Abstract
Children who have status epilepticus have continuous or rapidly repeating seizures that may be life-threatening and may cause life-long changes in brain and behavior. The extent to which status epilepticus causes deficits in auditory discrimination is unknown. A naturalistic auditory location discrimination method was used to evaluate this question using an animal model of status epilepticus. Male Sprague-Dawley rats were injected with saline on postnatal day (P) 20, or a convulsant dose of pilocarpine on P20 or P45. Pilocarpine on either day induced status epilepticus; status epilepticus at P45 resulted in CA3 cell loss and spontaneous seizures, whereas P20 rats had no cell loss or spontaneous seizures. Mature rats were trained with sound-source location and sound-silence discriminations. Control (saline P20) rats acquired both discriminations immediately. In status epilepticus (P20) rats, acquisition of the sound-source location discrimination was moderately impaired. Status epilepticus (P45) rats failed to acquire either sound-source location or sound-silence discriminations. Status epilepticus in rat causes an age-dependent, long-term impairment in auditory discrimination. This impairment may explain one cause of impaired auditory location discrimination in humans.
Keywords: auditory discrimination, epilepsy, seizures, age, hippocampus, lever press, rats
Status epilepticus (SE), an acutely life-threatening condition characterized by repetitive or prolonged seizures (Lowenstein & Alldredge, 1998), may ultimately cause behavioral impairment in rodents (Hoffman, Zhao, & Holmes, 2004) and humans who have developmental disabilities (Holmes, 2004). The impairments in behavior correspond to alterations in brain connectivity and excitatory neurotransmitter receptor distribution, and decreased neurogenesis. These alterations can occur in the absence of cell loss. Although impaired behavioral function and brain changes have been well-documented following SE, the mechanisms of seizure-induced dysfunction remain unclear (Holmes, 2004).
Severe impairment in learning and behavior has been obtained in animals by inducing SE with kainic acid (KA), an analog of the excitatory neurotransmitter glutamate (Holmes, Thompson, Marchi, & Feldman, 1988). However, neurobehavioral effects following KA administration may be caused by the direct neurotoxic damage of KA, or by the KA-induced seizures, or both. The present experiment used a cholinergic muscarinic agonist, pilocarpine (PI) hydrochloride, that induces seizures and seizure-related brain damage in rats following systemic administration (Cavalheiro et al., 1991; Cavalheiro et al., 1987; Cilio et al., 2003; Corsellis & Bruton, 1983). Shortly after PI injection, behavioral and electroencephalograph (EEG) SE ensues, followed by a progressive normalization of EEG and behavior. Chronic spontaneous recurrent seizures (SRS) develop following SE induced with PI in adolescent or adult rodents, but not in younger animals (Cavalheiro et al., 1991). Although cell counts show that SE causes a significant neuronal loss in the CA3 and CA1 subfields of the hippocampus in adult rats, this loss is due directly to the initial SE, because the resulting chronic seizures produce no additional cell loss in those regions (Gorter et al., 2003; Liu, Nagao, Desjardins, Gloor, & Avoli, 1994). (Other abberant alterations, discussed below, may continue after the initial SE; e.g., Cha, Akman, Siveira, Liu, & Holmes, 2004). The brain damage following PI-induced SE is similar to damage observed in human temporal lobe epilepsy (Corsellis & Bruton, 1983; Turski, Ikonomidou, Turski, Bortolotto, & Cavalheiro, 1989).
In the present study, auditory discrimination was tested in rats that were administered seizures either just before weaning (postnatal day 20; P20) or during adolescence (P45). Thus these results in the rat may be compared to the effects of SE in humans during infancy and adolescence (Spear, 2000). Adolescent and adult rats may develop hippocampal cell loss and SRS, and profound cognitive and behavioral impairments, whereas younger animals may not. SE in immature rats causes long-term behavioral deficits in nonauditory tasks, with a neuroprotective window occurring during early postnatal development in the rat in both KA and PI seizure models (Fisher, Sperber, & Moshe, 1998; Liu, Gatt, Werner, Mikati, & Holmes, 1994; Stafstrom, Chronopoulos, Thurber, Thompson, & Holmes, 1993; Stafstrom, Thompson, & Holmes, 1992; Thurber, Chronopoulos, Stafstrom, & Holmes, 1992).
Harrison (1984) demonstrated rapid acquisition of sound-source location discrimination (one trial) in normal rats. Using an analogous procedure, Neill, Alvarez, and Harrison (1989) demonstrated slow acquisition and erratic performance of sound-source location discrimination in adult humans who had chronic epilepsy and mental retardation, but the reasons for their impaired performance were obscured by confounding variables, such as chronic antiepileptic medications and uncontrolled histories. Hochester and Kelly (1981) demonstrated that children with temporal lobe epilepsy were able to localize a single source of clicks and perceive the leading sound as the sound source in pairs of clicks with temporal separation of less than 8 ms. However, in pairs of clicks with temporal separation of 8, 16, and 20 ms, the children did not perceive the leading sound as the source, as normal children did. This finding suggests that sound-source location discrimination may be impaired following seizure-induced brain injury in immature mammals. The present experiment tested this general hypothesis in rats using naturalistic auditory procedures and apparatus. Rapid conditioning of sound-source location discrimination occurs when the sound has complex spectral content (Harrison & Beecher, 1969), is novel at the start of training, and its source is located adjacent to the response site (Harrison, 1979, 1981; Neill & Harrison, 1987). Immediate acquisition of sound-source location discrimination also is obtained in squirrel and rhesus monkeys, cats, dogs, and bats when these naturalistic features are used (Harrison, 1984, 1992).
To assess the long-term effects of early onset SE on sound-source location discrimination, our rats began preliminary training on P105, which is comparable to adulthood in humans. We hypothesized that SE early in life would cause a long-term impairment in sound-source location discrimination, and that the nature and degree of impairment would depend upon the age of the rat at the time of SE.
Method
Subjects
The subjects were 11 male Sprague-Dawley rats (Charles River Laboratories, Cambridge, MA), 15 days (n = 7) and 40 days (n = 4) old at the start of the experiment. The younger rats were weaned at age 21 days. Rats were housed individually in plastic cages on a standard 12 ∶ 12 hr light/dark cycle. During development, rats had free access to food and water. Approximately 2 weeks before preliminary training began, rats were fed 10 to 12.5 g per day so that each animal would weigh 80% of its ad libitum weight before preliminary training began on P105.
