Abstract
How the nervous system encodes learning and memory processes has interested researchers for 100 years. Over this span of time, a number of basic neuroscience methods has been developed to explore the relationship between learning and the brain, including brain lesion, stimulation, pharmacology, anatomy, imaging, and recording techniques. In this paper, we summarize how different research approaches can be employed to generate converging data that speak to how structures and systems in the brain are involved in simple associative learning. To accomplish this, we review data regarding the involvement of a particular region of cerebellar cortex (Larsell's lobule HVI) in the widely used paradigm of classical eyeblink conditioning. We also present new data on the role of lobule HVI in eyeblink conditioning generated by combining temporary brain inactivation and single-cell recording methods, an approach that looks promising for further advancing our understanding of relationships between brain and behavior.
Keywords: classical conditioning, associative learning, cerebellum, brainstem, neuroscience methods, interpositus nucleus, eyelid conditioning
We have written this article with two purposes in mind. First, we want to provide readers with an overview of how a variety of techniques are used to explore the involvement of structures and systems in the brain in encoding a relatively specific learned behavior, in this case, the involvement of the cerebellum in classical eyeblink conditioning. Second, we want to provide a glimpse of the difficulties inherent in trying to reach a consensus of opinion about what a given brain structure or system contributes to a specific behavior. We do so by providing a summary of our current understanding of what one brain area, lobule HVI of the cerebellar cortex, contributes to the conditioning process. Our overall goal is to convince the reader that a joint behavioral and neuroscience approach to the study of learning, using a variety of levels of analysis and methods, is an effective way to advance our understanding of the science of learning.
For nearly thirty years, in several laboratories, experiments have been conducted to establish a causal relation between the cerebellum and simple forms of motor learning. Although much of the empirical progress has been achieved using the rabbit classical eyeblink conditioning paradigm, the work of Thach, Ito, Lisberger and others also has contributed to our understanding of cerebellar contributions to motor learning, as exemplified by work in the monkey and rabbit on the long-term and short-term adaptation of the vestibulo-ocular reflex (e.g., Lisberger, Pavelko, Bronte-Stewart, & Stone, 1994). Despite the advances that have occurred both theoretically and empirically, there is still much work to be done to more firmly identify and define the brain-behavior relationships that are the basis of adaptive movement and behavioral change involving the cerebellum, as well as other brain systems.
As reviewed below, much of the basic neural circuitry that appears to be critical for acquisition and performance of the classically conditioned eyeblink response has been identified. Instead of reviewing this body of work in-depth, we have chosen to focus on the involvement of one specific region of the cerebellum, Larsell's lobule HVI of cerebellar cortex. We do so to illustrate how multiple methods and research approaches are commonly used by neuroscientists to gather converging evidence concerning the biological mechanisms of learning. We hope to highlight some of the common problems inherent in basic neuroscience research as applied to the study of learning, problems that can sometimes lead to contradictory findings and spirited debate.
The Eyeblink Conditioning Paradigm
We start with a brief presentation of the eyeblink classical conditioning procedure. Arguably, more is known about the neurobiological correlates of eyeblink classical conditioning than any other learning paradigm. This is due largely to the rich behavioral database that was available for use by neuroscientists who began studying the brain correlates of eyeblink conditioning in the 1970s. Parametric manipulations of the basic eyeblink conditioning procedure produced predictable and well-documented results thus making it easier to interpret the results of neurobiological experiments that used brain lesion, recording, stimulation, and pharmacological techniques.
Although the study of human eyeblink conditioning predated the work on rabbit eyeblink conditioning, the rabbit has eclipsed humans as the species most often used in eyeblink conditioning experiments. The rabbit was introduced for use as an animal model of eyeblink conditioning by Isidore Gormezano and his colleagues in work done at Indiana University and the University of Iowa during the early 1960s (see Gormezano, 1966; Gormezano, Kehoe, & Marshall, 1983, for reviews). Because rabbits tolerate restraint well and rarely show spontaneous eyeblinks (unlike humans and other animals), they have proven to be an ideal animal subject. Although techniques have been developed to investigate eyeblink conditioning in the rat and the mouse (e.g., Chen, Bao, Lockard, Kim, & Thompson, 1996; Skelton, 1988; Stanton, Freeman, & Skelton, 1992), the majority of neurobiological studies conducted thus far have employed rabbits as subjects.
