJ strains were substantially longer than the other mouse types (over 10 s, compared to 3 to 5 s for the other four mouse types). In the two-lever procedure (Figure 5), the DBA/2J, C3H/HeJ, and CD-1 mice initially had longer lever-hopper durations than the other strains. The greater variability observed in 129X1/SvJ mice from the two-lever procedure (Figure 5, top right panel) is probably a function of the relatively few subjects observed here (see Table 2). C57BL/6J, BALB/cJ, and CD-1 mice from both procedures had shorter lever-hopper durations than the other strains. BALB/cJ and CD-1 mice, in particular, frequently emitted lever-hopper durations whose log10 values were less than zero (i.e., less than 1 s)—indicating that these mice were particularly fast at this component of the lever-press to reinforcement to lever-press cycle.
Hopper Durations
C57BL/6J and C3H/HeJ mice—during both the one- and two-lever procedures (Figures 6 and 7, left panels)—had longer hopper durations than did the other mouse types. The 129X1/SvJ mouse from the two-lever procedure (Figure 7, top right panel) emitted four hopper durations that were shorter than the specified scale, which is reflected in the sharp “dips” of the LOWESS smooth, beginning after response position 25. Although DBA/2J mice from the one-lever procedure (see Figure 6, top middle panel) and C57BL/6J mice from the two-lever procedure (see Figure 7, top left panel) tended to exhibit shorter hopper durations across successive responses, most of the functions were nonmonotonic. Overall, these data show that the C57BL/6J strain and, to a larger extent, the C3H/HeJ strain, were slow at this component of the cycle. In all mouse types, the hopper durations changed either very little, or not at all, relative to the lever-hopper durations.
Hopper-lever Durations
Figures 8 and 9 show the hopper-lever durations in the one- and two-lever procedure, respectively. These data were often more variable than the lever-hopper and hopper durations (compare the scatter of points relative to the LOWESS line). With the exception of the BALB/cJ strain and CD-1 stock, the shapes of the functions are less consistent across the two procedures and other mouse types. In addition, the hopper-lever durations usually were longer than the lever-hopper durations (compare Figures 4 and 5 vs. Figures 8 and 9). Nevertheless, hopper-lever durations tended to decrease among mouse types across the first 100 responses of both lever procedures.
Locomotor Activity and Lever Pressing
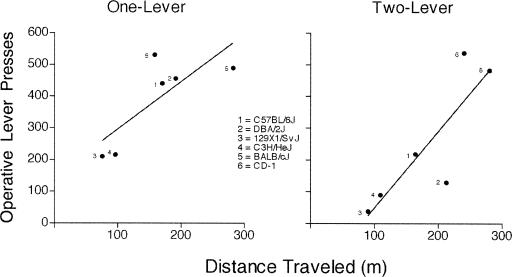
Mice from the one- and two-lever procedures were assessed in the force-plate actometers, 2 to 5 days before acquisition training. Figure 10 shows the mean distance traveled in the actometer, plotted against the mean number of lever presses in both acquisition sessions in each mouse type. The number to the left of each data point denotes the type of mouse. Lines of best fit are first-order polynomial functions.
Figure 10. Relation between locomotor activity and lever-press acquisition in one- (left panel) and two-lever (right panel) procedures.
Distance traveled (mean) in one 120-min session, as measured on the force-plate actometer, plotted as a function of the average number of operative lever presses in the first and second session. Each curve depicts a first-order polynomial fitted to the data.
One-way ANOVA indicated significant differences in distance traveled among mouse types in the one- (F[5, 29] = 17.26, p < .001) and two-lever (F[5, 30] = 23.04, p < .001) procedures. With the exception of the CD-1 stock, these differences were consistent between mice from the one- and two-lever procedures. Rank order of distance traveled—from least to most—in mice from the one-lever procedure was: 129X1/SvJ < C3H/HeJ < CD-1 < C57BL/6J < DBA/2J < BALB/cJ; rank order of distance traveled in mice from the two-lever procedure was: 129X1/SvJ < C3H/HeJ < C57BL/6J < DBA/2J < CD-1 < BALB/cJ. Straight line fits for the one- (Figure 10, left panel) and two-lever (Figure 10, right panel) procedure suggested a relation between distance traveled in the force-plate actometer and number of operative lever presses during acquisition. The R2 values of the fits were 0.61 and 0.73 for the one- and two-lever procedure, respectively. The lack of a simple one-to-one linear relation between these measures indicates that locomotor activity cannot completely predict lever-press acquisition; nevertheless, locomotor activity contributes to lever-press acquisition.