Electrodes
In order to record the EEG of the rats following injections, bipolar electrodes were implanted in all animals (Holmes & Thompson, 1988; Holmes et al., 1988). Rats were anesthetized with 50 mg/kg of pentobarbital sodium intraperitoneally, and electrodes were stereotaxically implanted into the right ventral hippocampus using the following brain coordinates: AP = 0.12; ML = 0.45; DV = 0.40 cm for P15 rats (Sherwood & Timiras, 1970) and AP = 0.32; ML = 0.5; DV = 0.65 cm for P40 rats (Paxinos & Watson, 1982). Electrodes were anchored to the skull using stainless-steel retaining screws and dental acrylic. The rats were allowed 5 days for surgical recovery before injections were administered.
Injections
PI (Pilocarpine hydrochloride) (Sigma, St. Louis, MO) was freshly dissolved in 0.9% saline and administered i.p. on either P20 (200 mg/kg), or P45 (380 mg/kg). The surviving rats are referred to as PI-P20 (n = 3), and PI-P45 (n = 4), respectively. Doses of PI were given to PI-P20 and PI-P45 rats that produced SE for approximately 5.5 hr in each age group, but resulted in a mortality of 30% or less. Although the use of different doses of PI may appear to be a confounding variable, these doses were selected because they produced nearly equivalent seizures in PI-P20 and PI-P45 rats. If equal doses of PI had been administered to both age groups, either the PI-P20 rats would not have survived at the higher dose, or the PI-P45 rats would not have had SE at the lower dose. Control rats (SA-P20) received an equal-volume i.p. injection of SA (normal saline) on P20 (n = 4). Only one saline control condition was used because parallel research found no evidence of histological or long-term behavioral changes due to a saline injection on P45 (Liu, Gatt, et al., 1994).
EEG and Observation of Seizures
The hippocampal EEG was recorded through the implanted electrodes with a Grass Model 6 EEG apparatus. EEGs were recorded for 10 min before and for 4 to 6 hr immediately after injections. During EEG recordings, rats were observed and behaviors were scored every 10 min for the first 2 hr after PI injection, then every 15 min.
Each P45 rat was videotaped for 138 hr in 6-hr sessions, alternating weekly between daytime (9:00 a.m. to 3:00 p.m.) and nighttime (6:00 p.m. to 12:00 a.m.) over 35 days, beginning 1 day after injection. Each P20 rat was videotaped for 240 hr (in 6-hr sessions) over 60 days, beginning 1 day after injection. The P20 rats had more monitoring than the P45 rats because there was a longer interval between PI administration and behavioral testing. SRS were counted when rats exhibited forelimb clonus with rearing and falling. All videotapes were viewed by an observer blind to treatment groups. Intraobserver reliability was 100%.
Other Testing
On postnatal day 80, all rats were subjected to a series of behavioral tests (not reported here), including water maze, handling, and open field tests (see Liu, Gatt, et al., 1994).
Behavioral Apparatus
The experimental chamber consisted of a wire cloth cage, 30 cm deep by 34 cm wide by 30 cm high, with two response levers mounted 5 cm from the corner and 5 cm above the cage floor. A speaker (Radio Shack® Model 40-130) was mounted above each lever. Aluminum funnels cemented over the openings of the speakers provided small (1 cm) sources of sound adjacent to the top of each lever. An automatic liquid dipper feeder was mounted at the center of the opposite wall, 0.5 cm above the floor. The feeder operated for 5 s, and delivered 0.1 cc of diluted sweetened condensed milk (3 volumes water to 1 volume of milk) each time. A 5-W houselight, mounted on top of the wire mesh chamber, was powered via a high-capacitance direct current circuit to prevent stimulus-correlated lighting artifacts. The experimental chamber was housed inside an acoustical chamber that isolated the rat from the solid-state stimulus and control equipment. The experiment was programmed to run 1 rat at a time using an Apple® IIe computer, a custom-built digital interface, and 28-V DC solenoid-driver relays for the houselight and feeder.
Auditory Stimulus
The sound stimulus consisted of bursts of broadband noise (40 ms on, 60 ms off, rise/fall time < 1 ms). Sound with complex spectral content (broadband noise with a rapid rise/fall time) was chosen for the stimulus because sine waves (pure tones) with slow rise times are not part of the rat's natural environment, and sine waves do not control auditory behavior as effectively (Harrison & Beecher, 1969). During discrimination training, sound bursts were used instead of continuous sound because sound bursts produce acquisition of sound-source location discrimination more rapidly and maintain higher asymptotic levels of accuracy than continuous sound (Guen, 1987). Pulsed sounds elicit a greater cardiac orienting response than continuous sound in both preweanling and adult rat (Richardson, Hess, & Campbell, 1994).
The noise had a continuous acoustic spectrum from 4 kHz to 50 kHz (Neill & Harrison, 1987). The intensities were set to 78 db sound pressure level (SPL, “A” scale), 1 cm from each speaker, using continuous (nonbursting) stimuli. The intensities of the continuous signals, 48 dB SPL at the center of the cage, were measured with a Radio Shack® sound level meter calibrated with a Bruel and Kjar pistonphone (4220). The signal (Coulbourn (CB) model S81-02) was gated in left and right channels by two separate rise/fall gates (CB model S84-04). Signals were amplified in each channel by separate amplifiers (CB model S82-24) and the background noise levels of the power amplifiers were reduced below the rat's auditory threshold by connecting the speakers to the amplifiers via –20 dB attenuators.
Preliminary Training
The speakers were disconnected and grounded during preliminary training, which began on P105. The rats were trained to go to the feeder when it operated, and lever pressing was then established by differential reinforcement of successive approximations (“shaping”). After responding on both levers was established, responses were reinforced by deliveries of milk on a variable-interval (VI) schedule of reinforcement, which was increased gradually from VI 15 s to VI 45 s. Availability of reinforcement was adjusted whenever a side bias developed so that the rats responded equally on both levers. For the remainder of training, the availability of reinforcement alternated from side to side in a predetermined pseudorandom order, such that no more than three reinforcers were available consecutively on one side. Sessions occurred 7 nights per week, at approximately the same time for each rat. Preliminary training was completed within seven sessions in all but 1 rat (R10, for which preliminary training took 10 sessions).
The percentage of responses on the adjacent lever per session was calculated by dividing the total number of adjacent responses per session by the total number of trials per session, and then multiplying by 100. During baseline, this percentage was based on the data collected with the same identical procedure as during sound sessions, but with the speakers disconnected.