A number of variations of eyeblink conditioning has been used with considerable success. However, delay conditioning with a relatively short interstimulus interval (ISI) has been used most commonly in neuroscience experiments. A typical eyeblink conditioning trial entails the presentation of an auditory (tone or white noise) stimulus as a conditioned stimulus (CS) that precedes, by 250–1000 ms, the presentation of a corneal air puff or peri-orbital shock as an unconditioned stimulus (US). Compared to other Pavlovian conditioning procedures, this is a relatively short ISI. In fact CS-US intervals longer than 2 s or so typically do not produce eyeblink conditioning. The US is normally presented for 100 ms and often coterminates with the CS. During a normal training session on a given day a subject might experience 50–200 trials. The intertrial interval usually is randomized within some pre-determined range (e.g., 20–40 s). It also is common practice to intersperse among the paired CS-US presentations, periodic CS-alone and US-alone trials. The CS-alone trials provide opportunities to measure characteristics of the conditioned response (CR) executed without contamination by the US or UR. The US-alone trials provide UR amplitude (i.e., reflex) data that are uncontaminated by the anticipatory CS.
There are several measures of conditioning available for analysis but the most often reported are: 1) percentage of trials containing a CR (by block or session and often grouped as paired trials and CS-alone trials), 2) CR amplitude, and 3) CR timing measures (e.g., CR onset latency and peak CR amplitude). Eyeblinks have been measured in a variety of ways including transducing nictitating membrane or external eyelid movements by mechanical or electrical means or recording electromyographic (EMG) activity from muscles involved in the production of eyeblinks. Most investigators treat these response-measurement techniques as completely interchangeable. There are data, however, that suggest they may not be. McCormick, Lavond, and Thompson (1982) concomitantly recorded nictitating membrane movements and EMG activity in a group of rabbits and found a general correspondence in the two measurements. They reported correlations between measurement techniques as high as 0.99 when response magnitude and onset latency were considered. In another study, however, Garcia, Mauk, Weidemann, and Kehoe (2003) compared upper eyelid responses recorded with an infrared LED system with nictitating membrane responses recorded with a photoelectric transducer. They showed that while magnitude and likelihood measurements were interchangeable, response amplitude, onset latency, and peak latency measures were not.
Often there is considerable variability among subjects in acquisition rate, but most animals begin exhibiting CRs on the second day of training, and asymptotic levels of learning (80–100% CRs) are usually attained by the fourth or fifth acquisition session. Conditioned response timing data are important because one of the more remarkable and distinguishing features of rabbit eyeblink conditioning is the precise timing of the CR from its inception. This can be seen on CS-alone trials where the peak CR-amplitude occurs at the time when the animal expects US delivery (e.g., about 500 ms after CS onset when training with a 500 ms ISI). Notwithstanding its relative simplicity, this basic associative-learning paradigm has generated a wealth of data on a variety of conditioning processes and phenomena including, but not limited to, backward conditioning, trace conditioning, extinction effects, time and stimulus discrimination training, latent inhibition, conditioned inhibition, stimulus intensity effects, sensory preconditioning, and compound stimulus effects (see Gormezano et al., 1983; Moore, 2002, for reviews). When the paradigm has been used in tandem with some of the neurobiological techniques described below, it has helped provide insights into the neural mechanisms associated with these important conditioning phenomena.
Brief Overview of the Critical Neural Circuitry Involved in Eyeblink Conditioning
Over the years, converging data from studies that have used a variety of basic neuroscience methods have uncovered a brainstem and cerebellar circuit that appears to be critical for the acquisition and performance of classically conditioned eyeblink responses. The neurobiological techniques that have been used to establish relationships between the brain and eyeblink conditioning include: 1) neural recordings of brain activity during either the acquisition, retention, and/or extinction phases of conditioning; 2) permanent electrolytic or chemical lesions; 3) temporary inactivation of brain areas via chemical infusion or brain cooling; 4) electrical stimulation directed at specific brain sites using biologically relevant parameters to simulate CS and US input normally activated by peripheral stimuli; and 5) anatomical tract-tracing techniques to establish connectivity between brain areas.
