Abstract
Working memory, the ability to temporarily retain task-relevant information across a delay, is frequently investigated using delayed matching-to-sample (DMTS) or delayed Go/No-Go tasks (DGNG). In DMTS tasks, sample cues instruct the animal which type of response has to be executed at the end of a delay. Typically, performance decreases with increasing delay duration, indicating that working memory fades across a delay. However, no such performance decrease has been found when the sample cues exist of present vs. absent stimuli, suggesting that pigeons do not rely on working memory, but seem to respond by default in those trials. We trained 3 pigeons in a DGNG task and found a similar default response pattern: The diverging slopes of the retention functions on correct Go and No-Go trials suggested that pigeons by default omitted their response following No-Go stimuli, but actively retained task-relevant information across the delay for successful responses on Go trials. We conducted single-cell recordings in the avian nidopallium caudolaterale, a structure comparable to the mammalian prefrontal cortex. On Go trials, many neurons displayed sustained elevated activity during the delay preceding the response, replicating previous findings and suggesting that task-relevant information was neurally represented and maintained across the delay. However, the same units did not show enhanced delay activity preceding correct response suppressions in No-Go trials. This activation-inactivation pattern presumably constitutes a neural correlate of the default response strategy observed in the DGNG task.
Keywords: working memory, electrophysiology, NCL, PFC, delayed matching to sample, Go/No-Go, default response, mandibulation, pigeons
The relation between behaviorism and cognitive neuroscience traditionally has been somewhat ambivalent. Whereas numerous behaviorists limited scientific conclusions to a formal description of observable laws and variables, and refrained from speculating about the “black box” between stimulus and response (Skinner, 1938), many cognitive neuroscientists attempted exactly this: Revealing the mediating internal processes between sensory and motor function. In the past few decades, however, frequent conciliating positions emerged, emphasizing the mutual benefit that both groups would gain from improved cooperation (cf. Zentall, 2001). In the present paper, we present a neuroscientific study that yields conclusions about the nature of what is coded in a memory task. With this paper, we hope to illustrate how combining the domains of neuroscience and behavior in the pursuit of investigating memory processes can allow for valuable insights at a different explanatory level than could be accomplished by either of these approaches alone.
A good example of a phenomenon that has been investigated extensively by both behaviorists and cognitive neuroscientists is working memory. Working memory refers to the animal's ability to temporarily maintain task-relevant information across a temporal delay to perform a goal-directed behavior. Although a large range of different paradigms are used to assess working-memory function, the two most-commonly employed tasks are delayed matching-to-sample (DMTS; Colombo, Cottle, & Frost, 2003; Colombo, Frost, & Steedman, 2001; Fuster, Bauer, & Jervey, 1982; Goldman-Rakic, 1995; Rosenkilde, Bauer, & Fuster, 1981; White, Ruske, & Colombo, 1996) and delayed Go/No-Go tasks (DGNG; Diekamp, Kalt, & Güntürkün, 2002; Kalt, Diekamp, & Güntürkün, 1999; Rosenkilde, et al., 1981; Tremblay & Schultz, 2000). In a typical DMTS task, several sample stimuli are associated with distinct comparison stimuli. Usually, one sample stimulus is presented to the animal at trial onset that is then followed by a delay of varying duration and the presentation of several comparison stimuli. A response to the comparison stimulus associated with the previously presented sample stimulus is reinforced. In a typical DGNG task, a Go or No-Go sample stimulus is followed by a delay of varying duration and then by a signal after which a response has to be executed or withheld. In both DMTS and DGNG tasks, the animal has to maintain the task-relevant information across the delay in order to choose the correct response.
Zentall, Urcuioli, Jagielo, & Jackson-Smith (1989) reported that, in a DMTS task, pigeons maintained a retrospective representation of the sample rather than a prospective, anticipatory representation of the comparison stimulus. Typically, the probability of a correct response declines with increasing delay length, suggesting a delay-dependent decay of the retrospective retention of the sample stimulus (Grant, 1991; Sherburne & Zentall, 1993). However, when sample stimuli consisted of the presence versus the absence of an event (e.g., food vs. no-food, Grant, 1991; or hue vs. no-hue, Sherburne & Zentall, 1993), the normally decreasing slope of the retention function was flatter or even absent after no-food and no-hue samples compared to food and hue samples. This finding has been interpreted as evidence that animals respond by default to no-feature samples because responding by default requires minimal working memory capacity and is as such only marginally affected by increasing delays.
In the present study, we trained pigeons in a DGNG task and found a similar retention pattern. In this task, a sample stimulus (red or green circle displayed on a screen) was presented at the beginning of a trial, followed by a delay of varying duration, indicated by a white light below the screen. The end of the delay, as cued by offset of the light, prompted the onset of the response on Go trials (mandibulation) or the inhibition of a response on No-Go trials, respectively. Our behavioral results show that the probability of a correct response after presentation of a Go stimulus decreased with increasing delay length; however, the probability of a correct response–rejection on No-Go trials appeared to be unaffected by delay duration. Based on the findings by Grant, Sherburne, Zentall and colleagues (Grant, 1991; Sherburne & Zentall, 1993), it is tempting to assume that animals retained the task-relevant information in memory across the delay, but by default refrained from responding following presentation of the No-Go stimulus.
A wealth of electrophysiological recording data collected over the past three decades has revealed that stimulus-specific single units in the primate dorsolateral prefrontal cortex (Fuster, 1973, 1995; Goldman-Rakic, 1995, 1996; Miller, Erickson & Desimone, 1996; Quintana & Fuster, 1992; Tremblay & Schultz, 2000), and the equivalent structure in the pigeon brain (Diekamp et al., 2002; Kalt et al., 1999) play a crucial role in working memory function. These studies showed that the activity of the so-called delay neurons was significantly enhanced during the delay phase in DMTS or DGNG tasks, that their activity correlated with working-memory performance, and that the same type of pharmacological intervention that affected working memory performance also altered the discharge pattern of delay neurons (Müller, von Cramon, & Pollmann, 1998; Sawaguchi & Goldman-Rakic, 1994; Sawaguchi, Matsumura, & Kubota, 1988; Williams & Goldman-Rakic, 1995). In addition to these empirical studies, an abundance of computational and theoretical work (Durstewitz, Kelc, & Güntürkün, 1999; Durstewitz & Seamans, 2002; Durstewitz, Seamans, & Sejnowski, 2000a, 2000b; Goldman-Rakic, 1996) suggests that delay neurons are indeed an integral part of a neural network responsible for maintaining a task-relevant stimulus representation across a delay and shielding this representation against interference.