Sound Discrimination Training
The auditory stimulus was novel at the start of discrimination training, because preexposure to the sound retards acquisition of sound-source location discrimination (Harrison, 1979; Neill & Harrison, 1987). On the day before sound-discrimination training, baseline data were collected to determine if the rats' responding was at chance levels. Although the sound was not presented in these sessions, the computer collected data in terms of trials. These data were used to calculate the percentage of “adjacent responses” exactly as they were used during discrimination training, and they provided a baseline against which the acquisition of the discrimination could be judged.
On the first day of sound-source location discrimination training, before the speakers were connected, each rat received a warm-up period, consisting of 10 trials presented with a variable 45-s intertrial interval, to ensure that there was no side bias, and so the rats were ready to respond to the novel sounds. The speakers then were connected to the amplifiers, and discrete sound trials were presented on a variable 45-s intertrial interval schedule composed of an arithmetic progression of 25 intervals ranging from 2 to 90 s. During each trial, the pulsing sound (“S+”) was presented either through the right or the left speaker. A response on the lever adjacent to the sounding speaker operated the feeder for 5 s, turned off the pulsing sound, and started the next intertrial interval. A response on the nonadjacent lever turned off the sound and started the next intertrial interval. The sound stimulus alternated from side to side in a predetermined pseudorandom order across trials, with the restriction that there were no more than three repetitions of a stimulus on one side in succession. The stimulus was presented 20 times on each side, for a total of 40 trials in each daily session. If an animal became motionless and unresponsive, it was allowed 30 min to make a response before it was removed from the chamber. Behaviors were monitored via closed-circuit video by the first author. Sound-source location discrimination training was conducted for 14 sessions.
Histology
After completion of behavioral testing, rats were anesthetized with ether and decapitated. Their brains were rapidly removed from the skulls and frozen by immersion in isopentane (−50°C) and stored at –80°C. The frozen brains were sectioned on a cryostat (−17°C) at 20 µm in the coronal plane. One out of five sections containing the hippocampus was mounted onto gelatin-coated slides and stained with cresyl violet. All slides were analyzed for major cell loss in the hippocampus.
Results
Epileptiform EEG and Seizure Behaviors Induced by Pilocarpine
Pilocarpine administration resulted in SE and a mortality rate of less than 30%. Fifteen min after injection, high-frequency spikes accompanied clonic jerks in both age groups. Thirty min after injection, continuous ictal discharges occurred concurrently with continuous head and forelimb clonus, and lasted up to 4 hr when EEG recording stopped. Additional descriptions of the effects of PI are published elsewhere (Liu, Gatt, et al., 1994).
The durations (in minutes) of SE in PI-P20 rats were: Rat R0 = 330, R1 = 350, R2 = 320 (mean = 333 min); for PI-P45 rats the durations were: Rat R0 = 360, R11 = 340, R15 = 310, R18 = 380 (mean = 347.5 min). Seizure durations of the two groups did not differ significantly, as indicated with tests of the means (t test), and the medians (Mann-Whitney Rank Sum Test).
Spontaneous Recurrent Seizures (SRS)
The PI-P45 rats had SRS, whereas the PI-P20 rats and saline P20 rats did not. These seizures resembled Stage 4 or 5 amygdala kindling. Table 1 displays the number of SRS for each rat.
Table 1. Average response latencies (s) for the last four sessions and number of spontaneous recurrent seizures (SRS).
| Rat | Injection | Average latency (SD) | Number of SRS |
| R7 | (SA-P20) | 1.95 (0.96) | 0 |
| R8 | (SA-P20) | 1.33 (0.54) | 0 |
| R9 | (SA-P20) | 1.37 (0.22) | 0 |
| R10 | (SA-P20) | 9.78 (7.68) | 0 |
| R0 | (PI-P20) | 3.61 (1.16) | 0 |
| R1 | (PI-P20) | 8.12 (3.72) | 0 |
| R2 | (PI-P20) | 4.91 (1.84) | 0 |
| R0 | (PI-P45) | 4.86 (5.90) | 3 |
| R11 | (PI-P45) | 7.70 (3.30) | 10 |
| R15 | (PI-P45) | 9.43 (11.95) | 8 |
| R18 | (PI-P45) | 6.84 (1.99) | 7 |
Note. Sessions with zero responses did not occur in the last four sessions.
Acquisition of Sound-Source Location Discrimination
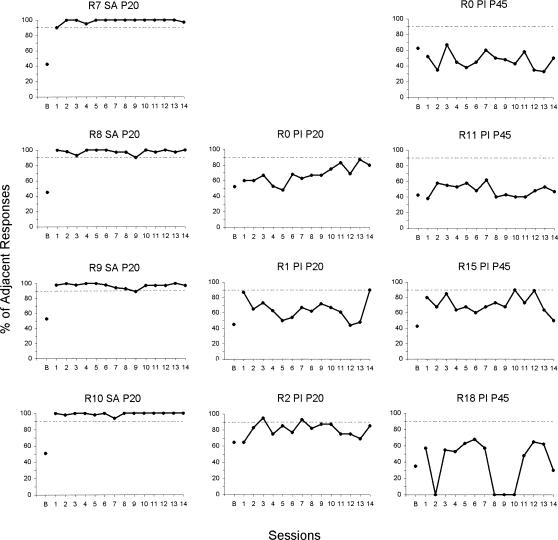
Performance in the sound-source location discrimination by saline control rats is shown in the left column of Figure 1 in terms of the percentage of adjacent (correct) responses for each session. Acquisition of this discrimination was extremely rapid, in some cases in one trial. Two saline rats (R8 and R10) acquired the discrimination with 0 errors (100% adjacent in the first session = one trial acquisition). One saline rat (R9) made one error in the first session, and another saline rat (R7) made four errors. A criterion level of 90% adjacent responding is highlighted by dashed lines in Figure 1. All saline rats responded at a minimum of 90% adjacent responses throughout training. The baseline percentages of no-sound responses are presented for all groups in Figure 1 (plotted above the point labeled B). These data points were within the range of chance (average = 48.8%).
Figure 1. Percentage of adjacent (correct) responses per session by 4 normal rats that had a saline injection on P20 (left column), 3 rats that had a pilocarpine-induced seizure on P20 (middle column), and 4 rats that had a pilocarpine-induced seizure on P45 (right column).
The point marked B gives the percentage of reinforced responses during the last session (VI 45 s) before the start of auditory discrimination training.
