Abstract
Dose-dependent changes in sensitivity to reinforcement were found when rats were treated with low, moderate, and high doses of the partial dopamine D1-type receptor agonist SKF38393 and with the nonselective dopamine agonist apomorphine, but did not change when rats were treated with similar doses of the selective dopamine D2-type receptor agonist quinpirole. Estimates of bias did not differ significantly across exposure to SKF38393 or quinpirole, but did change significantly at the high dose of apomorphine. Estimates of goodness of fit (r2) did not change significantly during quinpirole exposure. Poor goodness of fit was obtained for the high doses of SKF38393 and apomorphine. Decrements in absolute rates of responding were observed at the high dose of quinpirole and at the moderate and high doses of SKF38393 and apomorphine. Changes in r2 and absolute responding may be due to increases in stereotyped behavior during SKF38393 and apomorphine exposure that, in contrast to quinpirole, were distant from the response lever. The present data provide evidence that sensitivity to reward is affected more strongly by dopamine D1-like receptors rather than D2-like receptors, consistent with evidence from other studies investigating consummatory dopamine behavior and the tonic/phasic dopamine hypothesis.
Keywords: dopamine D1-like and D2-like receptors, quinpirole, SKF38393, apomorphine, matching, sensitivity to reward, lever press, rat
Dopamine (DA) traditionally has been considered the neurobiological substrate of reward. That is, DA release was the neural event correlated with the presentation of a reinforcer. Recent investigations, however, have challenged this role for dopamine (Farmer-Dougan, Dougan, Rokosik, Lewis, & Garris, 2004; Garris, Kilpatrick, Bunin, Walker, & Wightman, 1999; Salamone, Cousins, & Snyder, 1997; Schultz, 1998, 1999; Schultz, Apicella, & Ljungberg, 1993; Schultz, Dayan, & Montague, 1997; Schultz et al., 1995). For example, Garris et al. (1999) found inconsistencies in DA release during conditions that involved contingent versus noncontingent stimulation of the brain reward system (BRS). Schultz (1998) found evidence of DA neuronal activity for novel food or unpredictable CSs as well as DA neuronal spiking for the CS when food was predictable. Phillips, Stuber, Helen, Wightman, and Carelli (2003), using fast-scan voltammetry, found peaks of DA release just before the onset of a contingent lever press, but not to the discriminative stimulus, with a smaller DA peak coinciding with the delivery of IV cocaine.
Several models have emerged that recharacterize the relation between DA and rewarded behavior. Salamone et al. (1997) suggest that DA initially serves to activate an animal, resulting in higher activity levels leading to an increased probability of contact with reward. Schultz (1998) proposed that dopamine provides feedback regarding the opportunity for reward, and thus serves to activate the animal towards a probable reinforcer. A variety of behavioral and neurological data support the dopamine-as-activator hypothesis. Martin et al. (2004) compared contingent responding for electrical brain stimulation (EBS) to yoked EBS delivered noncontingently. Rats remained at or near the operant lever and engaged in high rates of lever pressing when contingent EBS was used. However, rats exposed to noncontingent EBS showed high rates of locomotion and moved away from the operant lever.
Other evidence suggests that DA may mediate some form of general “anticipatory” or appetitive responses, but not consummatory behavior (Ikemoto & Panksepp, 1999; Timberlake, 2001). For example, data indicate that DA agonists may alter responses related contingently to reward or the predictability of the reward (e.g., lever pressing, hoarding, place preference, or free foraging), but not the consummatory response itself (Kelley, Gauthier, & Lang, 1989; Kelley & Stinus, 1985; Salamone, Aberman, Sokolowski, & Cousins, 1999; Salamone, Cousins, McCullough, Carriero, & Berkowitz, 1994; Sokolowski & Salamone, 1998). Investigations also implicate DA as a potential neural substrate of novelty (Rebec, Christiensen, Guerra, & Bardo, 1997), or a feedback system for unpredictability (Salamone et al., 1997; Schultz, Tremblay, & Hollerman, 2000), incentive motivation (Bindra, 1978; Ikemoto & Panksepp, 1999), or salience (Pitts & Horvitz, 2000; Redgrave, Prescott, & Gurney, 1999; Spanagel & Weiss, 1999). The picture becomes even more complicated when we consider that there are multiple receptors for DA, each with a potentially distinct role in regulating behavior.
The Role of Dopamine D1-like and D2-like Receptors in Behavior
Molecular characterization techniques have identified the protein sequences for numerous DA receptor subtypes, which have been grouped into five major subtypes within two broad categories (Missale, Nash, Robinson, Jaber, & Caron, 1998). The D1-like receptor family includes the D1 and D5 receptors, whereas the D2, D3, and D4 receptors are grouped into the D2-like family. Stimulation of the D1-like receptors by DA increases the activity of the enzyme adenylate cyclase (positively coupled to cAMP) whereas activating the D2-like receptors decreases (negatively coupled to cAMP) or has no effect on adenylate cyclase activity. Because adenylate cyclase synthesizes cyclic AMP (the first substrate in a ubiquitous second messenger system within cells), changes in activity at these DA receptors can have multiple effects on neuronal function. Activation of the D2-like autoreceptors appears to modulate the level of tonic dopamine in the synapse and thus influence dopamine levels over a longer time horizon.
Schultz (1998) notes very different reinforcement functions for D1-like versus D2-like receptors. He suggests that dopamine neurotransmission may have at least two distinct functions in the brain: (a) the phasic processing of appetitive behavior or behaviors related to the prediction of reward, modulated primarily by postsynaptic D1-like receptors, and (b) the enabling and maintaining of general search behaviors via changes in synaptic dopamine controlled by D2-like autoreceptors. This enabling occurs in the absence of temporal coding regarding the predictability of reward.
According to Schultz (1998), because D1-like receptors are localized mainly along and around the periphery of dopaminergic synapses, high transient concentrations of dopamine after phasic impulse bursts would effect D1-like receptors in the immediate vicinity of the active release sites but not necessarily those along the periphery of the synapse. In contrast, high synaptic concentrations of dopamine should activate and even saturate the D2-like autoreceptors which are clustered within the synapse. D2-like receptors would thus remain partially activated when the ambient dopamine concentration returns to baseline after phasic increases. D1-like receptors, according to Schultz, should activate general appetitive and alerting behaviors. In contrast, D2-like receptors may be tied to the enabling and/or maintaining of more focused responses that occur when reward is predictable or contingent.
Further evidence for differential behavioral effects for D1-like versus D2-like receptors comes from Kurylo (2004). He suggests that, because of the way the D2-like receptors regulate dopaminergic release, these receptors should be responsible for perseveration of operant behavior. Activation of general appetitive behaviors, including operant responding, occurs with an increase in DA levels in the nucleus accumbens. Decreases or omission of behavior occur with a phasic decrease in tonic DA activity, regulated by the D2-like receptors. Thus activation of the D2-like receptors appears to prolong and maintain general appetitive, search, or operant behavior and may produce perseveration of a single response. Use of a D2 agonist such as quinpirole should, then, produce behavioral perseveration and prolonging of operant responding whereas use of a D1-like agonist such as SKF38393 should produce increases in general search that may interrupt ongoing operant responses (Kurylo, 2004).
Several studies support Schultz's and Kurylo's hypotheses. For example, Wachtel, Brooderson, and White (1992) found that SKF38393 (a partial D1 agonist) increased the amount of time rats spent grooming in a dose-dependent manner, whereas quinpirole (a D2 agonist) was found to induce excessive “checking” behavior similar to behavior found in obsessive-compulsive disorders (Szechtman, Sulis, & Eilam, 1998). Katz and Witkin (1992) found that SKF38393 and quinpirole differentially affected preferences for a nonselective DA agonist. Using a drug-discrimination task, squirrel monkeys were trained with food reinforcement to press one of two levers after administration of IV cocaine or saline. When delivered IM, quinpirole produced dose-related increases in cocaine-appropriate responding whereas SKF38393 failed to produce any significant cocaine-appropriate responding (Katz & Witkin, 1992).
Eilam, Clements, and Szechtman (1991) investigated the differential effects of SKF38393 and quinpirole on stereotyped locomotion in rats. When treated with quinpirole, the rats moved repeatedly in a limited area of an open field activity box. When treated with quinpirole and low doses of SKF38393, the area of the activity box in which the rat moved was expanded, and the rat moved through the center as well as around the periphery of the box. When the dose of SKF38393 was increased, the initial locomotor behavior was similar to that of the low doses. Subsequently, however, the animal stopped moving altogether and remained in a corner of the field.
Eilam, Talangbayan, Canaran, and Szechtman (1992) examined changes in locomotion, mouthing, and snout contact versus snout bout duration when rats were exposed to quinpirole (0.03 mg/kg and 0.5 mg/kg), SKF38393 (1.25 and 40.0 mg/kg), or combinations of the two. Low doses of quinpirole reduced locomotion and snout contact frequency where high doses increased these behaviors. High doses of quinpirole also increased mouthing and the duration of snout contact bouts, suggesting perseveration and stereotypy. Grooming was inhibited by both doses of quinpirole.
A different pattern of behaviors emerged from SKF38393 exposure. SKF38393 reduced locomotion and snout contact and attenuated the effects of the high dose of quinpirole. When given with the high dose of quinpirole, SKF38393 enhanced both mouthing and duration of snout contact bouts above that observed with the high dose of quinpirole alone. Grooming was stimulated by SKF38393, either alone or in combination with quinpirole. Eilam et al. (1992) suggest that their data support the oppositional model of D1-D2 receptor interactions. Their data further support the hypothesis that D1-like and D2-like receptors may differentially affect behavior.
In summary, there certainly is support for the role of DA as a behavioral activator. More specifically, differential activation of D1-like and D2-like receptors may elicit different behavioral topography. Further, there are hints of a potential oppositional interaction between the two receptors. However, little is known about how manipulation of these receptors might affect operant behavior and, in particular, choice behavior. It is possible that some behaviors modulated by D1-like or D2-like receptors may improve sensitivity to reinforcement, whereas other behaviors modulated by these receptors may interrupt or reduce sensitivity to reinforcement, particularly during choice situations using variable-interval schedules of reinforcement. The present paper explores such a relation.
The Matching Law as a Model for Examining D1-like and D2-like Effects
The matching law (Baum, 1974; Herrnstein, 1961, 1970) has been an extremely influential quantitative model of operant responding. The matching law has been particularly important because it allows assessment of the sensitivity of behavior to reinforcement. For that reason, it also provides a potential model for analyzing the effects of DA on behavior because activation of D1-like and D2-like receptors should have differential effects on the sensitivity of behavior to reinforcement.
According to Baum's (1974) extension of Herrnstein's (1961) matching law, the sensitivity of behavior to changes in reinforcement may be estimated using Equation 1. Specifically, when subjects respond on a concurrent schedule of reinforcement, the relation between response allocation and reinforcer allocation is described by the following relation:
| 1 |
where P1 and P2 are the absolute rates of responding to the two alternatives, and R1 and R2 represent the absolute rates of reinforcement for two alternatives. The parameter a represents the sensitivity of the behavior ratio (P1/P2) to changes in the reinforcement ratio (R1/R2). The b parameter represents bias, or the animal's tendency to choose one response manipulandum or reinforcer alternative over the other, independent of the reinforcement value of the two choices. Several studies have successfully used the matching paradigm to investigate sensitivity to reinforcement during drug exposure (e.g., Egli, Schaal, Thompson, & Clearly, 1992; Martinetti, Andrzejewski, Hineline, & Lewis, 2000), or when the reinforcer choice consisted of two pharmacological agents or a pharmacological agent and food (Anderson & Woolverton, 2000; Belke & Neubauer, 1997; McMillan & Hardwick, 2000; McMillan, Li, & Snodgrass, 1998; Woolverton & Alling, 1999).
The model has great potential for examining the role of dopamine D1-like and D2-like receptors in choice responding and reward. Specifically, the sensitivity parameter (a) should either increase or remain unchanged when animals are exposed to the D2-agonist quinpirole. This is because D2 receptors are thought to mediate focal search behavior such as operant lever pressing. Conversely, the sensitivity parameter should decrease during exposure to the D1-like SKF38393 or the nonselective DA agonist apomorphine. This is because D1-like receptors are thought to mediate general search behaviors that are more likely directed away from the response manipulandum. An increase in behavior directed away from the manipulandum should be reflected in a decreased sensitivity to reinforcement.