If pigeons indeed reject their response by default on No-Go trials in a DGNG task, but rely on memorized task-relevant information on Go trials, then we would expect to find working-memory-related delay activity on correct Go responses, but not on correct response rejections. Here, we conducted single-cell recordings in the nidopallium caudolaterale (NCL), a structure functionally comparable to the mammalian prefrontal cortex (Durstewitz, Kröner, & Güntürkün, 1999; Kröner & Güntürkün, 1999; Mogensen & Divac, 1982, 1993; Waldmann & Güntürkün, 1993) while pigeons performed a DGNG task. It is to be noted that the NCL was termed neostriatum caudolaterale prior to the revision of the avian brain nomenclature (Reiner et al., 2004). We hypothesized to find a neural activation pattern reflecting the pigeons’ presumed default response strategy. Parts of the results have been published elsewhere (Diekamp et al., 2002).
Method
Subjects and Surgery
Three naive pigeons (Columba livia), 3 to 5 years of age and unknown sex, were used in this experiment. They were obtained from local breeders and raised in the institute's own aviary, and during the time of training and testing, housed in a cage (40 cm by 40 cm by 40 cm) in a colony room. The animals had access to food ad libitum in their home cage at all times. For training and testing, they were put on a water deprivation schedule; that is, they were water deprived 24 to 36 hr prior to each training or testing session. Water was always available ad libitum within 20 min after each session and between testing days.
For surgery, pigeons were anaesthetized with a mixture of ketamine (Ketavet, Pharmacia & Upjohn, Germany; 40 mg/kg i.m.) and xylazine (Rompun, Bayer, Germany; 8 mg/kg i.m.). A recording chamber was implanted stereotactically and fixed with dental acrylic over the posterolateral skull. A < 1 mm trephine hole was made within the inner circle of the recording chamber that was subsequently sealed with bone wax. This position allowed recording from the NCL within the borders defined by Kröner and Güntürkün (1999), that is, at the coordinates A 4.25–7.5, L 2.5–7.5 and about 1.0 mm to 1.5 mm below the surface of the brain, according to the pigeon brain atlas by Karten and Hodos (1967). In addition, a head-fixation block was implanted medially to the recording chamber to attach the head to the recording stereotaxis and prevent head movements of the animal during recording sessions. The pigeons were allowed to recover fully from surgery before continuing the training.
All subjects were kept and treated according to the German guidelines for the care and use of animals in neuroscience, and the research was approved by the national committee of the State of Nordrhein Westfalen, Germany.
Apparatus
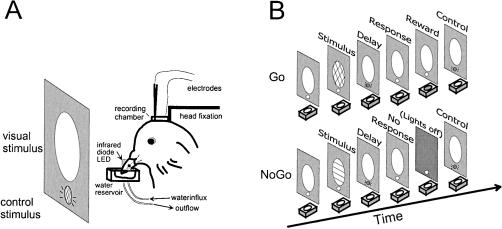
The pigeons were restrained by a loose cloth bag and placed on a foam couch in front of a translucent screen (25 cm high and 30 cm wide). A small white light (4°) below the stimulus screen was used as a cue indicating the onset or offset of a trial (see below). All stimuli could be detected by the animal with the eyes placed in lateral viewing position and without shifting gaze. The animal's head was fixed into stereotaxic coordinates by the head-fixation block that was reversibly attached to the stereotaxic frame (Figure 1A). Beak movements were monitored with an infrared light emitting diode (BPW 23) and a photodetector (BPW 40) that were positioned to the sides of the beak. The output from the photodetector was converted into TTL pulses and fed into a computer controlling the behavioral experiment. Reinforcement consisted of a drop of water that was presented in a small aluminum container (1 cm by 1 cm by 2 cm3). The container was adjusted into position such that the tip of the beak was 3 mm below the water surface. The water amount delivered as reinforcement was controlled by the computer through two electromagnetic valves (Kuhnke 65111). The influx and efflux valves were timed such that about 0.15 ml of water was available for about 3 s during which the pigeon was allowed to drink.
Figure 1. Experimental setup and behavioral task.
(A): Stereotaxis and apparatus. The pigeon was placed in front of a screen for stimulus presentation with its head fixed in a stereotaxis for electrophysiological recording. The beak was placed above a small water supplier, and beak movements were controlled with an infrared diode LED attached just above the water reservoir. (B): Sequence of events. Go trials (upper panel) were initiated with the presentation of the Go stimulus. The pigeon had to mandibulate during the response period following a delay of varying duration (0.6 s to 1.4 s). Correct Go responses (hits) were reinforced by access to water; incorrect misses had no consequence. No-Go trials (lower panel) were initiated with the presentation of a No-Go stimulus, followed by a delay. Pigeons had to refrain from responding during the response period. Correct response rejections had no consequences; incorrect false alarms were punished by a brief light-off period.
Procedure
Pigeons were trained on a delayed Go/No-Go task (Figure 1B). Each trial began with the presentation of a colored visual Go (red) or No-Go (green) stimulus for 500 ms (presented in random order; stimulus size of about 35° of visual angle) on the screen located in front of the pigeon. The offset of the Go/No-Go stimulus was followed by a delay period of varying duration, indicated by the small white light below the stimulus screen. The delay period remained constant in a block of 40 to 60 trials but varied from 0.6 to 1.4 s between blocks of trials and sessions. At the end of the delay period, the white delay light was switched off, and the pigeon was required to respond on Go trials, or refrain from responding on No-Go trials, within a 1.5 s period. The pigeons indicated their response by opening and closing the beak five times, or by suppressing beak openings, during the response interval, respectively. Correct responses on go trials (“hit”) were reinforced with water. Incorrect go trials (“miss”) had no consequences. Beak movements during the response period of No-Go trials (“false alarm”) led to a mild penalty consisting of a 3 s time-out with all lights turned off, whereas the suppression of beak movements on No-Go trials (“correct rejection”) again had no consequences. Each response period was followed by an intertrial interval of 5 to 10 s with randomly varying duration. Trials lasted approximately 14 to 19 s. Each block of 40 trials consisted of 20 Go and 20 No-Go trials that were presented in random order. Animals were trained and tested with up to five blocks per day. Blocks were separated by pauses of about 10 min.
Prior to electrophysiological recording, pigeons were trained until they reached a criterion of 70% correct responses in three successive blocks within a single day, (correct Go + correct No-Go)/total number of trials. Except for two recording sessions, we did not use long delays of 1.2 s and 1.4 s during electrophysiological recording sessions because each session would have lasted too long with the extra time needed for the electrophysiological procedures and the preceding training blocks.
To manage the task successfully in Go trials, pigeons had to either remember the previously presented color during the delay or form and maintain a motor plan for future actions. To control for the visual stimulation during the delay, the white light was also presented during the intertrial interval, 4.5 s after the reinforcer or 2.0 s after the end of the response period. The analysis of behavioral and neural activity in response to the white light during the delay and during this control period was used to assess whether sensory stimulation without working memory load was sufficient to evoke neuronal responses.