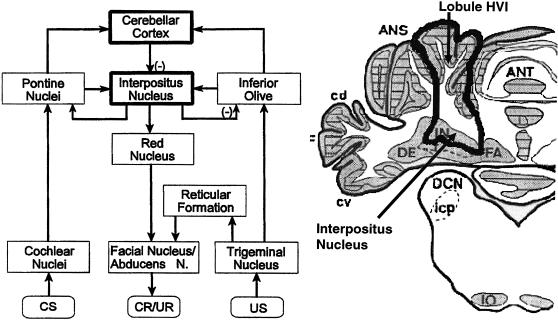
Figure 1 provides a schematic summary of the basic neural circuitry thought to be critically involved in classical eyeblink conditioning, a circuit that was delineated using converging data collected during dozens of experiments that used many different methods. We provide a brief overview of this extensively documented system. Interested readers should see Steinmetz (2000) or Steinmetz, Kim, and Thompson (2002) for more complete reviews.
Figure 1. The left panel shows a schematic of the basic brainstem and cerebellar circuit hypothesized to be involved in the acquisition and performance of classically conditioned eyeblink responses.
Critical plasticity that forms the basis of conditioned responding is thought to occur in the cerebellar cortex and interpositus nucleus where convergence of the CS and US occurs. The negative signs shown in parentheses indicate the locations in the circuit where inhibitory synaptic contacts occur. The right panel shows a schematic of a coronal section through the rabbit cerebellum and brainstem. Lobule HVI and the interpositus nucleus (IN) are outlined in black. These regions are thought to be involved in eyeblink conditioning. [Key abbreviations: ANS, ansiform lobe; DCN, dorsal cochlear nucleus; DE, dentate nucleus; IN, interpositus nucleus; FA, fastigial nucleus; ANT, anterior lobe]
The essential circuit for eyeblink conditioning appears to include regions of the brainstem and cerebellum and not higher brain areas. Normally, CSs used during eyeblink conditioning are projected along modality-specific inputs to activate specific regions of the basilar pontine nuclei. For example, a tone CS is projected from the periphery to the dorsal cochlear nuclei and then in turn to the dorsolateral and lateral pontine nuclei. The CS information is then projected to various areas of the cerebellum via the axons of pontine neurons known as mossy fibers. The USs used in eyeblink conditioning are projected to the trigeminal nuclear complex, which then relays input to two different brain regions. First, the trigeminal nucleus sends output to motor neurons responsible for the generation of eyeblinks via a relay area in the brainstem. This route provides a lower brainstem reflex circuit that can operate independently of higher brain areas. Second, the trigeminal nucleus sends output to the rostromedial portion of the dorsal accessory olive. Olivary neurons then project their axons as climbing fibers to discrete regions of the cerebellum.
A popular working model is that changes in the brain responsible for the acquisition and performance of classically conditioned eyeblink responses occur in areas of the cerebellum that receive convergent CS and US inputs from the pontine and olivary regions, respectively. Three cerebellar areas where convergent input appears to arrive have been identified: Larsell's lobule HVI of cerebellar cortex (the target area for this review), discrete regions of the anterior lobe of cerebellar cortex, and the interpositus nucleus. It appears that interpositus neurons change their firing rates and patterns of discharge due to the convergent CS and US inputs during conditioning and that these neurons then drive brainstem motor neurons that activate eyeblinking (the conditioned response or CR). The red nucleus serves as a relay between the interpositus nucleus and brainstem motor nuclei and is likely a site for important integration (e.g., descending inputs). This model contends that plasticity (i.e., learning-related changes in neuronal function) in the cerebellar cortical areas modulates activity in the deep cerebellar nuclei to provide response gain and response timing. The interpositus nucleus appears also to provide important feedback to the inferior olive and pontine nuclei. It seems likely that these inputs are very important for dynamic changes in responding seen during and after conditioning occurs.