This was the focus of the present study. Three groups of rats were exposed to a series of five to seven concurrent variable-interval variable-interval (conc VI VI) schedules. The rats were administered three doses of a partial D1 agonist, SKF38393, a D2 agonist, quinpirole, or a nonselective DA agonist, apomorphine, once stability was reached on each schedule. Changes in sensitivity to reward, bias, and an evaluation of the goodness of fit (r2) to the matching equation were assessed. SKF38393, a partial D1 agonist, and quinpirole, a D2 agonist, were chosen because of the large number of studies available for comparison with the present results.
Experiment 1: Exposure to Skf38393 and Quinpirole
Method
Subjects
Six male Sprague-Dawley rats, approximately 120 days old, served as subjects. Rats were individually housed on a 12∶12 hr light/dark cycle with ad libitum access to water. Animals were maintained at a minimum of 80% ad libitum body weight throughout the duration of the experiment. Animal care followed the guidelines advised by the Guide for the Care and Use of Laboratory Animals, and the study was approved by the Institutional Animal Care and Use Committee of Illinois State University.
Drugs/Injection Procedure
SKF38393 was dissolved in a 20∶1 solution of distilled water for the high and medium dose (20.0 mg/kg and 10.0 mg/kg, respectively) and in a 5∶1 solution of distilled water for the low dose (1.0 mg/kg). Quinpirole hydrochloride was dissolved in a 1∶1 solution of distilled water for the high, medium, and low dose (1.0 mg/kg, 0.5 mg/kg, and 0.25 mg/kg, respectively). All injections were given subcutaneously under the loose skin of the neck of the rat. Doses were identical to doses used in similar studies of discrimination and exploratory behavior (Belke & Neubauer, 1997; Eilam et al., 1992; Gao & Cutler, 1993; Katz & Witkin, 1992; Szechtman et al., 1998; Wachtel et al., 1992).
Apparatus
Two BRS/LVE (Behavioral Research Systems, Leheigh Valley Equipment) two-lever test chambers made of clear 7.0 mm thick plastic walls measuring 30 cm high (26.8 cm from grid floor to top) by 21.0 cm wide by 17.0 cm long were used. The chambers were set on insulated shelving and screened from the surrounding room. A 6-W white light located at the top of the chamber provided general illumination. The stainless-steel levers were 5.1 cm wide and were located 4.0 cm from the grid floor and 3.5 cm at midpoint from each sidewall. A force of at least 0.39 N was required to activate the lever. Presses on both the left lever and the right lever produced delivery of food pellets according to the reinforcement schedule. Food pellets (45-mg Noyes sucrose pellets) were accessible through an opening located 1.0 cm to the left of the right lever.
Procedure
The rats were randomly divided into two groups of 3 prior to the start of the experiment. The SKF38393 group was injected with the partial D1 agonist SKF38393 during drug days (described below). Rats in the quinpirole group were injected with the D2 agonist quinpirole during drug days. After being divided into groups, all rats were shaped to press one of the two response levers for food reinforcement, using the method of successive approximations. When responding was reliably established, they were shaped to press the other lever. Finally, the rats were exposed to an alternating FR 1 schedule, which required responding to alternate between the two levers. The experiment proper began once the rats were reliably responding on both levers and reliably alternating between the levers.
During the experiment proper, each rat was exposed to a series of seven different concurrent reinforcement schedules. The seven schedules were conc VI 15 s VI 15 s; conc VI 15 s VI 30 s; conc VI 15 s VI 60 s; conc VI 30 s VI 15 s; conc VI 60 s VI 15 s; conc VI 60 s VI 30 s; and conc VI 120 s VI 60 s. The order of schedule presentation was counterbalanced across animals to minimize systematic order effects. Each of the concurrent schedules was in effect for a number of consecutive days, which can be divided into baseline days and drug days. During baseline days, the rats pressed the levers for food on the appropriate concurrent schedule. There were no injections during baseline. Baseline days continued until behavior was stable or until 15 sessions had been conducted on that schedule, whichever came first. Stability was defined as 3 consecutive days with no new high or low in the absolute response rate on either lever.
Drug days occurred immediately after baseline days, and were scheduled over a 7-day period as follows: Saline session, no-injection session, drug-dose 1 session, no-injection session, drug-dose 2 session, no-injection session, and drug-dose 3 session. During saline sessions, each animal received a subcutaneous injection of sterile saline solution (0.5 ml/kg). During drug-dose sessions, each rat in the SKF38393 group received either a low (1.0 mg/kg), medium (10.0 mg/kg), or high (20.0 mg/kg) dose of SKF38393. Each rat in the quinpirole group received either a low (0.25 mg/kg), medium (0.50 mg/kg), or high (1.0 mg/kg) dose of quinpirole. The order of dosages was randomized to minimize systematic order effects. The saline session always occurred first, and a no-injection session always occurred between drug sessions.
All sessions were 30 min in duration, and were conducted daily. Reinforcement consisted of a single food pellet. On drug days, injections were given 20 min prior to the session. The rats remained in a plastic holding tub between the injection and the session. The rats also were kept in the plastic tubs prior to the session on baseline days.
Video Analysis
To assess changes in the topography of behavior during exposure to SKF38393 and quinpirole, one rat was videotaped during each baseline, saline, and drug-exposure session for each schedule. The videotaped session was then coded using time-sampling. Behavior was observed and coded using a 15-s point-in-time procedure. Behaviors coded included: sniff, lick, chew (other than food), groom, rear, and locomotion.
Results and Discussion
Absolute rates of response were calculated for all rats on all schedules, conditions, and response levers by dividing the number of presses on that lever during a session by the total session duration. The rates during baseline sessions were measured over the final 3 days on the schedule. Rates for saline and drug conditions were calculated over a single day because each injection dose was only done once for each schedule. In a similar way, absolute rates of reinforcement were calculated for each rat on each lever on each schedule in each condition by dividing the number of reinforcers obtained from a particular lever by the total session time.
Relative rates of response were calculated by dividing the absolute response rate on the left lever by absolute response rate for the right lever. Relative rates of reinforcement were calculated in a similar manner. Logarithmic transformations (base 10) were computed for both relative rates of response and relative rates of reinforcement. The log relative response rates were averaged across animals and plotted as a function of mean relative reinforcement rate for both quinpirole (Figure 1) and SKF38393 (Figure 2) groups. A linear regression then was computed for the data in both figures, which yields a fit to the generalized matching law (Equation 1) including estimates of sensitivity to reinforcement (the a parameter), bias (the b parameter), and the goodness of fit for the model (the r2 value). Parameter estimates for the group data are shown in the figures, with estimates for each individual rat shown in Table 1. The discussion below concentrates on statistical analyses of group means, which is a departure from traditional behavior analysis. A statistical approach was taken because the experimental design, in which each rat was exposed to each dosage/schedule combination for only a single session, produced considerable behavioral variability. A statistical approach is appropriate for variable data because such variability is factored into the analysis.
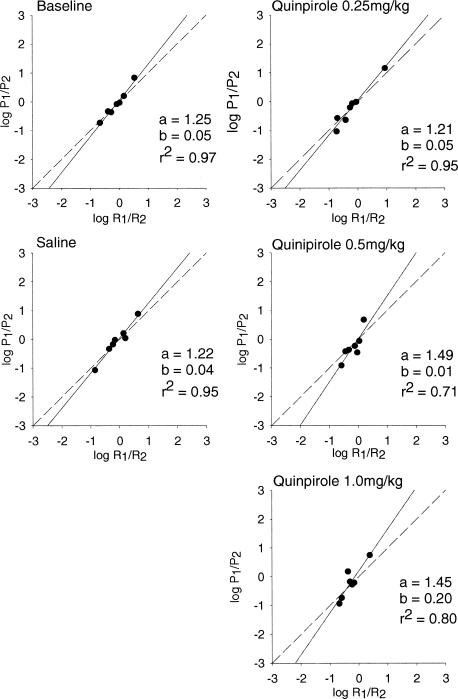
Figure 1. Mean estimates of sensitivity to reinforcement (matching), bias, and goodness of fit for quinpirole rats.
Estimates of matching and bias are based on mean relative rates of responding. The log of P1/P2 is plotted on the y axis, and the log of R1/R2 is plotted on the x axis. The dashed line represents hypothetical matching, and the solid line represents the best-fitting linear regression to the obtained data.
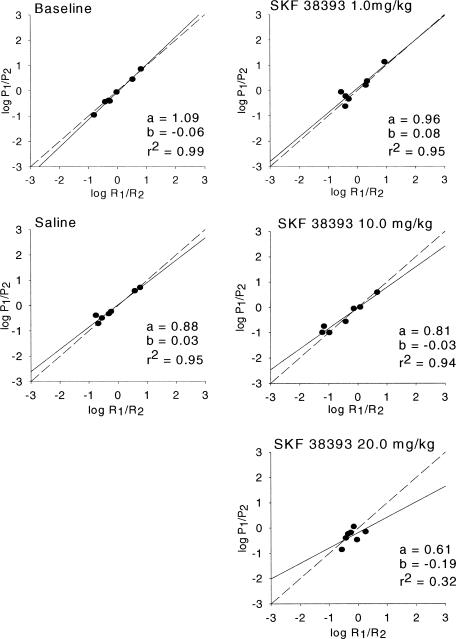
Figure 2. Mean estimates of sensitivity to reinforcement (matching), bias, and goodness of fit for SKF38393 rats.
Estimates of matching and bias are based on mean relative rates of responding. The log of P1/P2 is plotted on the y axis, and the log of R1/R2 is plotted on the x axis. The dashed line represents hypothetical matching, and the solid line represents the best-fitting linear regression to the obtained data.
Table 1. Matching, bias, and r2 values for quinpirole and SKF38393.
| Rat | Condition | Matching (a) | Bias (b) | r2 |
| Quinpirole | ||||
| R963 | Baseline | 1.11 | 0.08 | 0.87 |
| Saline | 0.98 | 0.17 | 0.87 | |
| 0.25 mg/kg | 1.15 | –0.21 | 0.93 | |
| 0.5 mg/kg | 1.49 | 0.07 | 0.77 | |
| 1.0 mg/kg | 0.71 | 0.02 | 0.42 | |
| R965 | Baseline | 1.22 | 0.13 | 0.96 |
| Saline | 1.15 | 0.11 | 0.92 | |
| 0.25 mg/kg | 1.18 | 0.06 | 0.72 | |
| 0.5 mg/kg | 1.48 | 0.15 | 0.89 | |
| 1.0 mg/kg | 1.23 | –0.01 | 0.88 | |
| R966 | Baseline | 1.02 | –0.06 | 0.92 |
| Saline | 1.16 | –0.15 | 0.99 | |
| 0.25 mg/kg | 0.99 | –0.19 | 0.94 | |
| 0.5 mg/kg | 0.77 | –0.16 | 0.75 | |
| 1.0 mg/kg | 1.20 | 0.05 | 0.90 | |
| SKF38393 | ||||
| R831 | Baseline | 0.91 | 0.03 | 0.78 |
| Saline | 0.62 | 0.09 | 0.81 | |
| 1.0 mg/kg | 0.78 | 0.02 | 0.98 | |
| 10.0 mg/kg | 0.58 | 0.08 | 0.63 | |
| 20.0 mg/kg | 0.10 | –0.52 | 0.01 | |
| R837A | Baseline | 1.06 | −0.14 | 0.95 |
| Saline | 0.85 | −0.10 | 0.93 | |
| 1.0 mg/kg | 0.99 | 0.08 | 0.90 | |
| 10.0 mg/kg | 0.94 | −0.07 | 0.95 | |
| 20.0 mg/kg | 0.42 | −0.35 | 0.47 | |
| R837B | Baseline | 1.06 | −0.03 | 0.93 |
| Saline | 0.91 | −0.01 | 0.80 | |
| 1.0 mg/kg | 1.15 | 0.08 | 0.88 | |
| 10.0 mg/kg | 0.87 | −0.02 | 0.79 | |
| 20.0 mg/kg | 0.13 | −0.06 | 0.12 | |
As seen in Figure 1, Equation 1 provided a moderately good fit for all quinpirole conditions (baseline, saline, and the three drug doses), as evidenced by the relatively high r2 values. Overmatching (an a parameter greater than 1.0) was present in all conditions, with greater overmatching at the higher doses, when mean data (Figure 1) were considered. Overmatching predominated in the individual subject data (Table 1), occurring in 11 of the 15 conditions for individual subjects. Bias was minimal, with no systematic changes apparent across conditions. As seen in Figure 2 and in Table 1, SKF38393 had a greater overall effect on behavior. Although Equation 1 provided a good fit in baseline, saline, and the low (1.0 mg/kg) dose of SKF38393, the fit became quite poor at the highest dose (20.0 mg/kg). Likewise, undermatching (an a parameter less than 1.0) became more pronounced as dosage increased. As with quinpirole, bias was minimal with no systematic changes across conditions, although the individual subject data suggest a trend toward increasingly negative bias values as the dosage increased from 1.0 to 10.0 to 20.0 mg/kg.