Recording Techniques
Extracellular activity was recorded from single cells with glass-insulated, platinum-iridium electrodes (3 to 10 µm). Before each recording session, a < 1 mm trephine hole in the skull was made within the inner circle of the recording chamber by removing the bone wax over the NCL or adjacent regions, and was sealed with bone wax again at the end of the recording. Electrodes were advanced through the intact dura with a hydraulic microdrive roughly at an angle of 20° to the vertical plane at a position of A 4.25–7.5 and L 2.5–7.5 according to the brain atlas by Karten and Hodos (1967). Neural signals were amplified (DAM 80, WPI), filtered, and continuously monitored with an oscilloscope and a loudspeaker. Neuronal data were digitized at a sampling rate of 10 kHz and stored on computer. For each neuron, data were sampled over 30 to 60 Go and No-Go trials.
Analysis of Electrophysiological Data
Recorded spike data were analyzed off-line to isolate single unit activity from background noise using the spike sorting analysis module and cluster analysis of DataWave (EWB, DataWave Technologies, Longmont, CO). In a few cases, two units recorded from the same electrode could be isolated. The time of event of the spike was used for analysis. For each neuron, changes in neuronal activity related to the task were assessed by comparing the spike rates (spikes per second) during specific intervals of the task. The following task relevant intervals were defined for the analysis: spontaneous, 0 to 1.0 s; stimulus, 1.0 to 1.5 s; delay, 0.6 s, 0.8 s, 1.0 s duration starting at the end of the stimulus period; response, 3 s duration starting at the end of the delay period; control light, 500 ms interval 4.5 s after the reinforcer or 2 s after the response period.
Neuronal responses during the delay interval were compared with spontaneous activity (no stimulus, no working memory load) and with spike rates recorded during the control period (identical stimulus, no working memory load). For all neurons, Wilcoxon tests and analyses of variance (ANOVA) were performed on the average response rates. Different time intervals during each trial (spontaneous activity, stimulus, delay, response, and control interval) and response categories (hit, miss, correct rejection, and false alarm) were used as factors to analyze the effects on the activity of cells. Results were evaluated at p < 0.05, and if appropriate, post hoc multiple comparisons (Tukey's test) were applied.
To show the time course of changes in neuronal activity for the different response categories, we calculated normalized average population histograms. Spike counts for each 50-ms bin were normalized by the spontaneous activity of each cell and expressed as a percentage. Separate histograms were calculated for each population of neurons tested with the same delay duration.
Histology
On the last day of recordings, electrode tracks were marked by inserting a microelectrode stained with DyeI (Snodderly & Gur, 1995) at positions about 200 µm anterior, posterior, medial, and lateral to the outermost electrode penetrations. Animals were deeply anesthetized with Equithesin (3.1 ml/kg i.m.) and perfused transcardially with saline followed by 4% paraformaldehyde. The frozen brains were sectioned in a parasagittal plane alternating at 40 µm and 100 µm, mounted, and stained with cresyl violet and 4′,6′-diamidino-2-phenylindole, respectively. Electrode tracks and recording sites were localized in stained sections of the brain by histological verification under fluorescence (100 µm 4′,6′-diamidino-2-phenylindole stained sections) and light microscopy (40 µm cresyl violet stained sections) and by stereotaxic reconstruction. Neuronal data were sampled from locations covering the complete extent of the NCL.
Results
Behavior
All pigeons were trained to reach a criterion of at least 70% performance, (hit + rejections)/2, in three consecutive sessions. Due to everyday variations, the performance occasionally dropped below 70% in the recording sessions. The overall performance across all delays and all pigeons was 71.33 ± 1.24% correct (mean ± standard error). The individual performance across all delays was (mean ± standard error in percentage correct) 71.45 ± 6.62 (Pigeon 627), 70.6 ± 2.46 (Pigeon 754), and 71.66 ± 4.44 (Pigeon 865). All pigeons tended to perform significantly better on No-Go trials (94.62 ± 0.62% rejections) than on Go trials (48.04 ± 2.62% hits; t(111) = 16.14, p < 0.001, Student's t test for paired samples). The individual performance was (mean ± standard error in percentage correct): 46.27 ± 9.78 hits in Go trials and 96.63 ± 1.08 correct rejections in No-Go trials (Pigeon 627), 47.68 ± 2.91 hits and 93.53 ± 1.45 rejections (Pigeon 754), and 50.83 ± 5.71 hits and 92.5 ± 1.58 rejections (Pigeon 865).
Moreover, pigeons mandibulated significantly more often during the response period in Go trials compared to No-Go trials (hits vs. false alarms: t[111] = 17.238, p < 0.001, t test). There was no significant interindividual difference for any of the performance measures (i.e., hits, rejections, misses, and false alarms) among the animals at any of the delay conditions (all Fs < 1.24, all ps > 0.31; multivariate ANOVA for every delay condition with the between-subject factor “animal #” and the dependent variables of hits, rejections, misses, and false alarms). The data shown in Table 1 (the averaged individual and interindividual responses for each delay condition, pigeon and response category) confirm that the same performance pattern was evident in each pigeon.
Table 1. Individual mean performance correct (in %) ± standard error for each delay condition, pigeon and response category, and the interindividual mean performance (± standard error) across all animals for each delay condition.