Discussion
Our finding of differential acquisition of lever pressing in inbred and outbred mice is consistent with frequently reported findings of genetically based behavioral differences among different types of mutant mice (Crawley et al., 1997; Wehner, Radcliffe, & Bowers, 2001). The present data, however, indicate that procedural and behavioral variables, as well as genetic variables, affect response acquisition. Had acquisition been characterized from the results of the one-lever procedure, we would have concluded that the C57BL/6J, DBA/2J, BALB/cJ, and CD-1 groups learned to lever press equally quickly, or roughly so; but in the two-lever procedure, only the BALB/cJ and CD-1 mice acquired the response in a manner similar to their acquisition in the one-lever procedure. Although the interaction between mouse type and lever procedure was not significant by ANOVA criteria, both the cumulative records (Figures 2 and 3) and graphic differences between the means (Figure 1) suggest that the procedural variable of adding an inoperative lever negatively affected response acquisition in some strains, but not in others. The correspondence between locomotor activity in a force-plate actometer and response acquisition did not account for all the behavioral variance in this study; nevertheless, the data suggested a substantial positive relation between the variables. Despite the force-plate actometer being an improvement over photobeam apparatus, the relation between locomotor activity and operant acquisition should be examined with technology that allows the precise measurement of locomotor and operant behavior simultaneously. In addition, more mouse types need to be evaluated before any general conclusions are drawn regarding the relation between activity and operant acquisition.
Baron and Meltzer (2001) reported that all mice of the C57BL/6J, 129X1/SvJ, and C3H/HeJ strains emitted at least 50 operative nose pokes in their FR 1 acquisition session, whereas not all BALB/cByJ (closely related, but different from the BALB/cJ strain), DBA/2J, and CD-1 mice emitted 50 or more nose pokes. Their findings differ from the results of the present study (see Table 2). Specifically, only half of the mice from the 129X1/SvJ strain emitted 50 or more response cycles during the one-lever procedure, and only 1 of 6 129X1/SvJ mice emitted 50 or more response cycles in the two-lever procedure. In addition, all BALB/cJ and CD-1 mice during both lever procedures emitted at least 50 response cycles. Furthermore, the frequency of operative nose poking reported by Baron and Meltzer was substantially less than the frequency of operative lever pressing observed in the present study. These differences are probably attributable to procedural differences between the studies. In the former study, acquisition of nose poking occurred in a single (FR 1) session that terminated after 120 operative responses or 120 min, whichever occurred first. In the present study, acquisition of lever pressing could occur within two 120-min sessions, and these sessions did not terminate upon a criterion number of responses.
The duration data of the response-cycle components served as supplementary dependent variables to the conventional rate measure; it was an additional method of describing behavioral differences among strains and stock. Although these motor behaviors are functionally distinct from locomotor activity as measured in the force-plate actometer, both types of motor behavior likely contribute to operant acquisition. As an organism's behavior contacts (i.e., as the animal “explores”) its environment, the probability of learning new behavior-environment relations (e.g., ways of obtaining food in a novel environment) increases (see Figure 10). In addition, the more rapidly a response is differentiated (see Figures 4 through 9) with respect to these new relations, the more successful that organism may be (cf. Catania, 1998, pp. 32–33). Some evidence in favor of this interpretation was seen in the present study. For example, the relatively longer hopper durations observed in the C3H/HeJ strain might have contributed to its slower acquisition, relative to the BALB/cJ strain and CD-1 stock. The duration data of the present study, however, should be interpreted carefully because these data did not account for the differential attrition (see Table 2) among mouse types. Nevertheless, the finding that some of these mouse types showed tendencies to be slower during specific response components perhaps is worth further experimental analysis.