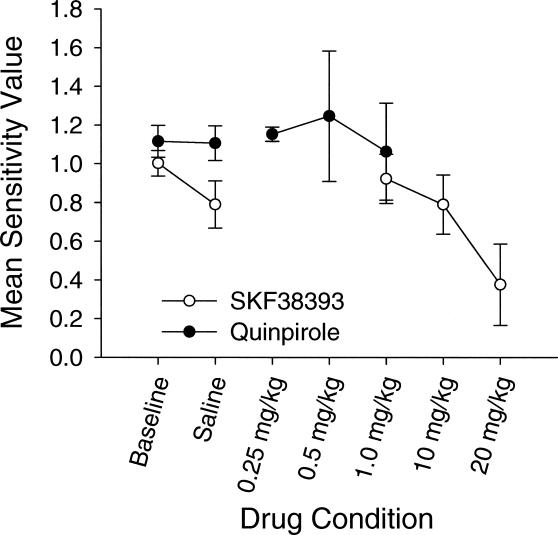
Mean values for each parameter and goodness of fit were calculated across animals using the data in Table 1. These are plotted in Figures 3, 4, and 5 for sensitivity, bias, and goodness of fit (r2 values), respectively. As seen in Figure 3, the mean sensitivity value was slightly higher during quinpirole injections than during baseline. For SKF38393 injections, the value of the sensitivity parameter decreased compared to baseline.
Figure 3. Mean estimates of sensitivity to reinforcement (matching) for the quinpirole and SKF38393 groups in Experiment 1.
Mean matching values are plotted on the y axis, and conditions are plotted on the x axis. Error bars represent the standard error of the mean.
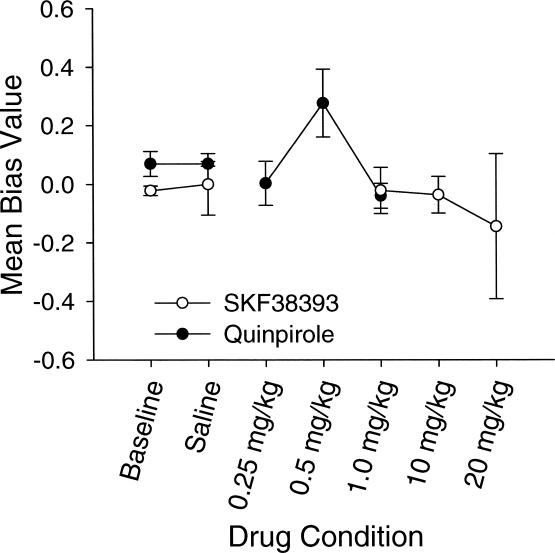
Figure 4. Mean estimates of bias for the quinpirole and SKF38393 groups in Experiment 1.
Mean bias values are plotted on the y axis, and conditions are plotted on the x axis. Error bars represent the standard error of the mean.
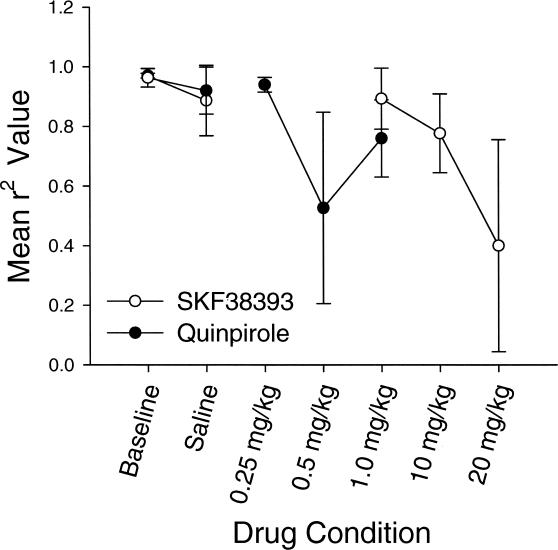
Figure 5. Mean estimates of goodness of fit (r2) for the quinpirole and SKF38393 groups in Experiment 1.
Mean r2 values are plotted on the y axis, and conditions are plotted on the x axis. Error bars represent the standard error of the mean.
Statistical analyses confirmed the visual analysis of Figure 3. First, a paired t test revealed no significant difference in sensitivity between baseline and saline conditions, t(2) = 0.126, p > .05. Subsequent analyses therefore include only baseline. A 2 × 4 (drug × dose) mixed design analysis of variance (ANOVA) revealed a significant main effect of drug, F(1, 4) = 277.492, p = .0001, and dose, F(3, 12) = 6.524, p < .007. There also was a significant interaction, F(3, 12) = 4.506, p = .024.
Additional statistical analyses examined the effects of each drug alone. When quinpirole was examined alone, there were no significant changes in sensitivity across the four dosage conditions, F(3, 6) = 0.33, p > .05. There were significant changes in sensitivity across the four SKF38393 dosage conditions, F(3, 6) = 36.224, p = .0001. Additional paired t tests showed significant differences between baseline and the high (20.0 mg/kg) SKF38393 dose, t(3) = 9.43, p = .001; between the low (0.1 mg/kg) SKF38393 and high (20.0 mg/kg) SKF38393 dose, t(3) = 5.587, p = .031, and between the moderate (10.0 mg/kg) SKF38393 and high (20.0 mg/kg) SKF38393 dose, t(3) = 7.716, p = .019.
As seen in Figure 4, the mean bias parameter stayed close to 0.0 during baseline and saline conditions. Bias did vary across drug doses. Results of a mixed design ANOVA revealed no statistically significant differences between groups, F(1, 4) = 0.51, p > .05, or across conditions, F(3, 12) = .645, p > .05. There was, however, a significant interaction, F(3, 12) = 4.285, p = .028. Rats treated with quinpirole showed a relatively strong bias towards the left lever at the intermediate dose (0.5 mg/kg). In contrast, rats treated with SKF38393 showed an increasing bias towards the right lever as the dosage increased.
As seen in Figure 5, goodness of fit to Equation 1 (r2 values) were high during baseline and saline conditions, but declined across drug conditions in a dose-dependent manner. A mixed-design ANOVA revealed no statistically significant difference between groups, F(1, 4) = 2.90, p < .05. There was, however, a statistically significant difference across drug dose conditions, F(3, 12) = 12.05, p = .001, as well as a significant interaction of condition by group, F(3, 12) = 7.95, p = .003.
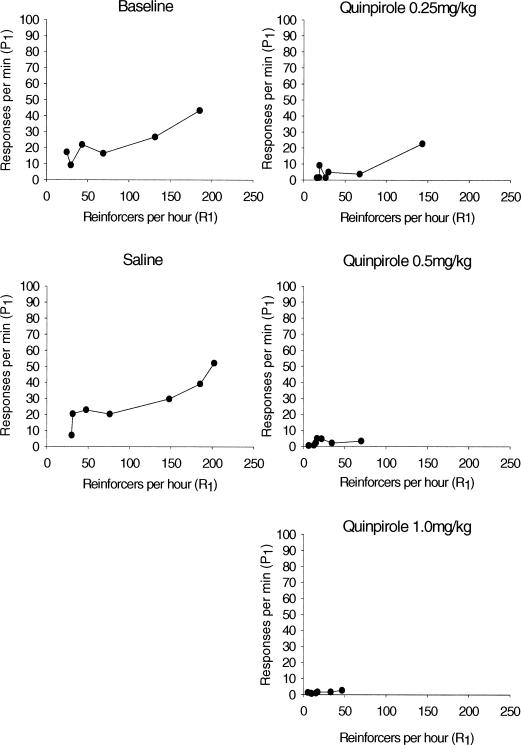
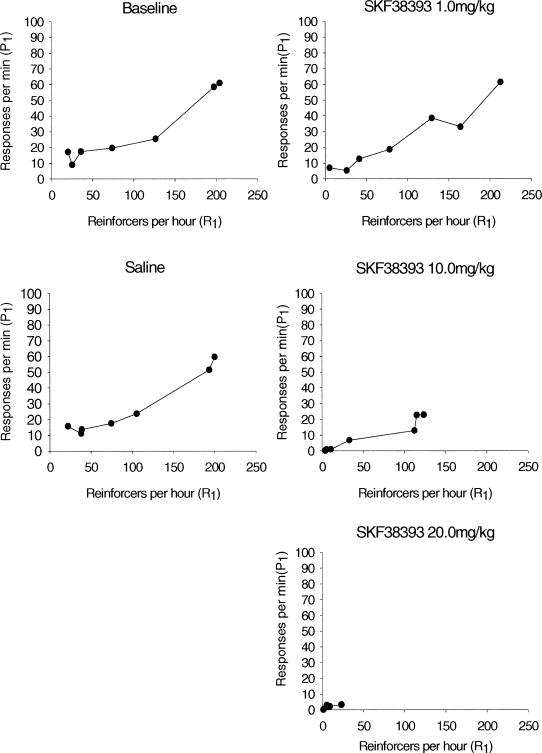
Mean absolute response rates for the left response lever are plotted as a function of obtained reinforcement rate for the quinpirole and the SKF38393 groups in Figures 6 and 7, respectively. Absolute rates for individual animals are shown in Appendix A. Responding to only one of the two response levers is plotted for sake of simplicity—results for the right lever parallel results for the left lever. As seen in Figure 6, rats in the quinpirole group showed a typical monotonic relation (e.g., Herrnstein, 1970) between response rate and reinforcement rate in the baseline and saline conditions, and at the lowest (0.25 mg/kg) dose. Both response rate and obtained reinforcement rate decreased substantially at the two highest drug doses. Figure 7 shows a similar pattern for SKF38393. Again, the typical monotonic function was found in baseline, saline, and low dose conditions, with substantial decreases in both response rate and obtained reinforcement rate at higher doses.
Figure 6. Mean absolute response rates (responses per minute) on P1 for quinpirole rats as a function of obtained reinforcers per hour (R1).
Figure 7. Mean absolute response rates (responses per minute) on P1 for SKF38393 rats as a function of obtained reinforcers per hour (R1).
The relation between the relative response rate data (Figures 1 and 2) and the absolute response rate data (Figures 6 and 7) is particularly interesting. Both quinpirole and SKF38393 rats displayed a marked decrease in absolute response rate at the higher drug doses. The quinpirole group continued to show a strong matching relation even at these reduced absolute rates. The SKF38393 group, in contrast, demonstrated decreased matching at the highest drug doses. This pattern of results suggests that the two agonists have quite different effects on behavior that warrant additional study.
Videotapes of individual sessions at a high, moderate, and low dose were made to examine the response topographies associated with the changes in lever-pressing behavior reported above. Videotapes of one session from each baseline or drug condition were obtained for each group across the five schedules. This session was coded using time-sampling (15-s intervals) across the session. Behavior categories included sniff, lick, groom, rear, and locomotion (behaviors that tended to occur away from the response lever), as well as focal search behaviors (those that occurred next to the operant lever), such as chewing the lever and lever pressing. Although videotapes from only 1 rat from each group were obtained, the tape analyses revealed some interesting differences in topography between the two drug conditions. The data suggested that the animals remained active during the drug sessions, but engaged in responses other than lever pressing. Thus the decrease in response rates at high doses did not occur because of a general suppression of motor responses.
The rat exposed to quinpirole appeared highly “focused” on the response lever and spent much of the session chewing on the lever. The chewing response did not, however, result in a “lever press” being counted. The rat also chewed frequently on the food cup in a perseverative manner. Rarely did the rat leave the area immediately in front of the lever or food cup. In contrast, the rat exposed to doses of SKF38393 showed high rates of searching, sniffing, and grooming. The rat also showed a high degree of stereotypy but remained near the middle or back of the operant chamber, spending more time sniffing and grooming than lever pressing. The duration of sniffing and grooming increased as the dose of SKF38393 was increased.