During 14 sessions of sound-source location discrimination training, the PI-P20 rats' asymptotic levels of performance were erratic and lower overall than those of the saline rats. As seen in the center column of Figure 1, Rat R0 (PI-P20) acquired the discrimination of the location of the sound sources slowly (i.e., a learning curve was present), and its best performance in the 14 sessions of training was 88% adjacent (Session 13). Rat R1's (PI-P20) performance in the first session reached 88% adjacent, but performance declined in 12 subsequent sessions to levels that were sporadically above chance (> 65% adjacent). Stimulus control was not evident again until Session 14 (90% adjacent). The only PI-P20 rat to perform above 90%, R2, performed at 95% adjacent in Session 3, and 93% in Session 7. However, its performance was also erratic, and ranged between 65% and 95% adjacent over the entire 14 days.
PI-P45 rats were profoundly impaired in sound-source location discrimination. They usually performed worse than PI-P20 rats. Three of 4 PI-P45 rats (R0, R11, and R18) did not show any sign of acquiring the sound-source location discrimination in 14 sessions of training (see Figure 1, right column). Rat R0 (PI-P45) performed the sound-source location discrimination consistently around chance levels (range, 35% to 66% adjacent) throughout the 14 sessions. Rat R11 (PI-P45) performed consistently at chance levels (range, 37.5% to 62.5% adjacent). Rat R15 (PI-P45) acquired the discrimination practically at asymptotic level in one session, 80% adjacent, and performed above chance levels throughout training. Four of 14 of Rat R15's sessions were performed at 80% adjacent or better, and it reached levels as high as 89% adjacent in Session 10 and 90% in Session 13. Rat R18 (PI-P45) operated the levers in only 71% (10/14) of training sessions (see Figure 1) and performed the sound-source location discrimination slightly above chance level in 40% (4/10) of these sessions (Session numbers 5, 6, 12, and 13).
Side Bias
Side bias may develop in rats that are not controlled by the location of the sound source. However, only Rat R11 (PI-P45) displayed a slight side bias, which became prominent in Sessions 9 to 13 (70 to 80% of responses on one lever).
Intertrial Responding
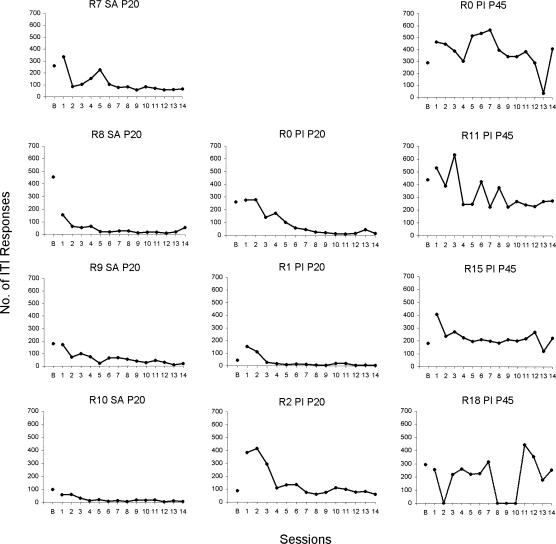
The behavior of the saline and PI-P20 rats was differentially controlled rapidly by the presence of sound (an S+) versus no sound (an S–). Responding during the intertrial interval declined to low rates by the end of the first discrimination training session in the saline rats and by Sessions 3 to 6 in the PI-P20 rats (see Figure 2). Thus the sound/no sound difference controlled the go/no-go aspect of behavior equally well for saline and PI-P20 rats.
Figure 2. Number of intertrial responses per session by 4 saline rats (left column), 3 rats that had a pilocarpine-induced seizure on P20 (middle column), and 4 rats that had a pilocarpine-induced seizure on P45 (right column).
The point marked B gives the number of intertrial responses during the last session (VI 45 s) before the start of auditory discrimination training.
PI-P45 rats responded at higher rates during the intertrial intervals than both the saline and the PI-P20 rats, demonstrating a lack of control of behavior by the sound/no sound difference. Two PI-P45 rats occasionally were immobile and unresponsive to the sounds, and made few or no lever presses for entire sessions. In four sessions, Rat R18 (PI-P45) did not respond at all, possibly because R18 experienced generalized seizures prior to these sessions (see Figure 2, R18, Sessions 2, 8, 9, and 10). Rat R0 (PI-P45) made few responses in Session 13, in marked contrast to its high rates of responding in other sessions (see Figure 2).
Response Latencies
Latencies to respond to the sound stimuli usually were greater in rats that had PI-induced SE, and this increase was greatest in rats that had SE later in development. The average response latencies were consistently small for SA-P20 Rats R7, R8 and R9 (see Table 1, which shows average response latencies and standard deviations of the last four sessions for all rats). These 3 saline rats' average response latencies were typical of normal animals studied in our laboratory, and elsewhere (cf. Harrison, 1981, p. 474).
Rat R10's (SA-P20) performance was unusual because of long response latencies and variability compared to the other saline rats (Table 1). This rat required 10 sessions of preliminary training, and initially displayed freezing in response to the sound of the liquid dipper operation. Still, Rat R10 acquired the sound-source location discrimination in one trial, and also acquired the sound/no-sound discrimination immediately. Two other saline rats, R7 and R9, were feeder trained in only one session, whereas Rat R8 was trained in four sessions.
Table 1 shows that 3 of the saline rats' latencies were less than latencies of PI-20 rats, and that latencies of the P45 rats were largest overall. The variability of response latencies was small in 3 of 4 saline rats, medium for PI-P20 rats, and largest for PI-45 rats.
The prolonged response latencies of PI-P20 and PI-P45 rats did not appear to be due simply to normal freezing responses. From time to time, these rats were nonresponsive when the discriminative stimulus occurred, possibly due to subtle (undetected) SRS. For example, in Session 13, Rat R0 (PI-P45) failed to respond to the auditory stimulus for 7 min on Trial 21 and was constantly in motion (biting, scratching, sniffing, climbing walls, pressing the levers, running, and rearing) during the remainder of the session. Rat R11 (PI-P45) also had long periods of unresponsiveness that alternated with hyperactivity. In Session 2, on Trial 19, this rat responded to the sound after a latency of 21 min 53 s. Rat R15 (PI-P45) stopped moving in Session 12 and became unresponsive to stimulation for 30 min. Rat R18 (PI-P45) was the most impaired animal, with consistently large average latencies, and several sessions (described above) in which responding was completely absent.
Some of the large response latencies of the PI-P20 and PI-P45 rats were due to incorrect orientation (e.g., animals would approach the nonoperating feeder instead of the sounding speaker). This occasional inability to respond to the appropriate location also was evident during presentation of the reinforcer, when PI-injected rats appeared to “get lost,” going to the corner of the cage instead of toward the operating feeder. This phenomenon also occurred during preliminary training, where PI-injected rats occasionally missed a reinforcer because they oriented to the wrong location for the duration of the feeder operation (5 s).