Baron and Meltzer (2001) reported that BALB/cByJ and CD-1 mice showed more locomotor activity than did the other mouse types—a finding that is generally consistent with ours; however, their 129X1/SvJ strain had nearly twice as much locomotor activity as did their C3H/HeJ strain. Furthermore, their DBA/2J strain only showed approximately 20% more locomotor activity than their C3H/HeJ strain. Conversely, on the force-plate actometer, the 129X1/SvJ strain showed slightly less locomotor activity than the C3H/HeJ strain, and the DBA/2J strain showed twice as much locomotor activity as the C3H/HeJ strain (see Figure 10). These quantitative discrepancies between the two studies may be attributed to the different apparatuses used. Baron and Meltzer assessed locomotor activity with a photobeam-based chamber that was a square of 10 cm on a side (floor area = 100 cm2) and it had a spatial resolution of 2.5 cm (i.e., photobeams were 2.5 cm apart), whereas the force-plate actometer used in the present study was a square of 28 cm on a side (floor area = 784 cm2) and it had a spatial resolution of 1 mm. With its larger floor area and higher spatial resolution, mice in the force-plate actometer would be expected to show more locomotor activity than they would in a smaller apparatus. Therefore, we observed approximately 10-fold more locomotor activity in the force-plate actometer than did Baron and Meltzer with a photobeam apparatus.
Compared with many of the behavioral procedures used in mouse genetics research, the acquisition procedure afforded several advantages. First, the instrumentation measured relatively discrete behavior in a wholly objective and quantitative manner. This contrasts with many of the behavioral procedures currently used in mouse genetics in which human observers are required to assess and quantify less-than-discrete behavior (e.g., rating scales for drug-induced stereotypies; see Karler, Bedingfield, Thai, & Calder, 1997). Second, automation may minimize the effect of experimenter intrusion. Because the extended presence of the experimenter in the experimental situation has been suspected to contribute to some of the behavioral differences observed among mouse labs (Fowler, Zarcone, & Vorontsova, 2001; see also Crabbe, Wahlsten, & Dudek, 1999, for an example of a failure to replicate mouse behavior in different labs), procedures that minimize the role of the experimenter may be advantageous. Finally, the data from the acquisition procedure suggested that lever pressing was established in a fairly rapid manner, more rapidly in fact than in the 4 hr nose-poke procedure of Baron and Meltzer (2001). Baron and Meltzer first exposed their mice to a single 120-min VT 60-s session in which nose-poke holes were blocked with steel panels. In the second session, removal of the steel panels and an FR 1 contingency allowed response acquisition to develop. Conversely, in the present study, the concomitant schedule of reinforcement and continuous access to the response levers in both sessions of the lever-press acquisition procedure allowed lever pressing to occur within the first session, rather than the second session. The rapidity of lever-press acquisition in the present study also compares favorably with other studies on the acquisition of positively reinforced behavior in mice (Mihalick et al., 2000; Zarcone, Chen, & Fowler, 2004), as well as with autoshaping procedures for nose poking (Vanover & Barrett, 1998) and lever pressing (McDonald et al., 1998) in mice.
Given that previous studies (e.g., Lattal & Abreu-Rodrigues, 1997) have shown that the continued presentation of response-independent food degrades the response-reinforcer correlation (e.g., behavior maintained by variable-interval schedules), one might expect that the concomitant VT 60-s FR 1 schedule of the current experiment would be less than optimal—especially for mice that acquire the FR contingency readily. An effective modification of the current acquisition procedure may be to discontinue the VT component once response rate reaches some threshold, and to reintroduce the VT component if rate falls below some threshold. The question remains, of course, whether schedule-appropriate behavior can be rapidly established according to more complex schedules of reinforcement—for example, multiple and mixed schedules that have large ratio and/or long interval requirements. Such studies may yield findings of interest to both neuroscientists and behavioral scientists while addressing questions involving the intricate interaction among genes, environment, and behavior.