Because video data were collected for only 1 animal in each group, caution should be used in interpreting the results. Still, the D2 agonist (quinpirole) appeared to elicit behaviors compatible with lever pressing, and resulted in the rat remaining close to the response lever. In contrast, exposure to increasing doses of the D1 agonist (SKF38393) appeared to increase more general search behaviors and grooming stereotypy that occurred more distant to the response lever. These differences in response topography may be related to the different effects the drugs had on lever-pressing behavior. Future experiments should examine these results.
Experiment 1 found that a partial D1 and selective D2 agonist had different effects on behavior on concurrent interval schedules. Experiment 2 examines behavior under similar schedules when rats are exposed to a nonselective DA agonist, apomorphine. Examining sensitivity to reward under a nonselective DA agonist allows comparison of the nonselective agonist to the more specific D1 and D2 agonists. This, in turn, allows determination of the dominate characteristic of general or nonselective DA agonist exposure, and may increase understanding of the relation between dopamine and reward sensitivity.
Experiment 2: Exposure to Apomporphine
Method
Subjects
Four male Sprague-Dawley rats, approximately 120 days old, served as subjects. Rats were housed and cared for in a manner identical to Experiment 1.
Drugs/Injection Procedure
Apomorphine was dissolved in a 5∶1 solution of distilled water for all doses (low 0.5 mg/kg, medium 1.0 mg/kg, and high 2.0 mg/kg, respectively). Injections were given subcutaneously under the loose skin of the neck of the rat.
Apparatus and Procedure
The experimental apparatus and procedure were identical to that used in Experiment 1 with the exception that only five concurrent reinforcement schedules were used, but with a wider range of schedule values. The schedules were conc VI 15 s VI 105 s, conc VI 30 s VI 90 s, conc VI 60 s VI 60 s, conc VI 90 s VI 30 s, and conc VI 105 s VI 15 s.
The pattern of baseline and injection sessions also was identical to Experiment 1. Baseline sessions were conducted for each schedule until responding stabilized. During baseline, rats pressed the lever for food but did not receive any injections. Following baseline, injections were arranged in the following order: Saline session, no-injection session, drug-dose 1 session, no-injection session, drug-dose 2 session, no-injection session, and drug-dose 3 session. The three drug doses were low (0.50 mg/kg), medium (1.0 mg/kg), and high (2.0 mg/kg) with the order counterbalanced across animals to minimize systematic order effects.
Daily experimental sessions were 30 min in duration. Injections were given 20 min prior to the start of the session. The rats remained in a plastic holding tub between the drug injection and the start of the session. The rats also were kept in the holding tub prior to the session on nondrug days.
Results and Discussion
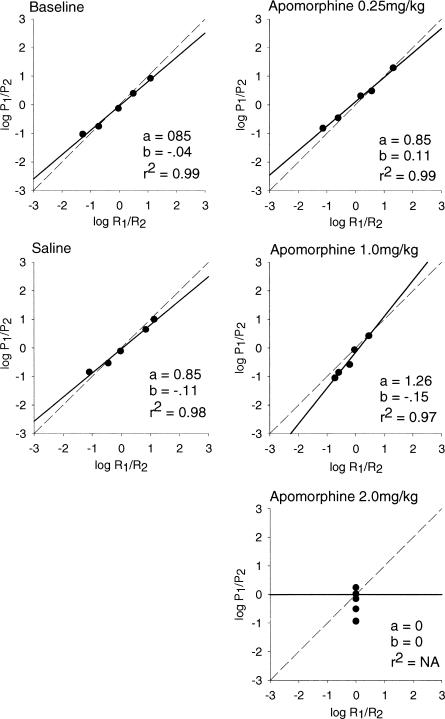
Both absolute and relative rates of response and reinforcement were calculated as in Experiment 1. Logarithmic transformations (base 10) were computed for both relative rates of response and relative rates of reinforcement. The log relative response rates were averaged across animals and plotted as a function of mean relative reinforcement rate in Figure 8. A linear regression was then computed which provides estimates of sensitivity to reinforcement (the a parameter), bias (the b parameter), and the goodness of fit for the generalized matching law (the r2 value). Parameter estimates for each individual rat are shown in Table 2. Note that responding virtually ceased at the high doses of apomorphine, making parameter estimates and interpretations problematic in that condition.
Figure 8. Mean estimates of sensitivity to reinforcement (matching), bias, and goodness of fit for apomorphine rats.
Estimates of matching and bias are based on mean relative rates of responding. The log of P1/P2 is plotted on the y axis, and the log of R1/R2 is plotted on the x axis. The dashed line represents hypothetical matching, and the solid line represents the best-fitting linear regression to the obtained data.
Table 2. Matching, bias, and r2 values for apomorphine.
| Rat | Condition | Matching (a) | Bias (b) | r2 |
| R834 | Baseline | 0.78 | 0.00 | 0.99 |
| Saline | 0.57 | −0.06 | 0.79 | |
| 0.5 mg/kg | 0.81 | 0.11 | 0.99 | |
| 1.0 mg/kg | 1.30 | 0.05 | 0.74 | |
| 2.0 mg/kg | 0.00 | 0.00 | 0.00 | |
| R835 | Baseline | 0.90 | −0.16 | 0.98 |
| Saline | 0.72 | −0.01 | 0.99 | |
| 0.5 mg/kg | 0.92 | −0.01 | 0.96 | |
| 1.0 mg/kg | 1.15 | −0.25 | −0.60 | |
| 2.0 mg/kg | 0.00 | 0.00 | 0.00 | |
| R836 | Baseline | 0.75 | 0.08 | 0.93 |
| Saline | 0.77 | 0.01 | 0.94 | |
| 0.5 mg/kg | 0.41 | −0.01 | 0.66 | |
| 1.0 mg/kg | 1.36 | −0.45 | 0.91 | |
| 2.0 mg/kg | 0.00 | 0.00 | 0.00 | |
| R969 | Baseline | 0.94 | −0.08 | 0.98 |
| Saline | 1.08 | −0.06 | 0.98 | |
| 0.5 mg/kg | 1.01 | 0.15 | 0.96 | |
| 1.0 mg/kg | 0.73 | 0.00 | 0.99 | |
| 2.0 mg/kg | −0.34 | 0.26 | 0.05 |
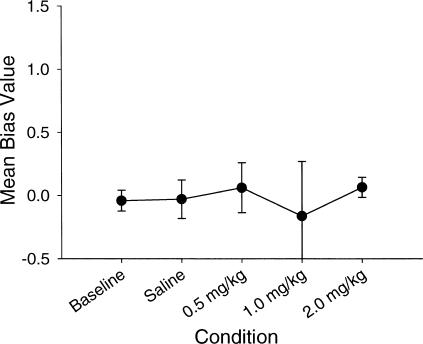
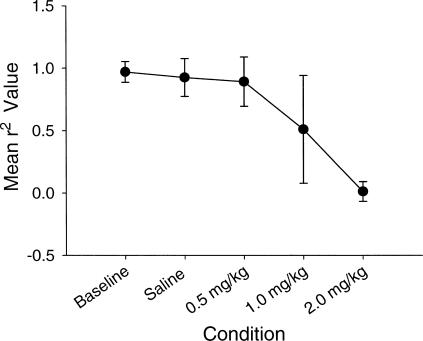
As seen in Figure 8, the mean r2 values were quite high in baseline conditions and at the two lowest drug doses. It was impossible to estimate r2 value at the highest dose because of the very low response and reinforcement rates. The sensitivity parameter showed slight undermatching in baseline conditions and at the low drug dose, with substantial overmatching at the moderate drug dose. Again, the parameter could not be estimated adequately at the highest drug dose. The bias parameter remained near 0.0 with no systematic changes over doses.
Figures 9, 10, and 11 plot changes in sensitivity, bias, and r2 respectively, expressed as means across individual animals. As seen in Figure 9, the mean sensitivity value suggested undermatching in the baseline, saline, and lowest dose conditions, and overmatching at the moderate dose condition. Sensitivity dropped off substantially in the high dose (2.0 mg/kg) condition, largely because of the low response rates in that condition. A repeated measures ANOVA revealed significant differences across the drug dose conditions, F(1, 3) = 14.718, p = .0001. Post hoc analyses using paired t tests and a Bonferroni-corrected significance level of .005 showed significant differences between baseline and the 2.0 mg/kg dose (p = .005), but not between saline and 2.0 mg/kg dose or between the 0.5 mg/kg and 2.0 mg/kg dose (p = .19 and .02, respectively).
Figure 9. Mean estimates of sensitivity to reinforcement (matching) for the apomorphine group in Experiment 2.
Mean bias values are plotted on the y axis, and conditions are plotted on the x axis. Error bars represent the standard error of the mean.
Figure 10. Mean estimates of bias for the apomorphine group in Experiment 2.
Mean bias values are plotted on the y axis, and conditions are plotted on the x axis. Error bars represent the standard error of the mean.
Figure 11. Mean estimates of goodness of fit (r2) for the apomorphine group in Experiment 2.
Mean r2 values are plotted on the y axis, and conditions are plotted on the x axis. Error bars represent the standard error of the mean.
As seen in Figure 10, the bias parameter remained close to a value of 0.0 over all conditions. Statistical analysis revealed no significant differences in bias across conditions.
As seen in Figure 11, the generalized matching law (Equation 1) provided a good fit for the data in the baseline, saline, and low (0.5 mg/kg) drug dose conditions. The mean r2 value declined markedly at moderate (1.0 mg/kg) and high (2.0 mg/kg) drug doses, F(1, 3) = 24.409, p = .001, although the results at the high drug dose should be viewed with caution because of the extremely low response rates in those conditions. Post hoc analyses using paired t tests (with the Bonferroni correction) determined that the saline was marginally different from the 2.0 mg/kg dose (p = .009), whereas the baseline dose was significantly different from the 2.0 mg/kg dose (p = .001). The 0.5 mg/kg dose also was found to differ significantly from the 2.0 mg/kg dose (p = .001).
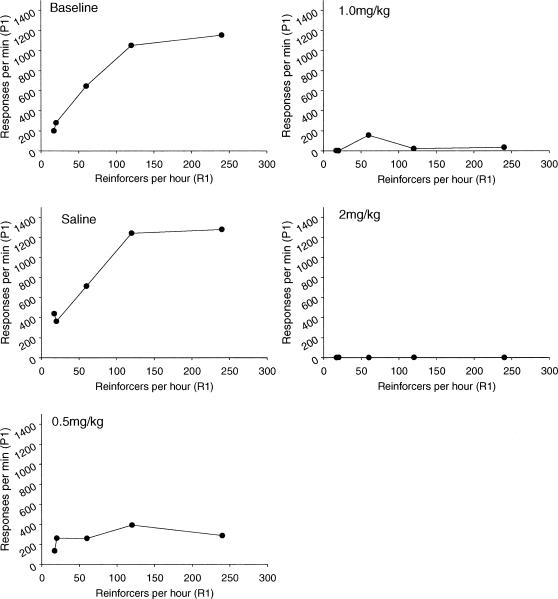
Mean absolute response rates are plotted as a function of mean obtained reinforcement rate in Figure 12. Absolute rates for individual animals are shown in Appendix B. As seen in Figure 12, the typical monotonic/hyperbolic relation between response rate and reinforcement rate was found during baseline and saline sessions. The hyperbolic relation also was found during the low drug dose, although responding was substantially lower. Response rates were extremely low at the two highest drug doses.
Figure 12. Mean absolute response rates (responses per minute) on P1 for apomorphine rats as a function of obtained reinforcers per hour (R1).
As in Experiment 1, videotapes of individual sessions at a high, moderate, and low dose for 1 rat were examined. Data suggested that again, as in Experiment 1, the rat was not motionless during the drug sessions, but engaged in responses other than lever pressing. At the 0.5 mg/kg dose of apomorphine, the rat sniffed at locations away from the response lever during 80% of observed intervals. At the 1.0 mg/kg and 2.0 mg/kg doses, the rat showed high rates of chewing on the floor grid or wall (97% and 76% of observed intervals, respectively). This pattern was similar to that observed in Experiment 1 for SKF38393 but different from the results observed for quinpirole.