There is generally good agreement that the interpositus nucleus is essential for eyeblink conditioning, a finding that was established using a variety of basic neuroscience methods. For example, permanent lesion and temporary inactivation studies have shown that removal of the interpositus nucleus abolishes eyeblink CRs and prevents learning of the response (e.g., Krupa, Thompson, & Thompson, 1993; Steinmetz, Logue, & Steinmetz, 1992). Also, recordings of the activity of interpositus neurons taken during conditioning have revealed populations of neurons that appear to fire when the CS is presented, including some with firing patterns that are closely related to performance of the CR (e.g., Berthier & Moore, 1986). And, electrical microstimulation of the interpositus nucleus produces discrete eyeblinks (McCormick & Thompson, 1984). These studies represent just a few of the many studies that have established the critical involvement of the interpositus nucleus in eyeblink conditioning, and collectively these experiments provide an excellent example of the power of this approach for advancing our understanding of brain–behavior relationships.
The specific role of the cerebellar cortex in conditioning is much more controversial, with models suggesting little or no role (e.g., Lavond & Kanzawa, 2001); models suggesting a critical, fundamental role for cortex (e.g., Attwell, Rahman, & Yeo, 2001); and models suggesting a co-involvement of interpositus nucleus and cortical regions in plasticity processes that underlie conditioning (e.g., Mauk & Donegan, 1997; Steinmetz, 2000). Several unresolved issues relevant to these models remain. Is plasticity established independently in the nucleus and cortical sites? Is the induction of plasticity in one region dependent on plasticity in the other (e.g., does deep nuclear plasticity require that cortical sites become plastic first, as suggested by Mauk and his colleagues)? Are both cerebellar sites critical for conditioning?
The interest in looking for plasticity in cerebellar cortex stemmed largely from the fact that several models of cerebellar function proposed plasticity at the synapses between the parallel fibers and Purkinje cells as a result of co-activation of mossy fibers and climbing fibers, the two sensory input systems that project information into the cerebellum (e.g., Albus, 1971; Marr, 1969). For the most part, these models proposed that co-activation of these fiber systems caused a long-term inhibition of Purkinje cells that in turn caused a disinhibition of deep nuclear neuronal activity (because all Purkinje cells inhibit deep nuclear cells). The long-term reduction in Purkinje cell excitability is called “long-term depression” (LTD). Indeed, a great deal of research has demonstrated a LTD effect at these synapses with conjunctive activation of climbing fibers and mossy fibers (Ekerot & Kano, 1985; Ito, 1989; Linden & Conner, 1991). Because mossy fiber (CS) and climbing fiber (US) co-activation appeared to be involved in classical eyeblink conditioning, it is possible that these models of cerebellar plasticity might be applicable to this type of learning. It should be noted that long-lasting excitatory plasticity also has been demonstrated in cerebellar cortex (e.g., Hirano, 1990; Jorntell & Ekerot, 2002), and possible potentiation processes at cerebellar synapses have begun to be considered in models of cerebellar function (e.g., Attwell et al., 2001; Medina & Mauk, 1999).
Lobule HVI and Eyeblink Classical Conditioning
To clarify how specific neuroscience techniques have been used together with eyeblink conditioning to study learning, we will concentrate on studies concerned with one brain region thought to be important for eyeblink conditioning—Larsell's lobule HVI of cerebellar cortex. Lobule HVI can be found in Figure 1, represented by the box labeled “Cerebellar Cortex” in the network schematic, and is also identified in the schematic of the coronal brain section (also in Figure 1). We will present data from a variety of laboratories concerning the role of lobule HVI in eyeblink conditioning, and in the process illustrate how a variety of behavioral neuroscience techniques have been used in an attempt to delineate the role of this cerebellar area in learning. Studies involving the techniques of permanent lesions, temporary inactivation, anatomical tract-tracing, and unit recording are described. We also present new data we have collected by combining two of these techniques: unit recording and temporary inactivation.
Lesion Studies of Lobule HVI: 1) Aspiration and Electrolytic Lesions
One means of determining whether a brain site is essential to the learning and/or performance of an adaptive response is to permanently remove or damage the area either prior to conditioning (to examine its effects on the course of acquisition) or after CRs have been well established (to examine its effects on the established conditioned response). This technique has been used extensively to study the neural correlates of eyeblink conditioning. For example, electrolytic or chemical lesions of the interpositus nucleus have been shown to permanently prevent conditioning or abolish learning (Steinmetz et al., 1992). Conversely, decerebrations or decortications that include all cerebral cortical tissue have proven to have little effect on delay eyeblink conditioning (e.g., Mauk & Thompson, 1987; Oakley & Russell, 1972).