Histology
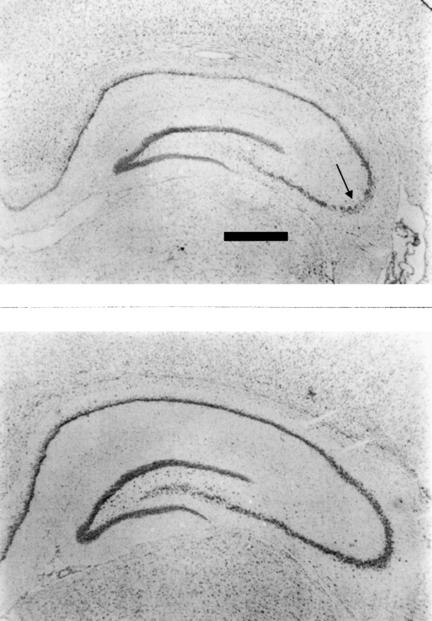
Gross cell loss was evident in the CA3 region of the hippocampus in all PI-P45 rats. No lesions were detected in PI-P20 rats or saline-treated rats (see Figure 3). Electrode placement was verified in all animals.
Figure 3. Histology. Photomicrographs of coronal sections of dorsal hippocampus.
Upper panel: Pilocarpine-P45 rat (R0). Note substantial cell loss (soma) in area CA3 (arrow). Lower panel: Pilocarpine-P20 rat (R1). No difference was detected between this slide and that of a saline (SA-P20) rat. Scale bar = 450 µm.
Discussion
Status epilepticus caused an age-dependent impairment in sound-source location discrimination and sound/no-sound discrimination. PI-P45 rats were most profoundly affected, probably due to SRS and severe hippocampal damage. The specific behavioral effects will be reviewed, followed by analysis of the particular adaptations that were likely disrupted by SE, acute and chronic effects of seizures, and implications for clinical intervention.
Sound-Source Location Discrimination
Saline (P20) rats acquired the sound-source location discrimination immediately, confirming prior experiments with normal rats (Burlile, Feldman, Craig, & Harrison, 1985; Harrison, 1979, 1981; Harrison, Downey, Segal, & Howe, 1971). The rapid acquisition by control animals shows that surgery did not cause auditory impairment. Immediate acquisition also suggests that the training method fits with the rat's behavioral adaptations to the natural environment (Harrison, 1988, 1992; Neill & Harrison, 1987).
SE induced by PI later in development caused greater impairment in acquisition and performance of the sound-source location discrimination. This finding supports the general conclusion that a neuroprotective window occurs during early postnatal development in the rat (Fisher et al., 1998; Holmes et al., 1988; Stafstrom et al., 1993; Stafstrom et al., 1992; Thurber et al., 1992). Another important new discovery was that one episode of SE at P20 caused a long-term impairment in acquisition of sound-source location discrimination, even in the absence of SRS and hippocampal cell loss. The PI-P20 rats were able to perform the sound-source location discrimination after extended training (data not shown), thus the behavioral effects of SE on P20 can be overcome by persistent behavioral intervention.
Intertrial Responding
Saline rats responded at relatively high rates during preliminary training on variable-interval schedules of reinforcement. After sound discrimination training began, responding during the silent intertrial intervals (i.e., the extinction component) declined rapidly to minimal levels. Other investigators obtained similar results (Burlile et al., 1985; Harrison, 1979, 1981). PI-P20 rats did not develop high levels of ITI responses. In contrast, PI-P45 rats made many more responses during the silent intertrial intervals. In addition, these animals were hyperactive in the operant chamber and in other settings (Liu, Gatt, et al., 1994). The operant hyperactivity is consistent with Harrigan, Peredery, and Persinger (1990) who injected adult rats with lithium plus PI, and trained their rats using differential-reinforcement-of-low-rate (DRL) schedules of reinforcement. Their seizure rats' IRTs did not conform to the demands of the DRL schedules after extended training. These findings, in concert, suggest that a single SE episode in an adolescent animal can cause hyperactive operant behavior.
Response Latencies
Three quarters of the saline control rats had short response latencies. The PI rats typically had longer response latencies, with the greatest latencies found in the PI-P45 rats. Occasional long latencies are also a characteristic feature of humans with intractable generalized seizures and mental retardation and are usually associated with generalized epileptiform EEG activity (Neill et al., 1989).
Disruption of Auditory Adaptations by Status Epilepticus
Here we consider how SE may have affected the following adaptations to the auditory world: auditory thresholds, detection of interaural time and intensity differences, and reinforcement of responses to novel sound sources.
The PI-P20 rats may have had slightly elevated auditory thresholds, as indicated by their lower than normal asymptotes of sound-source location discrimination. The PI-P45 rats may have had moderate to profound bilateral hearing loss, as suggested by their greater impairment in sound-source location discrimination and sound/no-sound discrimination. A similar effect was reported by Harrison (1981), who found that 30-month-old Sprague-Dawley rats had lower asymptotic levels of sound-source location discrimination than 12-month-old rats, which he attributed to changes in auditory threshold with age. It would be interesting to investigate behaviorally (Harrison & Turnock, 1975) or with auditory evoked potentials (Meeren, van Cappellen van Walsum, van Luijtelaar, & Coenen, 2001) whether SRS causes a change in auditory sensory thresholds. Patients with generalized epilepsy show abnormal auditory evoked potentials that correlate with the duration of epilepsy (Verleger, Lefebre, Wieschemeyer, & Kompf, 1997).
Animals localize sound sources primarily by detecting interaural amplitude and time differences (Gourevitch, 1987). In the present experiment, all PI-P20 rats and some PI-P45 rats could hear, as indicated by varying degrees of differential control of responding by the sound/no-sound difference, but their sound-source localization was moderately to severely impaired, possibly due to impairment in detection of interaural cues. Hochester and Kelly (1981) found that children with temporal lobe epileptic foci could localize single clicks, but when paired clicks with small time differences were used, the children were impaired in localizing the leading click, which depends upon interaural cues. Burlile et al. (1985) used a procedure similar to this study's to train normal and unilateral incudectomized rats. (Unilateral incudectomy removes the incus bone from one middle ear, and causes a high auditory threshold in one ear, effectively eliminating interaural cues). The incudectomized rats could not perform sound-source location discriminations, like the 2 most impaired PI-P45 rats in the present experiment. In concert, these results suggest that seizure-induced changes in brain may produce long-term deficits in acquisition of auditory spatial discriminations in rat and human due, in part, to impairment in interaural cue detection. Whether SE causes localization impairments by increasing auditory thresholds or by disrupting processing of interaural cues is an intriguing question for further investigation.