General Discussion
Differences in sensitivity to reward were found between rats treated with a partial D1 agonist, SKF38393, a D2 agonist quinpirole, and a nonselective dopamine agonist, apomorphine. Rats treated with quinpirole showed no significant differences in sensitivity to reward across drug-dose conditions. Rats treated with the D1 agonist SKF38393 did show significant changes in sensitivity to reward across drug dose. Data revealed a trend toward increased undermatching values across rats as dosage increased from baseline to 1.0 mg/kg and 10.0 mg/kg doses of SKF38393. However, treatment with SKF38393 at the 20.0 mg/kg dose produced very low sensitivity values, approaching zero in some of the individual subjects. Such low sensitivity values indicate that responding was not systematically related to reinforcement at that dose. Similarly, exposure to a nonselective dopamine agonist, apomorphine, resulted in slight improvement in sensitivity at 0.5 mg/kg doses, but decreased sensitivity to reinforcement at 1.0 mg/kg and 2.0 mg/kg doses.
Overall, the matching law provided a good fit of the data, with r2 values for most conditions remaining relatively high. No significant changes in r2 values were found between the SKF38393 and quinpirole groups or across drug conditions, but a very poor fit of the equation was found at high doses of apomorphine and SKF38393. Again, a significant interaction for r2 values was found between the D1-like and D2-like exposure groups: For rats treated with quinpirole, the r2 value suggested that the matching law provided a good fit to the data, regardless of dose. Examinations of the absolute rates of responding for these rats support this; although response rates slowed in a dose dependent manner, animals still matched to reward payoff.
In contrast, the matching law provided a good fit to the data for rats treated with SKF38393 at control, saline, and the 1.0 mg/kg and 10.0 mg/kg doses. However, the fit was very poor for the 20.0 mg/kg dose, suggesting that the allocation of responses was relatively random. Examinations of the absolute rates of responding support this: Response rates not only decreased in a dose-dependent manner, but rates of responding were extremely low, occasionally resulting in no more than one response per 30 min session, during the high dose condition. In addition, the schedule to which that response was allocated appeared to be random (Appendix A). Very similar results were found for moderate and high doses of apomorphine. Response rates greatly decreased, and at high doses of apomorphine resulted in only one lever press per session.
The present data support the use of the matching law as a model for reinforcement-related drug effects. Typically, changes in the sensitivity parameter (a) of the matching law mirrored the expected drug effects. Drugs effecting D1-like receptors (SKF38393 and apomorphine) caused the predicted decrease in the sensitivity parameter. The results for quinpirole were less clear. Drugs (such as quinpirole) effecting D2-like receptors should increase focal search behaviors, but also may produce perseverative behavior at higher doses. Such a pattern should result in an initial increase in sensitivity to reinforcement at low to moderate doses, and then a decrease in sensitivity as perseverative behaviors dominate at the higher doses. No such effect was found, although the data did trend in the predicted direction. The small number of rats in this group (N = 3) may be a factor. Note that 2 of the rats showed the predicted increase and then decrease across doses (baseline = 1.11 and 1.22, 0.25 mg/kg = 1.15 and 1.18, 0.5 mg/kg = 1.49 and 1.48, 1.0 mg/kg = 0.71 and 1.23). The 3rd rat showed similar sensitivity values at baseline and 0.25 mg/kg (1.02 and 0.99), a decrease during the 0.5 mg/kg dose (a = 0.77), but overmatching at the 1.0 mg/kg dose (1.20). In addition, the 3 rats showed relatively high baseline sensitivity values (1.11, 1.22, and 1.02), and thus there may have been a ceiling effect for detecting changes across the conditions. Replicating across a wider range of doses and with a larger number of animals may help resolve this issue.
Examination of videotapes of the drug sessions provided a clue to interpreting the changes observed in both sensitivity to reward and in absolute rates of responding. Rats treated with quinpirole appeared somewhat slowed and engaged in stereotyped chewing and sniffing of the lever. Although the rat appeared to move more slowly and chewing increased as the drug dose increased, the rat remained very near the response lever and rarely moved away from it. The rat chewed and sniffed both the lever (occasionally resulting in a lever press) and the food cup, in addition to pressing the response lever with the forepaw. In contrast, the rats treated with SKF38393 and apomorphine demonstrated large amounts of sniffing and grooming, especially at high doses. The rats remained quite distant from the response lever, with the grooming and sniffing apparently interrupting the lever pressing response. Thus it was not that rats ceased all responding, but that the form of the dominant response changed during drug exposure. This suggests that changes in sensitivity to reward may have been due to changes in the value of the scheduled and/or any unscheduled reinforcers. That is, other behaviors elicited by the D1 or D2 drug exposure (for example, sniffing and grooming for SKF38393 and perseverative nosing and chewing of the lever for quinpirole) may have taken on greater reinforcement value than operant responding. When treated with quinpirole, chewing increased. This added response was compatible with continued allocation of responses to the lever, as well as food consumption. In contrast, sniffing and grooming were incompatible with lever pressing for the animals treated with SKF38393 or apomorphine.
The use of SKF38393 and quinpirole in the present study merits brief comment. Many standard pharmacological assays indicate that SKF38393 behaves as a partial D1 agonist, but electrophysiological data suggest that SKF38393 may behave as a full agonist in vivo (Heidenreich, Mailman, Nichols, & Napier, 1995; Johansen, Hu, & White, 1991). It would seem unlikely that the use of a full D1 agonist would have produced markedly different results in these experiments. The DA agonist quinpirole has been shown to have affinity for both the D2 and D3 subtypes of D2-like DA receptors (Missale et al., 1998). Although it is important to distinguish between different effects mediated by these two members of the D2 subfamily, this consideration can only be addressed by additional studies with more selective agonists. Thus the present results with quinpirole should be considered as the effects of activating D2-like receptors in the matching paradigm.
The present data are consistent with Schultz's (1998) DA receptor hypothesis and other behavioral studies examining the effects of quinpirole and SKF38393. Eilam et al. (1991) found an inhibition of locomotion at high doses of both SKF38393 and quinpirole administration. During quinpirole exposure, repetitive movements, excessive checking, sniffing, and mouthing/chewing increased in a dose dependent manner (Eilam et al., 1991; Eilam et al., 1992; Gao & Cutler, 1993; Szechtman et al., 1998). Katz and Witkin (1992) found that quinpirole produced dose-related increases in cocaine-appropriate responding, whereas SKF38393 failed to produce any significant increases in cocaine-appropriate responding. The present data support these findings: Quinpirole exposure had no effect on undermatching, with a slight trend towards improved sensitivity to reward. The animals remained at or near the response lever, and engaged in chewing and sniffing of the lever rather than grooming or sniffing about the chamber. These repetitive movements allowed the animal to remain in close proximity to the response lever, thus resulting in no decrease in sensitivity to reward.
Similarly, the results from the SKF38393 and apomorphine exposure are consistent with Schultz's (1998) and Kurylo's (2004) hypothesis regarding differences in behavioral topography elicited by D1-like versus D2-like receptor stimulation. These results also support other investigations examining behavioral differences across D1-like and D2-like agonist drugs. Tinsley, Rebec, and Timberlake (2000) found facilitation of efficient search of an unbaited maze during D1 agonist exposure. Exposure to D1 agonists facilitated predatory search and contact with an artificial prey, whereas activation of D2 receptors had no effect. Data from the present study found similar changes in behavior during operant sessions. Locomotion with sniffing in the chamber increased during apomorphine and SKF38393 exposure, resulting in disruption of operant responding but consistent with search behavior.
The present data also support Kurylo's (2004) findings on the effects of quinpirole on operant responding. Kurylo found that quinpirole produced perseveration of several behaviors compatible with focused responding including excessive time in a drinking funnel when the reinforcer was present, and extended response time during extinction. He suggested that quinpirole produced perseveration of components of operant responding unrelated to general behavioral arousal, but were specific to the response-reward contingency. Thus the D2 receptor function may be to regulate the level of adaptability of a learned response to changing conditions. Such perseveration may allow the animal to remain focused on the response-reward contingency, ignore irrelevant stimuli, and thus increase sensitivity to reward. In contrast, the D1-like receptors' function may be to regulate general search for new or novel rewards or sources of reward. This would decrease sensitivity to reward in a given choice setting, but would increase the behavioral flexibility of an organism in a changing environment.
The results provide additional support that DA may serve as a general activator of behavior. More specifically, D1-like receptor agonists may serve to elicit general search whereas D2-like receptor agonists elicit more focused response repertoires consistent with operant responding or have little effect on appetitive search behavior. Both Schultz (1998) and Kurylo (2004) suggest a neural basis for these behaviors: D1-like receptors control the immediate release of dopamine into the synapse, resulting in bursts of dopamine as well as increases in behavioral activation. In contrast, D2-like receptors maintain tonic levels of dopamine and have little immediate activating effect. Instead, these receptors may regulate continuation or cessation of a given response in reaction to changes in the reinforcement setting. D1-like receptor activation, then, should result in general search behaviors that interfere with ongoing operant responses, and thus interfere with sensitivity to reward. D2-like receptor activation, however, should maintain ongoing operant responses and orientation towards the operant response, thus increasing sensitivity to reinforcement.
One way to investigate different behaviors elicited by D1-like and D2-like agonists may be to explicitly manipulate the number and sources of alternative reinforcement. Changes in competing responses can be predicted to result in a two-fold change: First, the rat is less likely to come in contact with operant responses that result in reward; second, competing responses may produce decreases in the value of the operant reward while increasing the value of alternative responses. Thus exposing rats to simple schedules of reinforcement while explicitly introducing competing reinforcers that may elicit search behaviors (e.g., chew blocks, shavings, or water bottles) should decrease sensitivity to reward during D1-like agonist exposure, but produce little disruption during D2-like agonist exposure. Observing changes in the response repertoire across drug exposure sessions would allow an accurate calculation of the value of “other” reinforcers. Using Herrnstein's (1970) equation to calculate Rο values for competing behaviors during drug exposure would allow quantitative evaluation of changes in response effort (k) as drug dose changed, and could be correlated with changes in reward sensitivity.
In conclusion, differences in sensitivity to reward were found between rats treated with the D2 agonist quinpirole and rats treated with the partial D1 agonist SKF38393 and a nonselective dopamine agonist, apomorphine. No significant changes in matching occurred across drug conditions for the rats treated with quinpirole, whereas matching was greatly compromised during exposure to high doses of SKF38393 and reduced at moderate doses and near zero for high doses of apomorphine. Estimates of bias did not differ significantly between SKF38393 and quinpirole exposure except at moderate doses of each. This, however, may have been due to relatively random responding rather than a tendency to prefer one response alternative.
Bias did shift during apomorphine exposure, with rats showing an increase in bias towards one response lever with increasing doses. Estimates of goodness of fit showed no significant decreases in fit across dose levels for quinpirole, a decrease in goodness of fit for 20.0 mg/kg doses of SKF38393, and a poor fit at 2.0 mg/kg apomorphine dose. Finally, it appears that changes in absolute rates of responding may be accounted for by changes in stereotyped behavior either near the response lever during quinpirole exposure, or distant from the response lever during SKF38393 or apomorphine exposure.
These data are consistent with the model of dopamine as a behavior activator, with D1-like receptors, but not D2-like receptors having a more active role in modulating general search and behavioral activation. Thus D1-like receptors can be predicted to have a more disruptive role in operant matching, suggesting that the value of reinforcement, and perhaps even the availability of reinforced responses, may be altered by stimulation of dopamine D1-like but not D2-like receptors.
Acknowledgments
This research was supported in part by a grant from the National Institute of Drug Abuse AREA Grant 1 R15 DA016343-01A1. We would like to thank Seshanand Chandrashekar and Yuliya Alexeeva for their comments on earlier versions of this manuscript.