| Delay length | Pigeon | Hit (correct go) | Miss (incorrect go) | Reject (correct no-go) | False alarm (incorrect no-go) |
| 600 ms | 627 | n/a | n/a | n/a | n/a |
| 754 | 53.77 ± 7.49 | 46.23 ± 7.49 | 93.89 ± 1.58 | 6.11 ± 1.58 | |
| 865 | 63.86 ± 8.58 | 36.15 ± 8.58 | 90.65 ± 3.14 | 9.36 ± 3.14 | |
| interindividual mean | 57.28 ± 5.70 | 42.72 ± 5.70 | 92.76 ± 1.50 | 7.24 ± 1.50 | |
| 800 ms | 627 | 97.44 ± 0.00 | 2.56 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 |
| 754 | 49.39 ± 4.84 | 50.61 ± 4.84 | 91.58 ± 3.74 | 8.42 ± 3.74 | |
| 865 | 46.95 ± 8.35 | 53.05 ± 8.35 | 91.97 ± 2.08 | 8.03 ± 2.08 | |
| interindividual mean | 50.28 ± 4.27 | 49.72 ± 4.27 | 91.90 ± 2.91 | 8.09 ± 2.91 | |
| 1000 ms | 627 | 45.30 ± 12.76 | 54.70 ± 12.76 | 97.78 ± 1.05 | 2.22 ± 1.05 |
| 754 | 44.62 ± 4.48 | 55.38 ± 4.48 | 95.19 ± 1.23 | 4.81 ± 1.23 | |
| 865 | 31.79 ± 14.26 | 68.21 ± 14.26 | 95.23 ± 2.79 | 4.78 ± 2.79 | |
| interindividual mean | 43.49 ± 4.09 | 56.51 ± 4.09 | 95.64 ± 0.95 | 4.37 ± 0.95 | |
| 1200 ms | 627 | 39.40 ± 17.53 | 60.60 ± 17.53 | 94.47 ± 2.37 | 5.54 ± 2.37 |
| 754 | 44.32 ± 11.89 | 55.69 ± 11.89 | 91.24 ± 5.95 | 8.76 ± 5.95 | |
| 865 | 50.00 ± 0.00 | 50.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | |
| interindividual mean | 42.15 ± 10.02 | 57.85 ± 10.02 | 93.80 ± 2.45 | 6.20 ± 2.45 | |
| 1400 ms | 627 | 44.74 ± 44.74 | 55.27 ± 4.74 | 97.37 ± 2.63 | 2.63 ± 2.63 |
| 754 | 15.00 ± 0.00 | 85.00 ± 0.00 | 100.00 ± 0.00 | 0.00 ± 0.00 | |
| 865 | n/a | n/a | n/a | n/a | |
| interindividual mean | 34.82 ± 27.66 | 65.18 ± 27.66 | 98.25 ± 1.75 | 1.75 ± 1.75 |
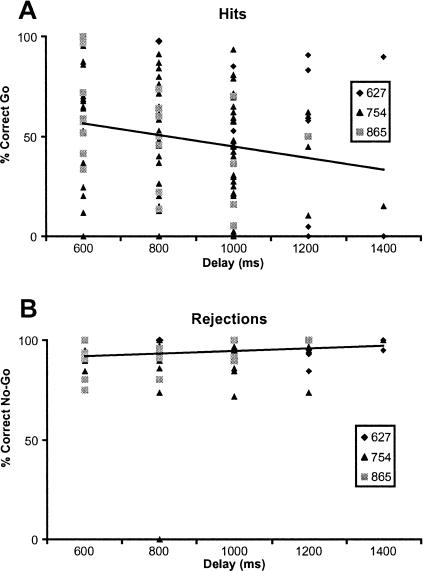
Figure panels 2A and 2B show the overall delay-dependent hit (Figure 2A) and rejection rates (Figure 2B) for each individual animal. Whereas the rejection rate appeared to be relatively stable and unaffected by delay length (range, 92.76% to 98.25%), the hit rate continuously decreased across the delays (from 57.28% at delay 600 ms to 34.82% at 1,400 ms; see also Table 1). It is to be noted that it was not possible to test every pigeon equally often at every delay length due to performance differences and electrophysiological constraints.
Figure 2. Behavioral results.
Individual hit and rejection performance for each pigeon, response frequency, and regression lines. (A): Hit performance (% correct) on Go trials as a function of delay duration. (B): Performance in No-Go trials (% correct rejections) as a function of delay duration.
To analyze the delay-dependency of hit and rejection rates, a repeated measures ANOVA was performed across all animals with response condition (hit vs. rejection) as a within-subject factor and delay (600 ms vs. 800 ms vs. 1,000 ms vs. 1,200 ms vs. 1,400 ms) as a between-subject factor. The ANOVA revealed that, as described above, there was a significant main effect for response condition, F(1,107) = 124.43, p < 0.001; however, the condition x delay interaction was not significant, F(4,107) = 1.478, p = 0.214.
It is possible that the ANOVA did not detect a significant interaction because of the higher overall performance on rejection trials compared to hit trials and the larger variance in hit performance level within the animals. To eliminate the performance difference effect and to determine a possible delay dependency, we fitted regression lines to the hit and rejection data of Figure 2. The fits showed a delay-dependent divergence of the retention functions: Whereas the rejection rate in No-Go trials was not significantly linked to delay length (y = 0.0004x + 90.5739; p = 0.161), the hit rate in Go trials was negatively correlated with delay duration (y = −0.0029x + 73.3482; p < 0.05), indicating that No-Go performance did not covary with delay duration, but Go performance decreased with longer delays.
Moreover, a Levene test for variance homogeneity revealed that the variance in hit trials was significantly larger than the variance in rejection trials, F(1,276) = 167.645, p < 0.001.
Electrophysiology
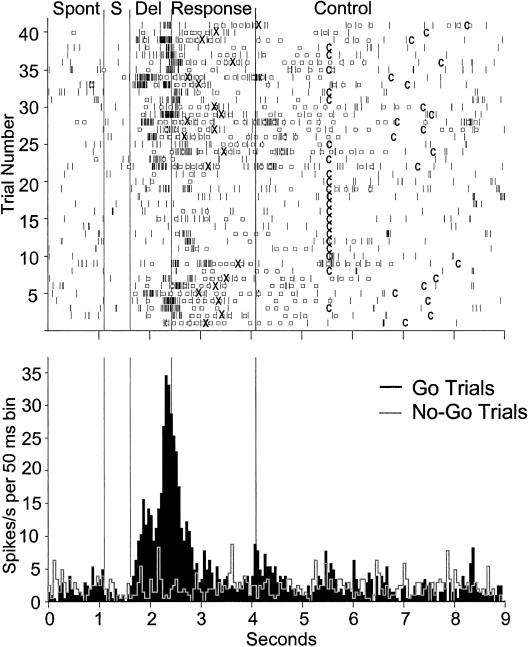
Single unit recordings were conducted from 163 NCL neurons while pigeons were performing the DGNG task. Based on electrode tracks and DyeI marks, the locations of these cells were verified histologically and found to be within the borders of the NCL. Results of the Wilcoxon tests comparing the spontaneous activity of a neuron with its activity during different periods of the task showed that neurons responded to different aspects of the behavioral task, the Go or No-Go stimulus, the reinforcer, the control light, and in addition, showed premotor activity (see also Kalt et al., 1999). In 19 neurons (12%), we observed a significant overall increase in their firing rate during the delay interval as compared to their spontaneous rates (all ps < 0.05; Wilcoxon tests comparing baseline discharge rate with the discharge rate during the delay interval). As illustrated in the raster plot in Figure 3, the increase in activity was most pronounced on successful Go trials, in which pigeons mandibulated during the given response period that then led to reinforcement. In contrast, neurons showed no increase in activity during the delay period on miss trials. Most notably, the neuronal activity during the delay period of No-Go trials was low and similar to the spontaneous rate, as shown in the peristimulus time histogram in Figure 3. In all cases, the neuronal activity was low during the presentation of the control light that was presented 2 s after the end of the response period or 4.5 s after the reinforcement with a drop of water. Note that this control light was presented to make sure that the increased activity during the delay was not due to the presentation of the very same light that in this case served to indicate the delay period.
Figure 3. Neuronal activity of a delay neuron recorded over 41 Go trials and 40 No-Go trials.