Acknowledgments
We thank Elena Vorontsova and Greg Osterhaus for their assistance with this experiment. We also thank the Biobehavioral Measurement Core and Ken Ratzlaff and John Ledford, of the Instrumentation Design Lab at the University of Kansas, for the design and construction of the operant interfacing equipment. Financial support was provided by the National Institute of Mental Health (MH 43429) and National Institute of Child Health and Human Development (HD 2528-37). Troy Zarcone is now at the University of Rochester Medical Center.
Footnotes
The terms strain and stock are often, but not universally, used in mouse genetics to differentiate inbred from outbred mice, respectively. An inbred strain results from at least 20 consecutive generations of full brother × sister mating; the genetic effect of such inbreeding is a strain in which at least 98.6% of the loci are homozygous in each mouse (Festing, 1979, pp. 5 & 54–56). Techniques for deriving outbred stocks are less clearly defined, but brother × sister mating is generally avoided (Silver, 1995, p. 43). Thus outbred stocks have more variable genes than do inbred strains, although the amount of variability can differ substantially among colonies (see Festing, 1993).
More than 2,500 inbred mouse strains currently exist, and these mice often have nearly identical names. This naming complexity is made more problematic because the mouse genetics literature is full of incomplete or inaccurate inbred strain designations. In order to be as clear as possible in describing our inbred mice, we have specified a strain's complete name and its unique stock number.
References
- Baron S.P, Meltzer L.T. Mouse strains differ under a simple schedule of operant learning. Behavioural Brain Research. 2001;118:143–152. doi: 10.1016/s0166-4328(00)00322-3. [DOI] [PubMed] [Google Scholar]
- Beck A, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig J.T, Festing M.F.W, Fisher E.M.C. Genealogies of mouse inbred strains. Nature Genetics. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behavioural Brain Research. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Benson E.S. Behavioral genetics: Meet molecular biology. Monitor on Psychology. 2004;35:42–45. [Google Scholar]
- Bucan M, Abel T. The mouse: Genetics meets behaviour. Nature Reviews Genetics. 2002;3:114–123. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- Byrne T, LeSage M.G, Poling A. Effects of chlorpromazine on rats' acquisition of lever-press responding with immediate and delayed reinforcement. Pharmacology Biochemistry and Behavior. 1997;58:31–35. doi: 10.1016/s0091-3057(96)00454-6. [DOI] [PubMed] [Google Scholar]
- Catania A.C. Learning. Saddle River, NJ: Prentice Hall; 1998. (4th ed.). [Google Scholar]
- Crabbe J.C, Wahlsten D, Dudek B.C. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. What's wrong with my mouse? New York: Wiley-Liss; 2000. [Google Scholar]
- Crawley J.N, Belknap J.K, Collins A, Crabbe J.C, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Festing M.F.W. Inbred strains in biomedical research. New York: Oxford University Press; 1979. [Google Scholar]
- Festing M.F.W. Genetic variation in outbred rats and mice and its implications for toxicological screening. Journal of Experimental Animal Science. 1993;35:210–220. [PubMed] [Google Scholar]
- Festing M.F.W. Warning: The use of heterogeneous mice may seriously damage your research. Neurobiology of Aging. 1999;20:237–244. doi: 10.1016/s0197-4580(99)00040-8. [DOI] [PubMed] [Google Scholar]
- Festing M.F.W, Fisher E.M.C. Mighty mice. Nature. 2000;404:815. doi: 10.1038/35009167. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman H. A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S.C, Birkestrand B.R, Chen R, Moss S.J, Vorontsova E, Wang G, Zarcone T.J. A force-plate actometer for quantitating rodent behaviors: Illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. Journal of Neuroscience Methods. 2001;107:107–124. doi: 10.1016/s0165-0270(01)00359-4. [DOI] [PubMed] [Google Scholar]