APPENDIX A
Absolute rates of responding for Experiment 1: Quinpirole and SKF38393 groups.
| Rat | Schedule | Condition | P1 | R1 | P2 | r2 |
| Quinpirole | ||||||
| R963 | VI 15 VI 15 | Baseline | 35.06 | 134.40 | 33.23 | 151.80 |
| Saline | 36.10 | 150.00 | 24.90 | 138.00 | ||
| 0.25 mg/kg | 1.20 | 19.80 | 2.87 | 54.00 | ||
| 0.5 mg/kg | 0.70 | 10.20 | 1.60 | 18.00 | ||
| 1.0 mg/kg | 1.37 | 28.20 | 1.40 | 13.80 | ||
| VI 15 VI 30 | Baseline | 67.73 | 192.60 | 40.43 | 70.20 | |
| Saline | 64.27 | 214.20 | 42.43 | 60.00 | ||
| 0.25 mg/kg | 1.07 | 7.80 | 6.10 | 61.80 | ||
| 0.5 mg/kg | 0.40 | 1.80 | 2.70 | 54.00 | ||
| 1.0 mg/kg | 0.20 | 1.80 | 2.67 | 60.00 | ||
| VI 15 VI 60 | Baseline | 74.98 | 204.60 | 28.28 | 38.40 | |
| Saline | 77.70 | 202.20 | 33.90 | 48.00 | ||
| 0.25 mg/kg | 51.67 | 184.20 | 2.73 | 16.20 | ||
| 0.5 mg/kg | 2.30 | 48.00 | 0.90 | 12.00 | ||
| 1.0 mg/kg | 1.20 | 18.00 | 3.40 | 31.80 | ||
| VI 30 VI 15 | Baseline | 8.60 | 45.00 | 76.47 | 206.40 | |
| Saline | 13.97 | 55.80 | 103.93 | 204.00 | ||
| 0.25 mg/kg | 0.37 | 1.80 | 7.30 | 151.80 | ||
| 0.5 mg/kg | 0.30 | 1.80 | 4.77 | 109.80 | ||
| 1.0 mg/kg | 0.17 | 4.25 | 0.73 | 30.00 | ||
| VI 60 VI 15 | Baseline | 12.68 | 24.60 | 199.20 | 71.88 | |
| Saline | 7.40 | 24.00 | 210.00 | 45.60 | ||
| 0.25 mg/kg | 0.47 | 1.80 | 102.00 | 5.27 | ||
| 0.5 mg/kg | 0.70 | 7.80 | 78.00 | 4.23 | ||
| 1.0 mg/kg | 0.40 | 4.20 | 72.00 | 2.87 | ||
| VI 60 VI 30 | Baseline | 28.44 | 39.60 | 51.19 | 107.40 | |
| Saline | 34.60 | 43.80 | 67.10 | 108.00 | ||
| 0.25 mg/kg | 2.67 | 18.00 | 7.13 | 84.00 | ||
| 0.5 mg/kg | 7.13 | 0.37 | 1.47 | 14.27 | ||
| 1.0 mg/kg | 0.20 | 1.80 | 5.36 | 70.20 | ||
| VI 120 VI 60 | Baseline | 17.27 | 19.20 | 49.92 | 57.60 | |
| Saline | 25.63 | 46.20 | 30.00 | 33.83 | ||
| 0.25 mg/kg | 12.20 | 19.00 | 28.33 | 52.20 | ||
| 0.5 mg/kg | 5.73 | 13.80 | 9.00 | 52.20 | ||
| 1.0 mg/kg | 0.77 | 4.20 | 12.33 | 60.00 | ||
| R965 | VI 15 VI 15 | Baseline | 11.30 | 102.00 | 19.50 | 154.20 |
| Saline | 15.30 | 102.00 | 13.20 | 144.00 | ||
| 0.25 mg/kg | 3.07 | 58.20 | 1.70 | 34.20 | ||
| 0.5 mg/kg | 1.27 | 24.00 | 1.30 | 31.80 | ||
| 1.0 mg/kg | 0.57 | 10.20 | 0.67 | 18.00 | ||
| VI 15 VI 30 | Baseline | 23.30 | 154.80 | 16.43 | 73.80 | |
| Saline | 14.17 | 126.00 | 10.50 | 72.00 | ||
| 0.25 mg/kg | 6.53 | 127.80 | 1.23 | 12.00 | ||
| 0.5 mg/kg | 3.93 | 91.80 | 0.73 | 4.20 | ||
| 1.0 mg/kg | 3.37 | 61.80 | 1.30 | 18.00 | ||
| VI 15 VI 60 | Baseline | 28.87 | 196.20 | 7.23 | 27.60 | |
| Saline | 32.87 | 196.20 | 10.10 | 28.20 | ||
| 0.25 mg/kg | 8.20 | 130.20 | 1.40 | 10.20 | ||
| 0.5 mg/kg | 3.47 | 90.00 | 0.43 | 1.80 | ||
| 1.0 mg/kg | 4.60 | 94.20 | 0.93 | 7.80 | ||
| VI 30 VI 15 | Baseline | 12.16 | 65.40 | 35.17 | 190.80 | |
| Saline | 12.73 | 70.20 | 46.53 | 192.00 | ||
| 0.25 mg/kg | 3.47 | 37.80 | 5.90 | 112.20 | ||
| 0.5 mg/kg | 0.73 | 7.80 | 1.50 | 49.80 | ||
| 1.0 mg/kg | 1.70 | 24.00 | 2.00 | 49.80 | ||
| VI 60 VI 15 | Baseline | 9.40 | 37.80 | 30.94 | 186.60 | |
| Saline | 7.03 | 46.20 | 34.27 | 199.80 | ||
| 0.25 mg/kg | 3.07 | 36.00 | 9.20 | 130.20 | ||
| 0.5 mg/kg | 5.47 | 31.80 | 6.87 | 90.00 | ||
| 1.0 mg/kg | 1.60 | 19.80 | 2.37 | 70.20 | ||
| VI 60 VI 30 | Baseline | 12.92 | 37.20 | 45.43 | 105.60 | |
| Saline | 11.30 | 58.80 | 48.73 | 96.00 | ||
| 0.25 mg/kg | 9.40 | 43.80 | 40.37 | 102.00 | ||
| 0.5 mg/kg | 4.37 | 42.00 | 8.63 | 67.80 | ||
| 1.0 mg/kg | 0.53 | 7.80 | 4.17 | 84.00 | ||
| VI 120 VI 60 | Baseline | 13.57 | 25.20 | 39.18 | 50.40 | |
| Saline | 13.50 | 22.20 | 39.00 | 54.00 | ||
| 0.25 mg/kg | 7.03 | 0.37 | 24.40 | 0.87 | ||
| 0.5 mg/kg | 4.73 | 16.00 | 30.26 | 31.00 | ||
| 1.0 mg/kg | 0.83 | 12.00 | 3.30 | 31.80 | ||
| R966 | VI 15 VI 15 | Baseline | 33.67 | 157.80 | 18.50 | 116.40 |
| Saline | 37.80 | 192.00 | 15.70 | 1.90 | ||
| 0.25 mg/kg | 0.07 | 1.80 | 7.00 | 70.20 | ||
| 0.5 mg/kg | 0.20 | 4.20 | 1.80 | 36.00 | ||
| 1.0 mg/kg | 0.90 | 7.80 | 3.80 | 61.80 | ||
| VI 15 VI 30 | Baseline | 38.70 | 208.80 | 11.93 | 16.20 | |
| Saline | 39.00 | 216.00 | 4.20 | 0.27 | ||
| 0.25 mg/kg | 3.73 | 67.80 | 1.47 | 6.00 | ||
| 0.5 mg/kg | 2.10 | 10.20 | 3.23 | 19.80 | ||
| 1.0 mg/kg | 1.40 | 36.00 | 1.83 | 7.80 | ||
| VI 15 VI 60 | Baseline | 45.81 | 195.60 | 11.93 | 23.40 | |
| Saline | 45.90 | 208.20 | 4.20 | 0.23 | ||
| 0.25 mg/kg | 8.47 | 115.80 | 1.47 | 6.00 | ||
| 0.5 mg/kg | 4.23 | 72.00 | 3.23 | 19.80 | ||
| 1.0 mg/kg | 2.03 | 28.20 | 1.83 | 7.80 | ||
| VI 30 VI 15 | Baseline | 28.56 | 96.00 | 21.67 | 136.80 | |
| Saline | 34.40 | 10.00 | 28.10 | 2.47 | ||
| 0.25 mg/kg | 0.87 | 16.20 | 1.27 | 25.80 | ||
| 0.5 mg/kg | 0.63 | 10.20 | 1.17 | 34.20 | ||
| 1.0 mg/kg | 0.03 | 1.80 | 0.03 | 1.80 | ||
| VI 60 VI 15 | Baseline | 5.38 | 26.40 | 34.57 | 181.20 | |
| Saline | 6.90 | 19.80 | 51.57 | 3.57 | ||
| 0.25 mg/kg | 0.87 | 10.20 | 7.43 | 90.00 | ||
| 0.5 mg/kg | 0.60 | 6.00 | 2.70 | 55.80 | ||
| 1.0 mg/kg | 0.47 | 4.20 | 5.93 | 85.80 | ||
| VI 60 VI 30 | Baseline | 24.37 | 52.80 | 25.42 | 85.80 | |
| Saline | 2.70 | 40.20 | 34.50 | 1.70 | ||
| 0.25 mg/kg | 2.83 | 28.20 | 0.77 | 12.00 | ||
| 0.5 mg/kg | 2.63 | 24.00 | 0.53 | 6.00 | ||
| 1.0 mg/kg | 4.20 | 42.00 | 1.40 | 6.00 | ||
| VI 120 VI 60 | Baseline | 20.78 | 29.40 | 34.74 | 49.20 | |
| Saline | 22.20 | 25.80 | 0.90 | 29.13 | ||
| 0.25 mg/kg | 8.47 | 37.80 | 4.83 | 24.00 | ||
| 0.5 mg/kg | 4.57 | 20.00 | 2.17 | 18.00 | ||
| 1.0 mg/kg | 0.33 | 2.37 | 0.03 | 0.30 | ||
| SKF38393 | ||||||
| R831 | VI 15 VI 15 | Baseline | 59.40 | 9.47 | 40.13 | 198.00 |
| Saline | 40.20 | 4.30 | 49.80 | 208.20 | ||
| 1.0 mg/kg | 156.00 | 39.40 | 27.40 | 142.20 | ||
| 10.0 mg/kg | 60.00 | 5.80 | 24.20 | 150.00 | ||
| 20.0 mg/kg | 12.00 | 0.73 | 6.10 | 84.00 | ||
| VI 15 VI 30 | Baseline | 78.46 | 189.60 | 26.38 | 75.60 | |
| Saline | 85.03 | 181.80 | 30.73 | 79.80 | ||
| 1.0 mg/kg | 62.03 | 166.20 | 17.30 | 72.00 | ||
| 10.0 mg/kg | 0.90 | 4.20 | 4.17 | 43.80 | ||
| 20.0 mg/kg | 0.03 | 1.80 | 1.57 | 42.00 | ||
| VI 15 VI 60 | Baseline | 78.47 | 202.80 | 10.60 | 36.60 | |
| Saline | 82.43 | 223.80 | 6.53 | 25.80 | ||
| 1.0 mg/kg | 72.43 | 199.80 | 12.03 | 37.80 | ||
| 10.0 mg/kg | 49.46 | 184.20 | 7.77 | 52.20 | ||
| 20.0 mg/kg | 0.03 | 1.80 | 0.73 | 12.00 | ||
| VI 30 VI 15 | Baseline | 18.96 | 55.80 | 56.76 | 194.40 | |
| Saline | 16.40 | 60.00 | 69.93 | 202.20 | ||
| 1.0 mg/kg | 25.17 | 78.00 | 54.63 | 180.00 | ||
| 10.0 mg/kg | 0.53 | 1.80 | 16.10 | 52.20 | ||
| 20.0 mg/kg | 6.33 | 1.80 | 2.07 | 54.00 | ||
| VI 60 VI 15 | Baseline | 8.43 | 19.80 | 76.26 | 216.60 | |
| Saline | 18.70 | 48.00 | 56.60 | 184.20 | ||
| 1.0 mg/kg | 9.43 | 36.00 | 57.57 | 193.80 | ||
| 10.0 mg/kg | 7.10 | 31.80 | 20.53 | 145.80 | ||
| 20.0 mg/kg | 0.03 | 1.80 | 0.03 | 1.80 | ||
| VI 60 VI 30 | Baseline | 15.30 | 53.40 | 60.06 | 106.20 | |
| Saline | 15.63 | 34.20 | 62.33 | 114.00 | ||
| 1.0 mg/kg | 14.00 | 46.20 | 63.77 | 10.00 | ||
| 10.0 mg/kg | 0.03 | 1.80 | 1.67 | 34.20 | ||
| 20.0 mg/kg | 0.03 | 0.03 | 0.23 | 1.00 | ||
| VI 120 VI 60 | Baseline | 11.19 | 14.40 | 57.37 | 61.80 | |
| Saline | 10.50 | 22.20 | 58.57 | 55.80 | ||
| 1.0 mg/kg | 0.33 | 1.80 | 1.33 | 1.80 | ||
| 10.0 mg/kg | 0.63 | 7.80 | 1.47 | 12.00 | ||
| 20.0 mg/kg | 8.83 | 13.80 | 54.00 | 67.80 | ||
| R837A | VI 15 VI 15 | Baseline | 45.83 | 172.20 | 10.80 | 70.80 |
| Saline | 49.60 | 162.00 | 13.97 | 102.00 | ||
| 1.0 mg/kg | 38.80 | 186.00 | 7.03 | 58.20 | ||
| 10.0 mg/kg | 20.90 | 154.20 | 7.40 | 72.00 | ||
| 20.0 mg/kg | 8.70 | 52.20 | 3.70 | 48.00 | ||
| VI 15 VI 30 | Baseline | 62.71 | 209.40 | 10.20 | 49.20 | |
| Saline | 55.80 | 216.00 | 6.80 | 40.20 | ||
| 1.0 mg/kg | 53.43 | 220.20 | 8.30 | 40.20 | ||
| 10.0 mg/kg | 27.30 | 168.00 | 7.50 | 43.80 | ||
| 20.0 mg/kg | 1.47 | 1.80 | 1.33 | 1.80 | ||
| VI 15 VI 60 | Baseline | 61.92 | 230.40 | 4.51 | 13.20 | |
| Saline | 38.80 | 196.20 | 8.23 | 36.00 | ||
| 1.0 mg/kg | 50.23 | 204.00 | 8.60 | 25.80 | ||
| 10.0 mg/kg | 16.23 | 132.00 | 1.47 | 10.20 | ||
| 20.0 mg/kg | 7.13 | 52.20 | 0.30 | 1.80 | ||
| VI 30 VI 15 | Baseline | 9.10 | 70.80 | 20.38 | 174.60 | |
| Saline | 8.27 | 73.80 | 20.87 | 172.20 | ||
| 1.0 mg/kg | 8.47 | 67.80 | 20.80 | 168.00 | ||
| 10.0 mg/kg | 2.07 | 28.20 | 4.63 | 54.00 | ||
| 20.0 mg/kg | 0.57 | 13.80 | 0.03 | 1.80 | ||