Orientation to novel stimuli is crucial for rapid acquisition of auditory discrimination in rat (Neill & Harrison, 1987). Without reinforcement of the novel stimulus-orientation-response relation, latent inhibition occurs (Lubow, 1973) and further auditory conditioning is slow in rats (Harrison, 1983). The hippocampal structures damaged following pilocarpine seizures are critical for auditory conditioning (Luntz-Leybman, Bickford, & Freedman, 1992; Sakurai, 1994) and memory (Squire, 1993). Even a relatively minor surgical manipulation of hippocampus causes a significant impairment in auditory conditioning in rats (Neill et al, 2001).
In PI-P45 rats, a continuous stream of hyperactive behaviors precluded reinforcement of orientation and exploration of the novel sound sources. PI-P45 rats also displayed persistent hyperactive exploration and locomotion in open field tests (Liu, Gatt, et al., 1994). Similar effects were found by Stafstrom et al. (1993). The amount of time spent investigating novel objects was significantly decreased by KA-induced SE at P30 and P60 in rat; no difference was found in rats exposed to SE at P5, P10 or P20 (Stafstrom et al., 1993).
Reinforcement of a response in the presence of sound increases the probability of orienting to and interacting with the sound source. The salience of the location of the sound source, and the biological predisposition to reapproach that location following reinforcement, is prepotent in rat (Harrison, 1979; Neill & Harrison, 1987), dog (Konorski, 1967; Lawicka, 1964, 1968), cat (Grastyan & Vereczkei, 1974), and monkey (Harrison et al., 1971). The relation between orientation and reinforcement may have been disrupted by SE, especially at P45. Although the PI-P45 rats often appeared to respond rapidly when a stimulus occurred, responses frequently were directed to the wrong location (e.g., toward the nonoperating feeder instead of the sounding speaker).
Acute Impairment Following Spontaneous Seizures
The durations of PI-induced seizures on P20 and P45 were not significantly different, yet SE on P45 caused SRS and profound impairment in auditory discrimination and lever pressing, resulting from either epileptiform discharge or prolonged postseizure effects. Short and long response latencies alternated in PI-P45 rats, and transient impairments in these animals could have occurred due to subtle recurrent seizures. Such epileptic activity would account for variability in performance of the sound-source location discrimination across trials. For example, Rat R15 (PI-P45) responded correctly to the sound-source locations for extended blocks of trials, only to become impaired later. PI-P45 rats had obvious SRS and became immobile and unresponsive for entire sessions. Rat R18 (PI-P45) did not respond during four sessions, and Rat R0 (PI-P45) made few responses in Session 13. These long-duration impairments could have been due to the postseizure effects of SRS, a common pattern in cats following kindling (Majkowski & Sobieszek, 1988) and in patients who have intractable epilepsy and mental retardation (Neill et al., 1989).
Chronic Impairment Following Status Epilepticus
In the present study, the PI-P45 rats, but not PI-P20 rats, had substantial cell loss (soma) in area CA3. Cell counting was not performed, so we cannot rule out the possibility of hippocampal cell loss in the P20 rats. Liu, Nagao, et al. (1994) showed that, although cell loss in dorsal hippocampus clearly was evident on visual inspection alone only with doses of PI of 380 mg/kg or greater, a computerized stereological estimation technique demonstrated a significant reduction in neuron density in the CA3 subregion at a lower dose (350 mg/kg). In the present study, only the hippocampus was analyzed histologically, but impairments in discrimination were probably due to damage in other structures as well (Harrigan, Peredery, & Persinger, 1990).
The question arises whether the hippocampal damage in the P45 rats was due to the initial SE, to SRS, or to both? Spontaneous recurrent seizures following SE enhance dentate gyrus neurogenesis, and the production of new neurons. These alterations may contribute to ongoing pathological changes months following SE (Cha et al., 2004). The so-called “two-hit” hypothesis suggests that SE may render the immature brain vulnerable to further seizure-induced injury (Hoffman et al., 2004; Koh, Storey, Santos, Mian, & Cole, 1999; Schmid, Tandon, Stafstrom, & Holmes, 1999). The present data are in agreement with this hypothesis, because only the PI-45 rats developed SRS and had obvious hippocampal damage with profound impairment of auditory localization.
Implications for Clinical Intervention
An analog of the present behavioral method has been used with adult humans who have histories of SE from infancy or childhood, poorly controlled epilepsy, and mental retardation (Neill et al., 1989). These subjects acquired the sound-source location discrimination slowly and erratically, in patterns remarkably similar to those in the present experiment. There is a strong association between early onset seizures, cognitive impairment, and developmental disorders (Besag, 2004; Holmes, 2004; Trevathan, 2004). Neill et al. (1996) demonstrated that brief serial seizures in rats caused a long-term impairment in auditory location discrimination using the go/no-go response paradigm. In concert, these findings may have special importance for evaluation and treatment of children who may have suffered from seizures. We hypothesize that the effects of seizures might not be noticed until later, when severe impairments in language and social behavior are noted. At that point, extensive preliminary training of developmentally delayed children (equivalent to magazine training and shaping, followed by extensive sound-source location training) may be justified because such training may greatly accelerate gains in social behaviors and language acquisition.
The deleterious effects of SE and epilepsy are compounded by the marked reduction in reinforcement density that victims of SE can experience. Low reinforcement density alone may be responsible for low rates of appropriate behavior. Conversely, reinforcement of auditory discrimination probably decreased the likelihood of abnormal brain electrical activity, and contributed to improved performance over time, in the auditory discrimination study of epileptic mentally retarded humans by Neill et al. (1989). Neill and Alvarez (1989) demonstrated that a high frequency of prompts and reinforcement for active behavior in the everyday environment suppresses epileptiform discharge by a factor of 10 in epileptic mentally retarded humans. It is therefore important to provide supplementary reinforcement in the daily lives of children who have such developmental disabilities, to ultimately increase their own ability to obtain maximum levels of reinforcers.
In conclusion, long-term damage due to SE can be so monumental that prevention is an important priority. Yang et al. (2000) were able to protect the hippocampus from seizure-induced damage using prenatal choline supplementation in rats. Future behavioral investigations should focus on investigating the impact of such effective preventive strategies on auditory discrimination.
Acknowledgments
This manuscript was completed during John Neill's sabbatical leave at the Brookhaven National Laboratory with S. J. Gately under National Aeronautics and Space Administration Grant T466-X. This research also was supported by Grants NS-27984 and NS-44295 from the National Institutes of Health to Gregory Holmes.