In the raster plot (top) only Go trials are shown, whereas in the histogram (bottom), spikes are accumulated across all Go (black) and No-Go (grey outline) trials. Vertical lines delineate different time intervals: spontaneous (SP), stimulus (S), delay (Del), response, and control intervals. The beginning of the control interval, which is embedded in the intertrial interval, depends on the animal's behavior and is indicated by a C. In the raster plot, spikes are indicated by short dark lines distributed over the duration of each trial. Open squares indicate responses of the pigeon (i.e., beak openings). Following five positive responses, reinforcement was delivered, indicated by an X. During the delay period, the neuronal activity was elevated in Go trials compared to No-Go trials and other intervals. In addition, the delay activity was increased only in correct Go trials (e.g., Trials 1 through 6), but not after incorrect misses (e.g., Trials 14 through 21).
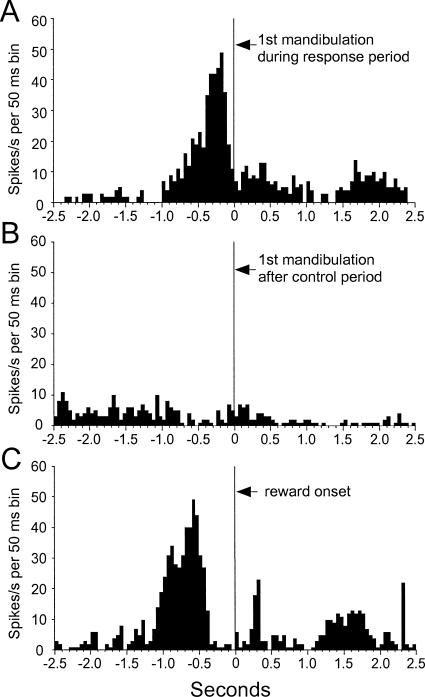
If the same spikes are realigned to the first mandibulation during the response period in Go trials (Figure 4A) or to a mandibulation after the control period (Figure 4B), it becomes clear that the increase in neuronal activity does not represent a premotor or motor response, which would be seen in both histograms (i.e., in all cases when the pigeon mandibulated). The peak about 200 ms prior to the first mandibulation (Figure 4A) matches the average latency of 233 ms between the end of the delay period and the first mandibulation response and thus represents the peak activity at the end of the delay period. The realignment of the spikes to the reinforcement (Figure 4C) demonstrates that the increase in activity also is not related to reward expectancy or reward delivery, as it stops several hundred milliseconds before reinforcer delivery. The very early peak, again, corresponds to the peak at the end of the delay, as the fifth mandibulation, resulting in reward, occurred on average 825 ms after the end of the delay period.
Figure 4. Neuronal activity of the delay neuron shown in Figure 3 with spikes aligned to the first mandibulation during the response interval (A), the first mandibulation occurring after the control period (B), and to the onset of reward (C).
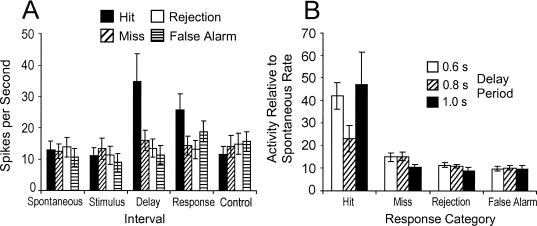
A repeated measures ANOVA with “trial interval” (spontaneous, stimulus, delay, response, and control interval) and “response condition” (hit, miss, correct rejection, and false alarm) as within-subject factors revealed that the different time intervals, F(4,72) = 12.5; p < 0.001, and response categories, F(3,54) = 6.4; p < 0.001, had a significant effect on the firing rate of these neurons (Figure 5A). More importantly, the interaction between these factors was significant, F(12,216) = 7.5; p < 0.001. Neurons showed relatively stable spike rates throughout the entire trial duration and across all trials with the exception of the delay period during hit trials (post hoc Tukey's tests; p < 0.05).
Figure 5. Activity of 19 delay neurons during different time intervals of the DGNG task (A) and during the delay interval with different delay lengths (B).
The neural activity of each unit was normalized to each unit's spontaneous activity (that equals one) and then to spikes per second, to compensate for the differences in the length of the intervals and the different number of trials for each neuron. A total of 310 hit, 179 miss, 452 correct rejection, and 34 false alarm trials were analyzed.
A separate repeated measures ANOVA tested the effects of different delay lengths on the neuronal activity during the delay interval (Figure 5B). Whereas the length of the delay interval had no significant effect on the firing rate of neurons, F(2,16) = 0.41; p = 0.67, clear differences occurred with respect to the performance and different trial types, F(3,48) = 7.28; p < 0.001. Planned comparisons revealed that firing rates during the delay period were significantly enhanced compared to spontaneous rate and the activity during the delay periods of miss, reject, and false alarm trials. In summary, our electrophysiological results clearly demonstrated that the neuronal activity of delay neurons depended on the type of the trial (i.e., Go versus No-Go trials). This activation-inactivation pattern occurred at all delay durations, and neither the pattern nor the firing amplitude appeared to be related to delay length.
Discussion
To date, it still is unknown what precisely is processed during the delay in delayed Go/No-Go tasks. At least three hypotheses are conceivable. Hypothesis 1 states that animals encode and retain the sample stimulus across the delay, and decide at the end of the delay period whether to respond (Go trials) or not (No-Go trials). This hypothesis is supported by evidence from DMTS tasks: Behavioral studies show that pigeons maintain a retrospective representation of the sample rather than a prospective, anticipatory representation of the comparison stimulus (Zentall et al., 1989). Consistent with this finding, many authors have shown that delay neurons in DMTS tasks are stimulus specific, hence suggesting that the representation of one particular sample stimulus was retrospectively maintained during the delay (Asaad, Rainer, & Miller, 1998; Fuster et al., 1982; Miller et al., 1996; Rainer, Asaad, & Miller, 1998).
Hypothesis 2 states that, in a DGNG task, the animal prepares the motor response during the delay following the sample stimulus, and executes the response at the end of the interval on Go trials, but actively inhibits the response on No-Go trials. Support for this hypothesis derives from work on neural motor control. A vast amount of literature reports evidence that changes in the neural delay signal relate to the preparation of the upcoming motor response (Funahashi & Kubota, 1994; Fuster, 1997; Rainer, Rao, & Miller, 1999; Riehle, Kornblum, & Requin, 1997; Riehle & Requin, 1989, 1995). In addition, single-unit studies on countermanding eye movements have begun to reveal the neural basis of suppressing an already prepared motor response (Schall, Hanes, & Taylor, 2000). Hypothesis 3, the default-response hypothesis, suggests that there is differential information processing on Go and No-Go trials. Whereas the Go cue triggers stimulus retention and/or motor preparation, the No-Go cue instructs the animal of neither retaining the stimulus across the delay, nor preparing the motor response, hence to show a default No-Go response.