| VI 60 VI 15 | Baseline | 14.81 | 42.00 | 26.56 | 169.80 | |
| Saline | 7.07 | 28.20 | 25.40 | 193.80 | ||
| 1.0 mg/kg | 1.83 | 19.80 | 0.03 | 1.80 | ||
| 10.0 mg/kg | 8.37 | 37.80 | 9.60 | 58.80 | ||
| 20.0 mg/kg | 6.50 | 24.00 | 25.63 | 211.80 | ||
| VI 60 VI 30 | Baseline | 8.39 | 40.20 | 22.20 | 107.40 | |
| Saline | 6.03 | 42.00 | 23.63 | 102.00 | ||
| 1.0 mg/kg | 8.43 | 43.80 | 38.93 | 108.00 | ||
| 10.0 mg/kg | 2.37 | 12.00 | 13.60 | 78.00 | ||
| 20.0 mg/kg | 0.43 | 1.80 | 0.33 | 1.80 | ||
| VI 120 VI 60 | Baseline | 12.74 | 22.20 | 15.03 | 43.80 | |
| Saline | 6.73 | 19.80 | 9.70 | 31.80 | ||
| 1.0 mg/kg | 7.86 | 0.20 | 17.76 | 0.93 | ||
| 10.0 mg/kg | 0.20 | 2.00 | 3.10 | 34.00 | ||
| 20.0 mg/kg | 0.17 | 1.80 | 0.40 | 6.00 | ||
| R837B | VI 15 VI 15 | Baseline | 21.07 | 148.20 | 25.63 | 154.20 |
| Saline | 17.30 | 114.00 | 28.03 | 178.20 | ||
| 1.0 mg/kg | 20.90 | 150.00 | 22.40 | 132.00 | ||
| 10.0 mg/kg | 11.90 | 121.80 | 21.40 | 150.00 | ||
| 20.0 mg/kg | 0.47 | 4.20 | 2.40 | 10.20 | ||
| VI 15 VI 30 | Baseline | 34.34 | 193.60 | 17.71 | 84.00 | |
| Saline | 38.27 | 202.20 | 15.50 | 66.00 | ||
| 1.0 mg/kg | 0.03 | 1.80 | 0.07 | 1.80 | ||
| 10.0 mg/kg | 40.40 | 198.00 | 18.00 | 72.00 | ||
| 20.0 mg/kg | 0.03 | 1.80 | 0.03 | 1.80 | ||
| VI 15 VI 60 | Baseline | 42.48 | 180.00 | 16.41 | 46.20 | |
| Saline | 33.20 | 160.20 | 17.37 | 54.00 | ||
| 1.0 mg/kg | 61.80 | 234.00 | 3.37 | 4.20 | ||
| 10.0 mg/kg | 2.30 | 28.20 | 1.67 | 22.20 | ||
| 20.0 mg/kg | 2.93 | 16.20 | 8.07 | 49.80 | ||
| VI 30 VI 15 | Baseline | 30.78 | 94.20 | 35.47 | 157.80 | |
| Saline | 28.00 | 90.00 | 41.10 | 166.20 | ||
| 1.0 mg/kg | 22.30 | 88.20 | 33.30 | 174.00 | ||
| 10.0 mg/kg | 0.30 | 1.80 | 3.60 | 60.00 | ||
| 20.0 mg/kg | 0.07 | 1.80 | 1.50 | 10.20 | ||
| VI 60 VI 15 | Baseline | 3.74 | 13.80 | 66.24 | 238.20 | |
| Saline | 7.73 | 37.80 | 74.63 | 202.20 | ||
| 1.0 mg/kg | 4.50 | 22.20 | 61.93 | 208.20 | ||
| 10.0 mg/kg | 4.67 | 30.00 | 26.20 | 144.00 | ||
| 20.0 mg/kg | 0.03 | 1.80 | 1.50 | 18.00 | ||
| VI 60 VI 30 | Baseline | 14.40 | 28.46 | 51.72 | 107.40 | |
| Saline | 40.20 | 19.30 | 49.30 | 115.80 | ||
| 1.0 mg/kg | 33.60 | 15.53 | 36.03 | 96.00 | ||
| 10.0 mg/kg | 0.30 | 1.80 | 0.77 | 13.80 | ||
| 20.0 mg/kg | 0.30 | 1.80 | 0.03 | 1.80 | ||
| VI 120 VI 60 | Baseline | 24.00 | 27.36 | 28.31 | 48.60 | |
| Saline | 24.00 | 30.03 | 31.23 | 54.00 | ||
| 1.0 mg/kg | 13.80 | 12.67 | 15.70 | 48.00 | ||
| 10.0 mg/kg | 1.80 | 1.80 | 1.50 | 20.00 | ||
| 20.0 mg/kg | 0.03 | 0.03 | 0.03 | 0.03 | ||
APPENDIX B
Absolute rates of responding for Experiment 2: Apomorphine group.
| Rat | Schedule | Condition | P1 | R1 | P2 | r2 |
| R834 | VI 105 VI 15 | Baseline | 7.00 | 13.33 | 32.17 | 93.00 |
| Saline | 9.23 | 10.00 | 32.90 | 96.00 | ||
| 0.5 mg/kg | 1.87 | 10.00 | 3.57 | 30.00 | ||
| 1.0 mg/kg | 0.20 | 1.00 | 0.43 | 7.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| VI 15 VI 105 | Baseline | 45.90 | 98.00 | 8.94 | 13.00 | |
| Saline | 50.23 | 103.00 | 11.83 | 10.00 | ||
| 0.5 mg/kg | 22.13 | 91.00 | 2.33 | 8.00 | ||
| 1.0 mg/kg | 0.50 | 5.00 | 0.07 | 1.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| VI 30 VI 90 | Baseline | 45.34 | 47.67 | 22.16 | 19.70 | |
| Saline | 45.80 | 49.00 | 18.77 | 16.00 | ||
| 0.5 mg/kg | 21.67 | 44.00 | 6.63 | 15.00 | ||
| 1.0 mg/kg | 0.00 | 0.10 | 0.00 | 0.10 | ||
| 2.0 mg/kg | 0.00 | 0.10 | 0.00 | 0.10 | ||
| VI 60 VI 60 | Baseline | 22.23 | 20.33 | 35.24 | 28.30 | |
| Saline | 30.03 | 29.00 | 30.03 | 26.00 | ||
| 0.5 mg/kg | 26.57 | 29.00 | 16.07 | 19.00 | ||
| 1.0 mg/kg | 20.63 | 17.00 | 49.00 | 50.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| VI 90 VI 30 | Baseline | 15.63 | 14.33 | 39.48 | 54.70 | |
| Saline | 18.00 | 47.00 | 31.70 | 22.00 | ||
| 0.5 mg/kg | 20.23 | 14.00 | 43.60 | 53.00 | ||
| 1.0 mg/kg | 0.00 | 0.10 | 0.33 | 1.00 | ||
| 2.0 mg/kg | 0.00 | 0.10 | 0.00 | 0.10 | ||
| R835 | VI 105 VI 15 | Baseline | 1.20 | 2.03 | 48.68 | 106.00 |
| Saline | 0.37 | 0.10 | 42.30 | 108.00 | ||
| 0.5 mg/kg | 1.07 | 4.00 | 24.30 | 85.00 | ||
| 1.0 mg/kg | 0.00 | 0.10 | 0.03 | 0.10 | ||
| 2.0 mg/kg | 0.00 | 0.10 | 0.03 | 0.10 | ||
| VI 15 VI 105 | Baseline | 26.76 | 95.33 | 5.77 | 8.00 | |
| Saline | 31.23 | 95.00 | 3.60 | 5.00 | ||
| 0.5 mg/kg | 10.97 | 74.00 | 0.40 | 1.00 | ||
| 1.0 mg/kg | 3.90 | 28.00 | 0.33 | 2.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.13 | 1.00 | ||
| VI 30 VI 90 | Baseline | 29.66 | 49.00 | 6.92 | 10.40 | |
| Saline | 42.80 | 56.00 | 1.87 | 1.00 | ||
| 0.5 mg/kg | 5.00 | 21.00 | 1.50 | 5.00 | ||
| 1.0 mg/kg | 0.00 | 0.10 | 0.00 | 0.10 | ||
| 2.0 mg/kg | 0.00 | 0.10 | 0.00 | 0.10 | ||
| VI 60 VI 60 | Baseline | 16.76 | 26.00 | 18.97 | 22.30 | |
| Saline | 20.17 | 22.00 | 25.07 | 28.00 | ||
| 0.5 mg/kg | 2.83 | 11.00 | 0.13 | 1.00 | ||
| 1.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| VI 90 VI 30 | Baseline | 2.34 | 4.67 | 47.60 | 53.70 | |
| Saline | 7.27 | 8.00 | 49.63 | 51.00 | ||
| 0.5 mg/kg | 3.77 | 7.00 | 18.53 | 45.00 | ||
| 1.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| R836 | VI 105 VI 15 | Baseline | 17.32 | 13.67 | 34.24 | 92.30 |
| Saline | 47.17 | 93.00 | 13.10 | 13.00 | ||
| 0.5 mg/kg | 14.17 | 14.00 | 18.40 | 72.00 | ||
| 1.0 mg/kg | 0.00 | 0.10 | 0.03 | 0.10 | ||
| 2.0 mg/kg | 0.00 | 0.10 | 0.03 | 0.10 | ||
| VI 15 VI 105 | Baseline | 43.46 | 101.33 | 4.62 | 7.67 | |
| Saline | 45.87 | 101.00 | 5.93 | 13.00 | ||
| 0.5 mg/kg | 2.03 | 10.00 | 3.57 | 13.00 | ||
| 1.0 mg/kg | 0.20 | 3.00 | 0.37 | 3.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| VI 30 VI 90 | Baseline | 47.86 | 49.00 | 18.10 | 18.30 | |
| Saline | 55.37 | 48.00 | 20.93 | 14.00 | ||
| 0.5 mg/kg | 18.37 | 34.00 | 9.17 | 11.00 | ||
| 1.0 mg/kg | 2.93 | 9.00 | 5.47 | 13.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| VI 60 VI 60 | Baseline | 30.52 | 23.00 | 35.93 | 26.30 | |
| Saline | 31.40 | 27.00 | 34.10 | 22.00 | ||
| 0.5 mg/kg | 2.87 | 4.00 | 5.70 | 19.00 | ||
| 1.0 mg/kg | 0.03 | 0.10 | 4.00 | 8.00 | ||
| 2.0 mg/kg | 0.03 | 0.10 | 0.03 | 0.10 | ||
| VI 90 VI 30 | Baseline | 10.18 | 12.67 | 40.76 | 55.70 | |
| Saline | 12.17 | 11.00 | 37.00 | 58.00 | ||
| 0.5 mg/kg | 8.13 | 14.00 | 16.43 | 47.00 | ||
| 1.0 mg/kg | 0.03 | 1.00 | 0.60 | 4.00 | ||
| 2.0 mg/kg | 0.30 | 1.00 | 0.27 | 1.00 | ||
| R969 | VI 105 VI 15 | Baseline | 1.10 | 2.03 | 38.17 | 102.00 |
| Saline | 1.87 | 6.00 | 40.67 | 101.00 | ||
| 0.5 mg/kg | 0.97 | 1.00 | 33.10 | 106.00 | ||
| 1.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 1.87 | 19.00 | ||
| VI 15 VI 105 | Baseline | 37.72 | 106.33 | 1.66 | 5.33 | |
| Saline | 43.23 | 109.00 | 1.23 | 5.00 | ||
| 0.5 mg/kg | 3.20 | 29.00 | 0.00 | 0.10 | ||
| 1.0 mg/kg | 0.03 | 0.10 | 0.03 | 0.10 | ||
| 2.0 mg/kg | 0.03 | 0.10 | 0.03 | 0.10 | ||
| VI 30 VI 90 | Baseline | 17.32 | 47.67 | 10.42 | 16.00 | |
| Saline | 21.70 | 48.00 | 8.67 | 12.00 | ||
| 0.5 mg/kg | 7.30 | 35.00 | 1.83 | 7.00 | ||
| 1.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| 2.0 mg/kg | 0.30 | 5.00 | 0.03 | 1.00 | ||
| VI 60 VI 60 | Baseline | 16.66 | 26.00 | 25.60 | 24.30 | |
| Saline | 13.70 | 22.00 | 28.67 | 29.00 | ||
| 0.5 mg/kg | 2.43 | 15.00 | 2.43 | 10.00 | ||
| 1.0 mg/kg | 0.03 | 1.00 | 0.03 | 1.00 | ||
| 2.0 mg/kg | 0.03 | 1.00 | 0.37 | 3.00 | ||
| VI 90 VI 30 | Baseline | 9.19 | 14.00 | 45.09 | 53.30 | |
| Saline | 10.93 | 14.00 | 41.90 | 54.00 | ||
| 0.5 mg/kg | 2.87 | 9.00 | 8.97 | 29.00 | ||
| 1.0 mg/kg | 0.03 | 0.10 | 0.30 | 2.00 | ||
| 2.0 mg/kg | 0.03 | 0.10 | 0.03 | 0.10 |
References
- Anderson K.G, Woolverton W.L. Concurrent variable-interval drug self-administration and the generalized matching law: A drug-class comparison. Behavioural Pharmacology. 2000;11:413–420. doi: 10.1097/00008877-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Baum W.M. On two types of deviations from the matching law: Bias and undermatching. Journal of the Experimental Analysis of Behavior. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke T.W, Neubauer J. A matching law analysis of the effect of amphetamine on responding reinforced by the opportunity to run. Psychological Record. 1997;47:483–498. [Google Scholar]
- Bindra D. How adaptive behavior is produced: A perceptual-motivational alternative to response-reinforcement. Behavioral and Brain Sciences. 1978;1:41–91. [Google Scholar]