Before discussing the use of permanent lesions in studying the role of Larsell's HVI in eyeblink conditioning it may be well to consider the advantages and disadvantages of this approach, in general. Brain lesions rarely obliterate a behavior under observation, one exception being the effects of interpositus nucleus lesions, which permanently abolish eyeblink CRs (Steinmetz et al., 1992). Much more typical are partial post-lesion effects. Partial effects are difficult to interpret because they may indicate either that the lesion was not large enough to produce a more global effect (i.e., only a portion of the target structure was destroyed by the lesion), or that a parallel brain structure or system is engaged in the behavior under observation. Even complete lesion effects are difficult to interpret. First, it is possible that the lesion destroyed fibers passing near or through the target structure, fibers that connect two other brain areas that are vital for behavioral function. Second, a positive lesion effect may result from the destruction of a brain area that serves as a mandatory efferent for an important brain area that resides upstream. An additional complication is that post-lesion deficits are often transient and, given time and/or additional training, can be overcome, further restricting any conclusions drawn from lesion data alone. For these reasons (and many others), as a general rule we draw very few conclusions from positive-lesion effect experiments. The primary contribution of lesion experiments is that they help identify brain regions that merit additional investigation.
Even conclusions derived from negative-result lesion experiments are not straightforward. If a researcher lesions a particular brain area (assuming for the moment that the lesion is complete based on histological examinations) and finds that there is no discernable effect upon any of a multitude of conditioned response measures, then we can conclude that the lesioned area is not required, under normal circumstances, for the learning/performance of the conditioned response. However, even if it is not required for its expression, the area may still play a role in the conditioned response, as is the case for the hippocampus. The hippocampus appears to be necessary for trace conditioning but not delay conditioning (Moyer, Deyo, & Disterhoft, 1990; Schmaltz & Theios, 1972; Solomon, Vander Schaaf, Thompson, &Weisz, 1986), yet strong learning-related activity in the hippocampus can still be seen during delay conditioning (e.g., Berger & Thompson, 1978; Sears & Steinmetz, 1990).
Despite the above caveats, the permanent lesion method has provided some information about the involvement of Larsell's lobule HVI in eyeblink conditioning. Relatively speaking, area HVI encompasses a large region of the cerebellum. As a result, the extent and completeness of the lesion, as determined from post-hoc histological reconstructions, is a salient issue when evaluating the results of lesion experiments. An additional complication is the fact that area HVI resides just a few millimeters above the anterior interpositus nucleus, a site that when damaged can produce a loss of conditioned responding. To complicate matters even further, many of the fibers that enter the interpositus nucleus from other brain areas do so from above the nucleus. Damage to the white matter above the nucleus can effectively cut off input to the nucleus. Thus, area HVI lesions deemed complete also may result in unintended collateral damage to the interpositus, which may not always be evident in histological analyses. Other issues that also merit consideration when evaluating lesion experiments are the amount and duration of impairment that occurred, and the conditioning measures that were most or least affected, such as effects on CR timing (e.g., Perrett, Ruiz, & Mauk, 1993).
Given the potential problems inherent in any lesion study, added to problems in trying to carefully lesion lobule HVI, it is easy to see why it has been difficult to reach a consensus on the role of area HVI in eyeblink conditioning when considering only lesion data. The first two papers that reported on the effects of lesioning area HVI effectively highlight some of the problems inherent to this form of research as two investigative groups came to dramatically different conclusions. Yeo, Hardiman, and Glickstein (1984) reported that small aspiration lesions of area HVI, that spared the underlying deep nuclei, completely abolished an established conditioned response and prevented its reacquisition despite additional training. McCormick and Thompson (1984), on the other hand, found that lesions of much of the ipsilateral hemisphere of the cerebellar cortex (including HVI) did not abolish the response. Cerebellar cortical lesions did appear to disturb the timing of the response in a few animals, an effect reported by Mauk and his colleagues over a decade later when the anterior lobe of the cerebellar cortex was lesioned (Perret et al., 1993). There were many methodological differences between these two studies, such as lesion size, strain of rabbits used, and training protocols, to name a few, and it is therefore very difficult to reconcile the different results.