Hypothesis 1 predicts stimulus-related delay activity on both Go and No-Go trials; Hypothesis 2 predicts delay activity related to motor preparation on both Go and No-Go trials, and a selective motor inhibition at the end of the delay on No-Go trials; and the default-response hypothesis, Hypothesis 3, predicts working-memory-related neural activation on Go trials, but not on No-Go trials, as pigeons omit their response by default. Our results are consistent with the third hypothesis because we observed such a differential neural activation-inactivation pattern and, furthermore, found behavioral evidence for a default response strategy on No-Go trials. A discussion of these two findings follows.
What is the behavioral evidence for a default response in the present data? Typically, the pigeon's ability to discriminate between the differential requirements on Go and No-Go trials was demonstrated by the fact that they mandibulated significantly more often on Go trials than on No-Go trials. Most interestingly, however, the probability of a correct response in Go trials decreased with increasing delay length although the rejection performance on No-Go trials did not covary with delay duration. Although the decrease in Go-performance was not very large—a fact that was presumably due to the small range of delay lengths (0.6 s to 1.4 s)—it still reached statistical significance.
A decreasing slope of the retention function is typical for a memorized representation of task-relevant information that fades with increasing delay length. The absence of the delay-dependent decrease in the retention function on correct No-Go trials therefore indicates that no or little memory was required for correct response suppressions. Hence, whereas pigeons presumably retained task-relevant information in memory across the delay on Go trials, they might have by default refrained from responding on No-Go trials.
Moreover, the difference in performance variance between hits and rejections is further evidence for the default-response hypothesis. Relying on working memory for successful task performance is more error-prone than responding by default. One would therefore expect a higher performance variance on tasks requiring working memory than on tasks that can be solved by default responding. The significantly higher variance in correct Go trials (Figure 2A) compared to correct No-Go trials (Figure 2B) therefore suggests that pigeons indeed required working memory to solve the Go tasks, but not the No-Go tasks.
Our explanation assumes an asymmetrical memory load on Go and No-Go trials. An alternative hypothesis, however, posits that the pigeons' response pattern in the present study was caused by an adjustment of the reinforcement-punishment ratio. Imagine the following scenarios in which pigeons indiscriminately perseverate on one response type: Responding on every trial (Go and No-Go) would have resulted in reinforcement on 50% of the trials (responses in Go trials), but also in a penalty in the remaining 50% of the trials (responses in No-Go trials). Alternatively, remaining silent on every trial would have had no consequence, appetitive or aversive, on any of the trials. This is, therefore, a more conservative strategy.
Increasing the delay duration generally leads to higher uncertainty about what is the required correct response. Therefore, pigeons might have opted for a progressively more conservative strategy at longer delay lengths (i.e., aimed to avoid punishments for the sake of missing reinforcers by reducing the overall response frequency). As a consequence, the probability of a hit response would decrease with increasing delay duration, but the probabilities of false alarms and correct rejections (all characterized by response omissions) should remain constant and independent of delay length.
Based on the current data, we cannot decide whether the decreasing retention function in Go trials is due to either fading memory or an increased tendency to reduce the response frequency with increasing delay length. However, in both scenarios, response omissions on No-Go trials would not require to retain and process task-relevant information during the delay. We therefore believe that, independent of what is causing the divergent retention functions, the mechanism producing a response rejection on No-Go trials indeed represents a default response strategy.
A detailed analysis of the single cell recordings in the NCL, the avian “prefrontal cortex” (Durstewitz et al., 1999; Kröner & Güntürkün, 1999, Mogensen & Divac, 1982, 1993; Waldmann & Güntürkün, 1993), relating neural activity to individual behavior, revealed that many neurons showed increased activity during the delay preceding correct hit responses on Go trials, replicating previous findings on delay neurons in NCL (Diekamp et al., 2002; Kalt et al., 1999) and entopallium (Colombo et al., 2001; note that the entopallium was previously termed ectostriatum prior to the revision of the avian nomenclature, Reiner et al., 2004). However, the same neurons showed no or little such delay activity before correct response suppressions on No-Go trials. This differential activation on Go and No-Go trials was observed in all delay conditions. There is substantial evidence that the increased neural activity during the response-preceding delay period is an essential property of the neural network producing working memory (Colombo et al., 2001; Diekamp et al., 2002; Durstewitz et al., 1999; Durstewitz & Seamans, 2002; Durstewitz et al., 2000a, 2000b; Fuster, 1973, 1995; Goldman-Rakic, 1995, 1996; Kalt et al., 1999; Quintana & Fuster, 1992). We therefore conclude that the lack of delay activity on correct No-Go trials indicates that these neurons did not code any task-relevant information and hence were not involved in working memory processes. Because default responses do not require working memory, we believe that the lack of delay activity in correct rejections thus constitutes a neural correlate of the pigeon's default response omission.
As an alternative explanation, the neural delay signal in Go trials might represent the correlate of preparing for the upcoming motor response, and not represent working memory. The lack of delay activity on No-Go trials, then, would merely be a correlate of not preparing for mandibulation. However, comparing correct with incorrect motor responses (hits vs. false alarms) revealed that there was no delay activity on false alarm trials, although a motor response was prepared in both conditions. It is unlikely, therefore, that delay activity in NCL related to motor preparation. Presumably, delay activity in NCL rather corresponded to factors unique to Go trials (i.e., an asymmetrical working memory load or a disparity in Go-stimulus representation).
It is noteworthy that correct responses to Go stimuli were mediated by positive reinforcement operant learning processes (correct responses on Go trials were reinforced), whereas correct response rejections after No-Go stimuli were mediated by avoidance learning (correct response suppressions on No-Go trials had no consequence, but incorrect false alarms were punished). Hence Go stimuli cued reinforcer delivery, whereas No-Go stimuli cued no reinforcement or even punishment. This reinforcement-punishment asymmetry might constitute a problem in interpreting the neural data. Numerous studies have shown that, in addition to working memory load, neural delay activity also can be modulated by the amount, the probability, and the subjective value of the anticipated reward, as well as by general motivational factors (Hikosaka & Watanabe, 2000; Kalenscher et al., 2005; Leon & Shadlen, 1999; Quintana & Fuster, 1992; Roesch & Olson, 2004; Schultz, Tremblay, & Hollerman, 2000; Tremblay & Schultz, 2000; Wallis & Miller, 2003; Watanabe, 1996; Watanabe, Hikosaka, Sakagami, & Shirakawa, 2002). Hence the absence of delay activity in the present task might not indicate lack of working memory, but might merely reflect the lack of reinforcer anticipation.