- Egli M, Schaal D.W, Thompson T, Clearly J. Opiod-induced response rate decrements in pigeons responding under variable-interval schedules: Reinforcement mechanisms. Behavioural Pharmacology. 1992;3:581–591. [PubMed] [Google Scholar]
- Eilam D, Clements K.V, Szechtman H. Differential effects of D1 and D2 dopamine agonists on stereotyped locomotion in rats. Behavioural Brain Research. 1991;45:117–124. doi: 10.1016/s0166-4328(05)80077-4. [DOI] [PubMed] [Google Scholar]
- Eilam D, Talangbayan H, Canaran G, Szechtman H. Dopaminergic control of locomotion, mouthing, snout contact, and grooming: Opposing roles of D-sub-1 and D-sub-2 receptors. Pharmacology. 1992;106:447–454. doi: 10.1007/BF02244813. [DOI] [PubMed] [Google Scholar]
- Farmer-Dougan V.A, Dougan J.D, Rokosik S, Lewis J, Garris P.A. Locomotion induced by non-contingent intracranial stimulation: Comparison to psychomotor stimulant. Behavioural Processes. 2004;67:245–261. doi: 10.1016/j.beproc.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Gao B, Cutler M.G. Effects of quinpirole on the behaviour shown by mice in the light-dark box and during social interactions. Neuropharmacology. 1993;32:93–100. doi: 10.1016/0028-3908(93)90134-o. [DOI] [PubMed] [Google Scholar]
- Garris P.A, Kilpatrick M, Bunin M.A, Walker Q.D, Wightman R.M. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Heidenreich B.A, Mailman R.B, Nichols D.E, Napier T.C. Partial and full dopamine D1 agonists produce comparable increases in ventral pallidal neuronal activity: Contribution of endogenous dopamine. Journal of Pharmacology and Experimental Therapeutics. 1995;273:516–525. [PubMed] [Google Scholar]
- Herrnstein R.J. Relative and absolute strength of response as a function of frequency of reinforcement. Journal of the Experimental Analysis of Behavior. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein R.J. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward seeking. Brain Research Reviews. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johansen P.A, Hu X.T, White F.J. Relationship between D1 dopamine receptors, adenylate cyclase, and the electrophysiological responses of rat nucleus accumbens neurons. Journal of Neural Transmission General Section. 1991;86:97–113. doi: 10.1007/BF01250571. [DOI] [PubMed] [Google Scholar]
- Katz J.L, Witkin J.M. Effects of quinpirole and SKF38393 alone and in combination in squirrel monkeys trained to discriminate cocaine. Psychopharmacology. 1992;107:217–220. doi: 10.1007/BF02245140. [DOI] [PubMed] [Google Scholar]
- Kelley A.E, Gauthier A.M, Lang C.G. Amphetamine microinjections into distinct striatal sub regions cause dissociable effects on motor and ingestive behavior. Behavioural Brain Research. 1989;35:27–39. doi: 10.1016/s0166-4328(89)80005-1. [DOI] [PubMed] [Google Scholar]
- Kelley A.E, Stinus L. Disappearance of hoarding behavior after 6-hyrdoxydopamine lesions of the mesolimbic dopamine neurons and its reinstatement with L-dopa. Behavioral Neuroscience. 1985;99:531–545. doi: 10.1037//0735-7044.99.3.531. [DOI] [PubMed] [Google Scholar]
- Kurylo D.D. Effects of quinpirole on operant conditioning: Perseveration of behavioral components. Behavioural Brain Research. 2004;155:117–124. doi: 10.1016/j.bbr.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Martin J.C, Dougan J.D, Wu Q, Stanisz L.A, Martyn S, Rokosik S, Garris P.A, Farmer-Dougan V.A. Locomotion induced by non-contingent intracranial electrical stimulation: Dopamine dependence and general characteristics. Behavioral Processes. 2004;67:131–146. doi: 10.1016/j.beproc.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Martinetti M.P, Andrzejewski M.E, Hineline P.N, Lewis M.J. Ethanol consumption and the matching law: A choice analysis using a limited-access paradigm. Experimental and Clinical Psychopharmacology. 2000;8:395–403. doi: 10.1037//1064-1297.8.3.395. [DOI] [PubMed] [Google Scholar]
- McMillan D.E, Hardwick W.C. Drug discrimination in rats under concurrent variable interval variable interval schedules. Journal of the Experimental Analysis of Behavior. 2000;73:103–120. doi: 10.1901/jeab.2000.73-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan D.E, Li M, Snodgrass S.H. Effects of drugs on concurrent variable interval schedule performance. Behavioral Pharmacology. 1998;9:663–670. doi: 10.1097/00008877-199812000-00002. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash S.R, Robinson S.W, Jaber M, Caron M.G. Dopamine receptors: From structure to function. Physiological Reviews. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Phillips P.E.M, Stuber G.D, Helen M.L.A.V, Wightman R.M, Carelli R.M. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pitts S.M, Horvitz J.C. Similar effects of D-sub-1/D-sub-2 receptor blockade on feeding and locomotor behavior. Pharmacology, Biochemistry and Behavior. 2000;65:433–438. doi: 10.1016/s0091-3057(99)00249-x. [DOI] [PubMed] [Google Scholar]
- Rebec G.V, Christensen J.R, Guerra C, Bardo M.T. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Research. 1997;776:61–67. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott T.J, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends in Neuroscience. 1999;22:146–151. doi: 10.1016/s0166-2236(98)01373-3. [DOI] [PubMed] [Google Scholar]
- Salamone J.D, Aberman J.E, Sokolowski J.D, Cousins M.S. Nucleus accumbens dopamine and rate of responding: Neurochemical and behavioral studies. Psychobiology. 1999;27:236–247. [Google Scholar]
- Salamone J.D, Cousins M.S, McCullough L.D, Carriero D.L, Berkowitz R.J. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacology, Biochemistry & Behavior. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Salamone J.D, Cousins M.S, Snyder B.J. Behavioral functions of nucleus accumbens dopamine: Empirical and conceptual problems with the anhedonia hypothesis. Neuroscience and Biobehavioral Reviews. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W. The reward signal of midbrain dopamine neurons. News in Physiological Science. 1999;14:249–255. doi: 10.1152/physiologyonline.1999.14.6.249. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. Journal of Neuroscience. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague P.R. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R, Ljungberg T, Mirenowicz J, Hollerman J.R, Dickinson A. Reward-related signals carried by dopamine neurons. In: Houk J.C, Davis J.L, Beiser D.G, editors. Models of information processing in the basal ganglia. Cambridge, MA: The MIT Press; 1995. pp. 233–248. [Google Scholar]
- Schultz W, Tremblay L, Hollerman J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–283. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sokolowski J.D, Salamone J.D. The role of accumbens dopamine in lever pressing and response allocation: Effects of 6-OHDA injected into core and dorsomedial shell. Pharmacology, Biochemistry & Behavior. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends in Neuroscience. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Sulis W, Eilam D. Quinpirole induces compulsive checking behavior in rats: A potential animal model of obsessive-compulsive disorder. Behavioral Neuroscience. 1998;112:1475–1485. doi: 10.1037//0735-7044.112.6.1475. [DOI] [PubMed] [Google Scholar]
- Timberlake W. Motivational modes in behavior systems. In: Mowrer R.R, Klein B.B, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 155–209. [Google Scholar]
- Tinsley M.R, Rebec G, Timberlake W. Facilitation of preparatory behavior: An artificial prey paradigm by D1-subfamily dopamine receptor activation. Behavioral Brain Research. 2000;114:23–30. doi: 10.1016/s0166-4328(00)00188-1. [DOI] [PubMed] [Google Scholar]
- Wachtel S.R, Brooderson R.J, White F.J. Parametric and pharmacological analyses of enhanced grooming response elicited by the D1 receptor agonist SKF38393 in the rat. Psychopharmacology. 1992;109:41–48. doi: 10.1007/BF02245478. [DOI] [PubMed] [Google Scholar]
- Woolverton W.L, Alling K. Choice under concurrent VI schedules: Comparison of behavior maintained by cocaine or food. Psychopharmacology. 1999;141:47–56. doi: 10.1007/s002130050805. [DOI] [PubMed] [Google Scholar]