As part of a series of cerebellar lesion experiments, Yeo and colleagues replicated their initial findings. In one study, Yeo, Hardiman, and Glickstein (1985a) lesioned area HVI after five days of training were given. The animals were allowed to recover, and then given five days of additional training. As before, they reported that small lesions of HVI either abolished the CR (n = 6 rabbits) or severely disrupted the CR (n = 4). The authors reported that histological analysis of the lesion site revealed that in no case were the deep nuclei damaged. Moreover, animals whose post-lesion conditioning was largely or completely unaffected by the lesion had considerable sparing of area HVI. Based on these data it was concluded that any sparing of HVI, especially at the base of the lobule and just above the dentate and interpositus nuclei, left the CR intact. Thus, Yeo and colleagues' lesion data was able to highlight a specific region of HVI as being particularly important to eyeblink conditioning.
In an effort to resolve the budding controversy, Lavond and colleagues (Lavond, Steinmetz, Yokaitis, & Thompson, 1987) adopted Yeo's conditioning parameters but extended the number of post-lesion training days from five to 10. Again, the authors reported taking great care to avoid damaging the interpositus nucleus during the lesion surgery. Under these conditions, Lavond and colleagues found a transient abolition of conditioned responding that lasted a few days. They found a more rapid recovery of CRs to an auditory CS than to a light CS, and a total recovery to both CSs by the tenth day of post-lesion training. Based on these findings, Lavond et al. suggested that the additional complexity intrinsic to Yeo's conditioning parameters (e.g., longer ISIs, two CS types, and more trials per training session) may have made the learning more vulnerable to lesion effects.
Lesion Studies and Lobule HVI: 2) Chemical Lesions and Inactivation
A variety of pharmacological agents are available either to permanently destroy neurons in a given brain area (e.g., kainic acid and ibotenic acid) or to temporarily inactivate an area (e.g., lidocaine, muscimol, picrotoxin, CNQX). In addition, brain cooling techniques, such as those used by Lavond and his colleagues (Clark, Zhang, & Lavond, 1992; Zhang, Ni, & Harper, 1986) have proven useful for temporarily halting neuronal activity in a discrete brain region. These chemical techniques are in many ways an improvement over aspiration and electrolytic methods. Permanent chemical lesions destroy neurons in the area of infusion with little damage to surrounding fibers. And, these lesions can be somewhat chemically selective for distinct populations of neurons. Areas of the brain can be inactivated during the time of the infusion and then reactivated at a different time. Importantly, both acquisition and performance can be assessed using these methods. Even though the use of chemical techniques has solved some problems inherent to lesion studies, some disadvantages of using this approach can be cited. Perhaps the major problem is that the infusion of chemicals into the brain is somewhat difficult to control and measuring the extent of spread of the chemicals can be tricky (especially in temporary inactivation studies).
To illustrate the power of the temporary inactivation procedure, we begin with a study by Krupa et al. (1993). Krupa et al. infused muscimol into the region of the interpositus nucleus of one group of rabbits and saline into a second group of rabbits during five days of standard delay eyeblink conditioning. Muscimol acts by binding to and activating GABAA receptors on neurons. The activation of GABA receptors in the brain produces inhibition of the neuron. That is, muscimol works by preventing neuronal firing which in turn blocks activation of pathways involving those neurons. They observed a normal learning curve for the control animals but no signs of CRs in the muscimol animals. This was expected because muscimol prevented neurons in the interpositus nucleus from firing. They next trained both groups of animals for an additional five days while infusing saline into both groups. Remarkably, they observed a normal acquisition function for the muscimol rabbits. That is, no savings of training were observed—it was as if the rabbits had received no previous paired training. These data demonstrate rather convincingly that critical plasticity processes that underlie eyeblink conditioning occurred in the deep nuclear region that was inactivated by the muscimol and not in other brain regions either afferent or efferent to the infusion zone. For purposes of this article, this study illustrates the power of the inactivation methods: behavior can be assessed both in the presence or absence of neuronal activity generated in the infused area.