- Fowler S.C, Zarcone T.J, Vorontsova E. Haloperidol-induced microcatalepsy differs in CD-1, BALB/c, and C57BL/6 mice. Experimental and Clinical Psychopharmacology. 2001;9:277–284. doi: 10.1037//1064-1297.9.3.277. [DOI] [PubMed] [Google Scholar]
- Fowler S.C, Zarcone T.J, Vorontsova E, Chen R. Motor and associative deficits in D2 dopamine receptor knockout mice. International Journal of Developmental Neuroscience. 2002;20:309–321. doi: 10.1016/s0736-5748(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Gene-targeting studies of mammalian behavior: Is it the mutation or the background genotype? Trends in Neuroscience. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- Heyser C.J, McDonald J.S, Beauchamp V, Koob G.F, Gold L.H. The effects of cocaine on operant responding for food in several strains of mice. Psychopharmacology. 1997;132:202–208. doi: 10.1007/s002130050337. [DOI] [PubMed] [Google Scholar]
- Karler R, Bedingfield J.B, Thai D.K, Calder L.D. The role of the frontal cortex in the mouse in behavioral sensitization to amphetamine. Brain Research. 1997;757:228–235. doi: 10.1016/s0006-8993(97)00221-7. [DOI] [PubMed] [Google Scholar]
- Knight J, Abbott A. Mouse genetics: Full house. Nature. 2002;417:785–786. doi: 10.1038/417785a. [DOI] [PubMed] [Google Scholar]
- Lattal K.A, Abreu-Rodrigues J. Response-independent events in the behavior stream. Journal of the Experimental Analysis of Behavior. 1997;68:375–398. doi: 10.1901/jeab.1997.68-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M.P, Wong R, Goldstein G, Weintraub B, Cheng S, Crawley J.N. Hyperactivity and learning deficits in transgenic mice bearing a human mutant thyroid hormone ß1 receptor gene. Learning and Memory. 1998;5:289–301. [PMC free article] [PubMed] [Google Scholar]
- Mihalick S.M, Langlois J.C, Krienke J.D, Dube W.V. An olfactory discrimination procedure for mice. Journal of the Experimental Analysis of Behavior. 2000;73:305–318. doi: 10.1901/jeab.2000.73-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Nagy Z.M, Misanin J.R. Visual perception in the retinal degenerate C3H mouse. Journal of Comparative and Physiological Psychology. 1970;72:306–310. doi: 10.1037/h0029465. [DOI] [PubMed] [Google Scholar]
- Phillips T.J, Crabbe J.C. Behavioral studies of genetic differences in alcohol action. In: Crabbe J.C, Harris R.A, editors. The genetic basis of alcohol and drug action. New York: Plenum Press; 1991. pp. 25–104. [Google Scholar]
- Sidman R.L, Green M.C. Retinal degeneration in the mouse: Location of the rd locus in linkage group XVII. The Journal of Heredity. 1965;56:23–29. doi: 10.1093/oxfordjournals.jhered.a107364. [DOI] [PubMed] [Google Scholar]
- Silver L.M. Mouse genetics: Concepts and applications. New York: Oxford University Press; 1995. [Google Scholar]
- Simpson E.M, Linder C.C, Sargent E.E, Davisson M.T, Mobraaten L.E, Sharp J.J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nature Genetics. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Vanover K.E, Barrett J.E. An automated learning and memory model in mice: Pharmacological and behavioral evaluation of an autoshaped response. Behavioural Pharmacology. 1998;9:273–283. [PubMed] [Google Scholar]
- Wang G, Fowler S.C. Concurrent quantification of tremor and depression of locomotor activity induced in rats by harmaline and physostigmine. Psychopharmacology. 2001;158:273–280. doi: 10.1007/s002130100882. [DOI] [PubMed] [Google Scholar]
- Wehner J.M, Radcliffe R.A, Bowers B.J. Quantitative genetics and mouse behavior. Annual Review of Neuroscience. 2001;24:845–867. doi: 10.1146/annurev.neuro.24.1.845. [DOI] [PubMed] [Google Scholar]
- Willner P. New York: Cambridge University Press; 1991. Behavioural models in psychopharmacology: Theoretical, industrial, and clinical perspectives. [Google Scholar]
- Zarcone T.J, Chen R, Fowler S.C. Differential acquisition of food-reinforced disk pressing by CD-1, BALB/cJ and C57BL/6J mice. Behavioural Brain Research. 2004;152:1–9. doi: 10.1016/j.bbr.2003.09.010. [DOI] [PubMed] [Google Scholar]