References
- Besag F.M.C. Behavioral aspects of pediatric epilepsy syndromes. Epilepsy and Behavior. 2004;5:S3–S13. doi: 10.1016/j.yebeh.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Burlile C.J, Feldman M.L, Craig C, Harrison J.M. Control of responding by the location of a sound: Role of binaural cues. Journal of the Experimental Analysis of Behavior. 1985;43:315–319. doi: 10.1901/jeab.1985.43-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro E.A, Leite J.P, Bortolotto Z.A, Turski W.A, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: Structure damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Cavalheiro E.A, Silva D.F, Turski W.A, Calderazzo-Filho L.S, Bortolotto Z.A, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Developmental Brain Research. 1987;37:43–58. doi: 10.1016/0165-3806(87)90227-6. [DOI] [PubMed] [Google Scholar]
- Cha B.H, Akman C, Silveira D.C, Liu X, Holmes G.L. Spontaneous recurrent seizure following SE enhances dentate gyrus neurogenesis. Brain & Development. 2004;26:394–397. doi: 10.1016/j.braindev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Cilio M.R, Sogawa Y, Cha B.H, Liu X, Huang L.T, Holmes G.L. Long-term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia. 2003;44:518–528. doi: 10.1046/j.1528-1157.2003.48802.x. [DOI] [PubMed] [Google Scholar]
- Corsellis J.A.N, Bruton C.J. Neuropathology of SE in humans. In: Delgado-Escueta A.V, Wasterlain C.G, Treiman D.M, Porter R.J, editors. SE. New York: Raven Press; 1983. pp. 129–139. [Google Scholar]
- Fisher P.D, Sperber E.F, Moshe S.L. Hippocampal sclerosis revisited. Brain &Development. 1998;20:563–573. doi: 10.1016/s0387-7604(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Gorter J.A, Conçalves Pereira P.M, van Vliet E.A, Aronica E, Lopes da Silva F.H, Lucassen P.J. Neuronal cell death in a rat model for mesial temporal lobe epilepsy is induced by the initial staus epilepticus and not by later repeated spontaneous seizures. Epilepsia. 2003;44:647–658. doi: 10.1046/j.1528-1157.2003.53902.x. [DOI] [PubMed] [Google Scholar]
- Gourevitch G. Binaural hearing in land mammals. In: Yost W.A, Gourevitch G, editors. Directional hearing. New York: Springer-Verlag; 1987. pp. 226–278. [Google Scholar]
- Grastyan E, Vereczkei L. Effects of spatial separation of the conditioned signal from reinforcement: A demonstration of the conditioned character of the orienting response or the orientational character of conditioning. Behavioral Biology. 1974;10:121–146. doi: 10.1016/s0091-6773(74)91725-8. [DOI] [PubMed] [Google Scholar]
- Guen M. Sound source position discrimination: Acquisition and performance as a function of continuous and pulsed sounds. Boston, MA: Boston University; 1987. Unpublished doctoral dissertation, [Google Scholar]
- Harrigan T, Peredery O, Persinger M.A. Failure to acquire an inhibitory task following seizure-induced brain damage. Perceptual and Motor Skills. 1990;70:268–270. doi: 10.2466/pms.1990.70.1.268. [DOI] [PubMed] [Google Scholar]
- Harrison J.M. The control of responding by sounds: Unusual effect of reinforcement. Journal of the Experimental Analysis of Behavior. 1979;32:167–181. doi: 10.1901/jeab.1979.32-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.M. Effects of age on acquisition and maintenance of a location discrimination in rats. Experimental Aging Research. 1981;7:467–476. doi: 10.1080/03610738108259825. [DOI] [PubMed] [Google Scholar]
- Harrison J.M. Effects of age on some behavioral characteristics of novel auditory stimuli in the rat. Experimental Aging Research. 1983;9:35–39. doi: 10.1080/03610738308258418. [DOI] [PubMed] [Google Scholar]
- Harrison J.M. The functional analysis of auditory discrimination. Journal of the Acoustical Society of America. 1984;75:1848–1854. doi: 10.1121/1.390985. [DOI] [PubMed] [Google Scholar]
- Harrison J.M. Control of responding by sounds of different quality: An evolutionary analysis. Journal of the Experimental Analysis of Behavior. 1988;50:521–539. doi: 10.1901/jeab.1988.50-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.M. Avoiding conflicts between the natural behavior of the animal and the demands of the discrimination experiments. Journal of the Acoustical Society of America. 1992;92:1331–1345. doi: 10.1121/1.403927. [DOI] [PubMed] [Google Scholar]
- Harrison J.M, Beecher M.D. Control of responding by the location of an auditory stimulus: Role of rise time of the stimulus. Journal of the Experimental Analysis of Behavior. 1969;12:217–227. doi: 10.1901/jeab.1969.12-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.M, Downey P, Segal M, Howe M. Control of responding by the location of auditory stimuli: Rapid acquisition in rat and monkey. Journal of the Experimental Analysis of Behavior. 1971;15:373–386. doi: 10.1901/jeab.1971.15-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J.M, Turnock M.T. Animal psychophysics: Improvements in the tracking method. Journal of the Experimental Analysis of Behavior. 1975;23:141–147. doi: 10.1901/jeab.1975.23-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochester M.E, Kelly J.B. The precedence effect and sound localization by children with temporal lobe epilepsy. Neuropsychologia. 1981;19:49–55. doi: 10.1016/0028-3932(81)90043-9. [DOI] [PubMed] [Google Scholar]
- Hoffman A.F, Zhao Q, Holmes G.L. Cognitive impairment following SE and recurrent seizures during early development: Support for the “two-hit hypothsis.”. Epilepsy & Behavior. 2004;5:873–877. doi: 10.1016/j.yebeh.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Holmes G.L. Effects of early seizures on later behavior and epileptogenicity. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:101–105. doi: 10.1002/mrdd.20019. [DOI] [PubMed] [Google Scholar]
- Holmes G.L, Thompson J.L. Effects of kainic acid on seizure susceptibility in the developing brain. Developmental Brain Research. 1988;39:51–59. doi: 10.1016/0165-3806(88)90066-1. [DOI] [PubMed] [Google Scholar]
- Holmes G.L, Thompson J.L, Marchi T, Feldman D.S. Behavioral effects of kainic acid administration on the immature brain. Epilepsia. 1988;29:721–730. doi: 10.1111/j.1528-1157.1988.tb04226.x. [DOI] [PubMed] [Google Scholar]
- Koh S, Storey T.W, Santos T.C, Mian A.Y, Cole A.J. Early-life seizures in rats increase susceptibility to seizure-induced brain injury in adulthood. Neurology. 1999;53:915–921. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrated activity of the brain: An interdisciplinary approach. Chicago, IL: Chicago University Press; 1967. [Google Scholar]