Muscimol and CNQX both have been used to temporarily disturb the cellular processes of Purkinje cells in lobule HVI. CNQX, when infused into a brain area, blocks excitatory AMPA/kainate receptors. These receptors are plentiful on the dendrites of Purkinje cells and are thought to play a prominent role in the activation of Purkinje cells by inputs from the brainstem. When CNQX was given before paired CS-US training, nictitating membrane conditioning was prevented (Attwell et al., 2001). When CNQX was given to well-trained animals it interfered with CR expression for 10–60 min postinfusion in a dose-dependent manner (Attwell, Rahman, Ivarsson, & Yeo, 1999). Based on these data, and post-hoc anatomical analyses of the spread of the CNQX, the region identified by the authors as critical to the learning and performance of the conditioned nictitating membrane response was the medial portion of rostral lobule HVI, which matched well with the area of lobule HVI that Yeo and his colleagues lesioned in their aspiration studies. At the cellular level, it has been suggested that desensitization of AMPA receptors at parallel fiber–Purkinje cell synapses may be the basis of at least some forms of LTD in cerebellar cortex. In simple terms, the binding of neurotransmitters at the desensitized Purkinje cell receptor is less likely to produce excitation in the neuron. As we detailed above, LTD is thought to be a key long-term plasticity process that may regulate excitability of neurons in the cerebellar cortex. These data suggest that these receptors (and perhaps the LTD mechanism) may be involved in the acquisition of eyeblink CRs.
Yeo and his colleagues also have reported that lobule HVI appears to be involved in post-training memory consolidation processes. Infusions of muscimol into lobule HVI immediately after each of five daily training sessions precluded the development of conditioned nictitating membrane responses (Attwell, Cooke, & Yeo, 2002). Interestingly, post-training muscimol infusions directed at the deep nuclei (including the interpositus nucleus) did not produce this effect. This might not be surprising, however, because the net effects of muscimol infusion into the deep nuclei, versus muscimol infusion into the cerebellar cortex, are quite different. Muscimol infusions into the deep nuclei essentially prevent activation of interpositus neurons. Muscimol infusions into the cerebellar cortex prevent Purkinje cell activation (presumably by activating inhibitory GABAergic basket and stellate cell synapses onto the Purkinje neurons). The loss of the normal tonic inhibitory Purkinje cell influence on deep nuclear cells may result in a hyperexcitable nucleus, which may in some fashion contribute to the disruption of consolidation. Nevertheless, the CNQX and muscimol infusion data provide evidence that Larsell's lobule HVI is playing an important role in classical eyeblink conditioning, either directly or indirectly.
Anatomical Studies of Lobule HVI Connectivity
A very important issue concerning the involvement of lobule HVI in eyeblink conditioning is whether or not the brain region connects with afferent and efferent areas known to be involved in conditioning. Some early studies explored patterns of retrograde degeneration of axonal connections to many areas of cerebellar cortex, including lobule HVI, after brainstem lesions (e.g., Brodal, 1940; Brodal & Jansen, 1946). These studies provided a general description of the patterns of projection of mossy fibers and climbing fibers from brainstem areas to well-defined areas of cerebellar cortex.
The first systematic study of connectivity of the eyeblink classical conditioning system was published by Yeo, Hardiman, and Glickstein (1985b). They studied anterograde and retrograde transport of wheatgerm-agglutinated horseradish peroxidase (HRP) following its injection into lobule HVI. HRP is an enzyme that is taken up by neurons and transported from one end to the other thus allowing one to trace neuronal projections from one brain area to another. Strong retrograde transport to the interpositus nucleus was observed with evidence of anterograde transport to many brainstem areas including the pontine nuclei and the inferior olivary complex. In another study, Steinmetz and Sengelaub (1992) infused choleratoxin-conjugated HRP into the interpositus nucleus and observed anterograde transport to the pontine nuclei and inferior olive as well as strong retrograde labeling to lobule HVI. Together these studies provide support for the idea that the pontine nuclei and inferior olive send projections to the interpositus nucleus and lobule HVI, and that lobule HVI projects axons to the interpositus nucleus. These studies provided anatomical evidence for connectivity in the neural network that has been hypothesized to underlie classical eyeblink conditioning, including connectivity of lobule HVI with other areas of the brain hypothesized to be involved in conditioning.