The delay neuron firing rate depicted in Figure 4C, however, showed no evidence of encoding reward or reward anticipation, since the enhanced response rate dropped to baseline level approximately 400 ms prior to reward delivery. Moreover, other studies using a symmetrically rewarded DGNG design (e.g., Tremblay & Schultz, 2000) have found single units with a similar activation-inactivation pattern, suggesting that neural inactivations in No-Go trials also occurred when correct response rejections were reinforced.
In addition, even if delay activity were modulated by reward anticipation, the default response conclusion still would hold. A closer examination of theoretical and empirical concepts of working memory reveals that reward anticipation is, in fact, an integral part of working-memory function. Most current definitions imply that working memory is a system linking perception, long-term memory, and action (Baddeley, 2003). These definitions suggest that working memory is more than simply the mere maintenance of the sample stimulus. As a central part of the perception-action cycle (Fuster, 2000), working memory includes the representation of currently relevant task-rules, goals, and choice-consequences (including reinforcers and punishers). In this sense, if delay activity in Go trials is a correlate of working-memory function, then it would not be surprising if neural delay activity was indeed influenced by the anticipation of reinforcement to some degree.
In fact, there is substantive theoretical and experimental support for this view. Numerous studies on the neural basis of working memory have shown that dopaminergic input to the prefrontal cortex, originating from mesencephalic structures, is necessary to establish working memory and delay neuron activity (Durstewitz & Seamans, 2002; Müller et al., 1998; Sawaguchi & Goldman-Rakic, 1994; Sawaguchi et al., 1988; Williams & Goldman-Rakic, 1995). Presumably the same dopaminergic midbrain neurons are activated by a cue predicting reinforcement (Montague & Berns, 2002; Montague, Dayan, & Sejnowksi, 1996; Schultz, 1997, 2002; Schultz, Dayan, & Montague, 1997; Waelti, Dickinson, & Schultz, 2001), and inter alia exert influence on the reactivity of prefrontal cortex cells (Roesch & Olson, 2004; Schultz et al., 2000; Watanabe, 1996). Hence there is substantial evidence that neural delay activity in prefrontal cortex/NCL is simultaneously modulated by a reward- and a working-memory-related dopamine signal. Although it is difficult to disentangle the different components, delay activity thus reflects both reward- and memory processing. Whereas delay activity on Go trials might represent a compound of stimulus-retention, task-relevant information, and reward anticipation, the lack of delay activity on No-Go trials in the present task suggests that pigeons neither retained the sample stimulus nor anticipated a reward—and thus met the characteristics of a response omission by default. Future work needs to quantify if, or to which degree, reward expectancy affects neural delay activity on Go trials.
Several studies (Kalaska & Crammond, 1995; Schultz et al., 2000; Tremblay & Schultz, 2000) found a similar activation-inactivation pattern in primate orbitofrontal, parietal, and premotor units. In these studies, neurons that were active on both Go and No-Go trials were considered reward-related (correct responses in both conditions were followed by reinforcement), but neurons that were active only on Go trials, but not on No-Go trials were classified as motor neurons. However, as argued in this paper, a differential activation-inactivation pattern might in fact not relate to motor preparation, but could as well be indicative of a default responding strategy—a possibility that had not been considered by the authors in the cited studies. A reinterpretation of this previous work might reveal valuable new information.
In summary, we found behavioral evidence that pigeons require working memory to perform on Go trials, but by default omit their response on No-Go trials. Electrophysiological recordings revealed that neural delay activity on Go trials was a correlate of working memory. The lack of neural activation preceding correct response omissions on No-Go trials suggests that none of the characteristic working-memory components was processed by the neurons. We therefore believe that the reported neural activation-inactivation pattern might hence represent the cellular basis of a default response strategy in a delayed Go/No-Go task. This is evidence for the third hypothesis, the default-response hypothesis, which suggests that responding in Go and No-Go trials involves qualitatively different information processing.
The present experiment combines classic behavioral techniques with recent neuroscientific methods. Our results ought to shed light on the mediating internal processes in a DGNG task and yield conclusions on a different explanatory level than could be provided by a purely neuroscientific or behavioral approach. By presenting interesting insights into the nature of what is coded in a delay task, we hope to present a study that reconciles classic behavioral research with cognitive neuroscience that investigators from both groups might find interesting and stimulating.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft. We thank W. Dreckmann, G. Steinrücke, and the other technicians in Bochum for technical assistance.
References
- Asaad W.F, Rainer G, Miller E.K. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–1407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Colombo M, Cottle A, Frost N. Degree of representation of the matching concept in pigeons (Columba livia). Journal of Comparative Psychology. 2003;117:246–256. doi: 10.1037/0735-7036.117.3.246. [DOI] [PubMed] [Google Scholar]
- Colombo M, Frost N, Steedman W. Responses of ectostriatal neurons during delayed matching-to-sample behavior in pigeons (Columba livia). Brain Research. 2001;917:55–66. doi: 10.1016/s0006-8993(01)02906-7. [DOI] [PubMed] [Google Scholar]
- Diekamp B, Kalt T, Güntürkün O. Working memory neurons in pigeons. Journal of Neuroscience. 2002;22:RC210. doi: 10.1523/JNEUROSCI.22-04-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Kelc M, Güntürkün O. A neurocomputational theory of the dopaminergic modulation of working memory functions. Journal of Neurocience. 1999;19:2807–2822. doi: 10.1523/JNEUROSCI.19-07-02807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Kröner S, Güntürkün O. The dopaminergic innvervation of the avian telencephalon. Progress in Neurobiology. 1999;59:161–195. doi: 10.1016/s0301-0082(98)00100-2. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans J.K. The computational role of dopamine D1 receptors in working memory. Neural Networks. 2002;15:561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans J.K, Sejnowksi T.J. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. Journal of Neurophysiology. 2000a;83:1733–1750. doi: 10.1152/jn.2000.83.3.1733. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans J.K, Sejnowksi T.J. Neurocomputational models of working memory. Nature Neuroscience. 2000b;3:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Kubota K. Working memory and prefrontal cortex. Neuroscience Research. 1994;21:1–11. doi: 10.1016/0168-0102(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Fuster J.M. Unit activity in prefrontal cortex during delayed-response performance: Neuronal correlates of transient memory. Journal of Neurophysiology. 1973;36:61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Fuster J.M. Memory in the cortex of the primate. Biological Research. 1995;28:59–72. [PubMed] [Google Scholar]
- Fuster J.M. Philadelphia: Lippincott-Raven; 1997. The prefrontal cortex: Anatomy, physiology, and neurophysiolofy of the frontal lobe. (3rd ed.). [Google Scholar]