- Lawicka W. The role of stimulus modality in successive discrimination and differentiation learning. Bulletin of the Academy of Polish Sciences. 1964;12:35–38. [Google Scholar]
- Lawicka W. Differing effectiveness of auditory quality and location cues in two forms of differentiation learning. Acta Neurobiologica Experimentalis. 1968;29:83–92. [PubMed] [Google Scholar]
- Liu Z.T, Gatt A, Werner S.J, Mikati M.A, Holmes G.L. Long-term behavioral deficits following pilocarpine seizures in immature rats. Epilepsy Research. 1994;19:191–204. doi: 10.1016/0920-1211(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Nagao T, Desjardins C.G, Gloor P, Avoli M. Quantitative neuronal loss evaluation in rats with pilocarpine seizures. Epilepsy Research. 1994;17:237–247. doi: 10.1016/0920-1211(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Lowenstein D.H, Alldredge B.K. Status Epilepticus. New England Journal of Medicine. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- Lubow R.E. Latent inhibition. Psychological Bulletin. 1973;79:398–407. doi: 10.1037/h0034425. [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V.L, Bickford P.C, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Research. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Majkowski J, Sobieszek A. Effects of hippocampal kindled afterdischarges and complex partial seizures on previously established avoidance response in cats. Acta Neurobiologica Experimentalis. 1988;48:295–309. [PubMed] [Google Scholar]
- Meeren H.K.M, van Cappellen van Walsum A.M, van Luijtelaar E.L.J.M, Coenen A.M.L. Auditory evoked potentials from auditory cortex, medial geniculate nucleus, and inferior colliculus during sleep-wake states and spike-wave discharges in the WAG/Rij rat. Brain Research. 2001;898:321–331. doi: 10.1016/s0006-8993(01)02209-0. [DOI] [PubMed] [Google Scholar]
- Neill J.C, Alvarez N. Effects of the everyday environment on epileptic activity in three mentally retarded individuals. Electroencephalography and Clinical Neurophysiology. 1989;72:198–206. doi: 10.1016/0013-4694(89)90244-7. [DOI] [PubMed] [Google Scholar]
- Neill J.C, Alvarez N, Harrison J.M. Intensive EEG and behavior monitoring of epilepsy in developmentally disabled individuals. Transitions in Mental Retardation. 1989;4:155–186. [Google Scholar]
- Neill J.C, Harrison J.M. Auditory discrimination: Konorski's Quality-Location effect. Journal of the Experimental Analysis of Behavior. 1987;48:81–95. doi: 10.1901/jeab.1987.48-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J.C, Liu Z, Sarkisian M, Tandon P, Yang Y, Stafstrom C, Holmes G.L. Recurrent seizures in immature rats: Effect on auditory and visual discrimination. Developmental Brain Research. 1996;95:283–292. doi: 10.1016/0165-3806(96)00099-5. [DOI] [PubMed] [Google Scholar]
- Neill J.C, Sarkisian M, Wang Y, Liu Z, Yu L, Tandon P, Zhang G, Holmes G, Geller A.I. Enhanced auditory reversal learning by genetic activation of protein kinase C in small groups of hippocampal neurons. Molecular Brain Research. 2001;93:127–136. doi: 10.1016/s0165-3806(01)00204-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Sydney, Australia: Academic Press; 1982. [Google Scholar]
- Richardson R, Hess M, Campbell B.A. The orienting response to brief auditory stimuli in preweanling and adult rats. Developmental Psychobiology. 1994;27:93–100. doi: 10.1002/dev.420270203. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Involvement of auditory cortical and hippocampal neurons in auditory working memory and reference memory in the rat. The Journal of Neuroscience. 1994;14:2606–2623. doi: 10.1523/JNEUROSCI.14-05-02606.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid R, Tandon P, Stafstrom C.E, Holmes G.L. Effects of neonatal seizures on subsequent seizure-induced brain injury. Neurology. 1999;53:1754–1761. doi: 10.1212/wnl.53.8.1754. [DOI] [PubMed] [Google Scholar]
- Sherwood N.M, Timiras P.S. A stereotaxic atlas of the developing rat brain. Berkeley, CA: University of California Press; 1970. [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neuroscience and Behavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squire L. The hippocampus and spatial memory. Transitions in Neuroscience. 1993;16:56–57. doi: 10.1016/0166-2236(93)90016-f. [DOI] [PubMed] [Google Scholar]
- Stafstrom C.E, Chronopoulos A, Thurber S, Thompson J.L, Holmes G.L. Age-dependent cognitive and behavioral deficits following kainic acid seizures. Epilepsia. 1993;34:420–432. doi: 10.1111/j.1528-1157.1993.tb02582.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom C.E, Thompson J.L, Holmes G.L. Kainic acid seizures in the developing brain: SE and spontaneous recurrent seizures. Developmental Brain Research. 1992;65:237–246. doi: 10.1016/0165-3806(92)90184-x. [DOI] [PubMed] [Google Scholar]
- Thurber S, Chronopoulos A, Stafstrom C.E, Holmes G.L. Behavioral effects of continuous hippocampal stimulation in the developing rat. Developmental Brain Research. 1992;68:35–40. doi: 10.1016/0165-3806(92)90245-r. [DOI] [PubMed] [Google Scholar]
- Trevathan E. Seizures and epilepsy among children with language regression and autistic spectrum disorders. Journal of Child Neurology. 2004;19((Suppl. 1)):S49–S57. doi: 10.1177/088307380401900106. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou E, Turski W.A, Bortolotto Z.A, Cavalheiro E.A. Review: Cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: A novel experimental model of intractable epilepsy. Synapse. 1989;3:154–171. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Verleger R, Lefebre C, Wieschemeyer R, Komf D. Event-related potentials suggest slowing of brain processes in generalized epilepsy and alterations of visual processing in patients with partial seizures. Cognitive Brain Research. 1997;5:205–219. doi: 10.1016/s0926-6410(96)00071-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu Z, Cermack J.M, Tandon P, Sarkisian M.R, Stafstrom C.E, Neill J.C, Blusztajn J.K, Holmes G.L. Protective effects of prenatal choline supplementation on seizure-induced memory impairment. The Journal of Neuroscience. 2000;20:RC109. doi: 10.1523/JNEUROSCI.20-22-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]