- Fuster J.M. Executive frontal functions. Experimental Brain Research. 2000;133:66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Fuster J.M, Bauer R.J, Jervey J.P. Cellular discharge in the dorsolateral prefrontal cortex of the monkey in cognitive tasks. Experimental Neurology. 1982;77:679–694. doi: 10.1016/0014-4886(82)90238-2. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the USA. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D.S. Symmetrical and asymmetrical coding of food and no-food samples in delayed matching in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:186–193. [Google Scholar]
- Hikoskaka K, Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Kalaska J.F, Crammond D.J. Deciding not to go: Neuronal correlates of response selection in a go/nogo task in primate premotor and parietal cortex. Cerebral Cortex. 1995;5:410–428. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kalenscher T, Windmann S, Rose J, Diekamp B, Güntürkün O, Colombo M. Single units in the pigeon brain integrate reward amount and time-to-reward in an impulsive choice task. Current Biology. 2005;15:594–602. doi: 10.1016/j.cub.2005.02.052. [DOI] [PubMed] [Google Scholar]
- Kalt T, Diekamp B, Güntürkün O. Single unit activity during a Go/NoGo task in the “prefrontal cortex” of pigeons. Brain Research. 1999;839:263–278. doi: 10.1016/s0006-8993(99)01727-8. [DOI] [PubMed] [Google Scholar]
- Karten H.J, Hodos W. Stereotaxic atlas of the brain of the pigeon (Columba livia) Baltimore: Johns Hopkins University Press; 1967. [Google Scholar]
- Kröner S, Güntürkün O. Afferent and efferent connections of the caudolateral neostriatum in the pigeon (Columba livia). Journal of Comparative Neurology. 1999;407:228–260. doi: 10.1002/(sici)1096-9861(19990503)407:2<228::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Leon M.I, Shadlen M.N. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999;24:415–425. doi: 10.1016/s0896-6273(00)80854-5. [DOI] [PubMed] [Google Scholar]
- Miller E.K, Erickson C.A, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. Journal of Neuroscience. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen J, Divac I. The prefrontal ‘cortex’ in the pigeon. Behavioral evidence. Brain Behavior and Evolution. 1982;21:60–66. doi: 10.1159/000121617. [DOI] [PubMed] [Google Scholar]
- Mogensen J, Divac I. Behavioural effects of ablation of the pigeon-equivalent of the mammalian prefrontal cortex. Behavioural Brain Research. 1993;55:101–107. doi: 10.1016/0166-4328(93)90012-f. [DOI] [PubMed] [Google Scholar]
- Montague P.R, Berns G.S. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–284. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- Montague P.R, Dayan P, Sejnowski T.J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. Journal of Neuroscience. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, von Cramon D.Y, Pollmann S. D1- versus D2-receptor modulation of visuospatial working memory in humans. Journal of Neuroscience. 1998;18:2720–2728. doi: 10.1523/JNEUROSCI.18-07-02720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J, Fuster J.M. Mnemonic and predictive functions of cortical networks in a memory task. Neuroreport. 1992;3:721–724. doi: 10.1097/00001756-199208000-00018. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad W.F, Miller E.K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Rainer G, Rao S.C, Miller E.K. Prospective coding for objects in primate prefrontal cortex. Journal of Neuroscience. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel D.J, Bruce L, Butler A, Csillag A, Kuenzel W, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. Journal of Comparative Neurology. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle A, Kornblum S, Requin J. Neuronal correlates of sensorimotor association in stimulus-response compatibility. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:1708–1726. doi: 10.1037//0096-1523.23.6.1708. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. Monkey primary motor and premotor cortex: Single-cell activity related to prior information about direction and extent of an intended arm movement. Journal of Neurophysiology. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- Riehle A, Requin J. Neuronal correlates of the specification of movement direction and force in four cortical areas of the monkey. Behavioural Brain Research. 1995;70:1–13. doi: 10.1016/0166-4328(94)00180-n. [DOI] [PubMed] [Google Scholar]
- Roesch M.R, Olson C.R. Neuronal activity related to reward value and motivation in primate prefrontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rosenkilde C.E, Bauer R.H, Fuster J.M. Single cell activity in ventral prefrontal cortex of behaving monkeys. Brain Research. 1981;209:375–394. doi: 10.1016/0006-8993(81)90160-8. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic P.S. The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. Journal of Neurophysiology. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Matsumara M, Kubota K. Dopamine enhances the neuronal activity of spatial short-term memory performance in the primate prefronatal cortex. Neuroscience Research. 1988;5:465–473. doi: 10.1016/0168-0102(88)90030-2. [DOI] [PubMed] [Google Scholar]
- Schall J.D, Hanes D.P, Taylor T.L. Neural control of behavior: Countermanding eye movements. Psychological Research. 2000;63:299–307. doi: 10.1007/s004269900008. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague P.R. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sherburne L.M, Zentall T.R. Coding of feature and no-feature events by pigeons performing a delayed conditional discrimination. Animal Learning & Behavior. 1993;21:92–100. [Google Scholar]
- Skinner B.F. The behavior of organisms: An experimental analysis. New York: Appleton-Century-Crofts; 1938. [Google Scholar]
- Snodderly D.M, Gur M. Organization of striate cortex of alert, trained monkeys (Macaca fascicularis): Ongoing activity, stimulus selectivity, and widths of receptive field activating regions. Journal of Neurophysiology. 1995;74:2100–2125. doi: 10.1152/jn.1995.74.5.2100. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during Go-NoGo performance in primate orbitofrontal cortex. Journal of Neurophysiology. 2000;83:1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Waldmann C, Güntürkün O. The dopaminergic innervation of the pigeon caudolateral forebrain: Immunocytochemical evidence for a ‘prefrontal cortex’ in birds? Brain Research. 1993;600:225–234. doi: 10.1016/0006-8993(93)91377-5. [DOI] [PubMed] [Google Scholar]
- Wallis J.D, Miller E.K. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Reward expectancy in primate prefrontal cortex. Nature. 1996;382:629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hikosaka K, Sakagami M, Shirakawa S. Coding and monitoring of motivational context in the primate prefrontal cortex. Journal of Neuroscience. 2002;22:2391–2400. doi: 10.1523/JNEUROSCI.22-06-02391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K.G, Ruske A.C, Colombo M. Memory procedures, performance and processes in pigeons. Cognitive Brain Research. 1996;2:309–317. doi: 10.1016/0926-6410(96)00016-x. [DOI] [PubMed] [Google Scholar]
- Williams G.V, Goldman-Rakic P.S. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Zentall T.R. The case for a cognitive approach to animal learning and behavior. Behavioural Processes. 2001;54:65–78. doi: 10.1016/s0376-6357(01)00150-4. [DOI] [PubMed] [Google Scholar]
- Zentall T.R, Urcuioli P.J, Jagielo J.A, Jackson-Smith P. Interaction of sample dimension and sample comparison mapping on pigeons' performance of delayed conditional discriminations. Animal Learning and Behavior. 1989;17:172–178. [Google Scholar]