Lobule HVI Recording Studies
Since unit recording techniques were developed to explore the involvement of the brain in learning and memory (e.g., Olds, Disterhoft, Segal, Kornblith, & Hirsh, 1972), unit recording studies have contributed immensely to our understanding of the neural substrates of learning and memory. In these studies, insulated recording electrodes with small exposed recording tips are lowered into discrete brain regions and the activity of neurons are sampled during acquisition and performance of learned behaviors. Neuronal recordings have been mainly of three types: multi-unit recording that involves sampling a population of neurons simultaneously; extracellular single unit recording that involves isolating the activity of one neuron at a time and recording the activity from outside of the neuron; and intracellular cell recording that involves recording neuronal currents with an electrode placed inside the cell. For several reasons, classical eyeblink conditioning has proven to be an ideal paradigm for the application of unit recording techniques. For example, the trial lengths are relatively short, usually 500–2000 ms, thus limiting the sampling period that must be examined; the presentations of stimuli are under the control of the investigator so the time of their occurrences are known; and discharge patterns of neurons can be correlated with the discrete eyeblink (CR or UR) that is executed.
Typically, neural recordings of brain activity during the learning and/or performance of the conditioned eyeblink response are used to identify changes in firing patterns that may drive or produce the behavior or at least contribute to some aspect of its occurrence. Several patterns of action potentials may be observed during the course of conditioning. If a change in firing rate is observed after CS or US presentation and appears to be time locked to the CS or US presentation and not to the eyeblink response, then it is likely that this brain region is involved in sensory processes related to the CS or US. This pattern is seen when recordings are taken from the dorsal cochlear nucleus during CS presentations and from the trigeminal nucleus during US presentations (Clark & Lavond, 1996; Steinmetz et al., 1987). Sometimes firing patterns prove to be time-locked to UR onset and also seem to encode response amplitude as well as timing. This pattern can be seen when recordings are taken from the facial nucleus, which contains motor neurons that are related to eye-blinking (Young, Cegavske, & Thompson, 1976).
Most often, investigators are interested in changes in firing patterns that are somehow related to variations in CRs such as changes in amplitude, timing, and topography. These are brain regions that may contain neurons that change their excitability as learning occurs (i.e., the so-called learning-related “plastic” neurons). If changes in firing patterns in a brain region occur at the same time as CR onset or follow CR onset it is likely that the neurons are not responsible for driving or generating the behavioral response (e.g., cellular responses that follow a CR may be receiving feedback information concerning execution of the behavioral response). Brain sites that have neurons that show firing pattern changes that precede the execution of the CR are candidate brain sites for learning-related plastic cells that form the cellular basis of the learning. Studies have shown that the interpositus nucleus contains cells that meet this requirement (McCormick & Thompson, 1984).
Several studies have examined the activity of lobule HVI neurons during eyeblink conditioning in hopes of delineating further the role this cerebellar area plays in motor learning. One of the earliest studies was conducted by Berthier and Moore (1986), who used electrophysiological methods to examine HVI Purkinje cells during retention of the classically conditioned nictitating membrane response. They conducted extracellular recordings from single Purkinje cells in well-trained animals to determine if HVI Purkinje cell activity was related causally to CR production. Their procedures employed discrimination training where a CS+ and a CS– were presented, to better isolate neural activity associated with CR production from activity related to stimulus presentations. Of the Purkinje cell activity associated with conditioned responding, the most frequent outcome was an increase in firing in anticipation of the CR. Other cells showed decreases in activity after CS presentation. The observation of both excitatory and inhibitory units is interesting when the influence that cerebellar cortex has on the deep cerebellar nuclei is considered. As we described above, Purkinje cells inhibit the deep cerebellar nuclei. Therefore, cells that increased their firing rate in relation to the CR inhibited neurons in the interpositus nucleus, while cells that decreased their firing rate increased the excitability of the interpositus nucleus neurons. In other words, the inhibitory lobule HVI Purkinje cells should promote CR-related interpositus activity, while excitatory lobule HVI Purkinje cells should depress CR-related interpositus activity. The Berthier and Moore data suggested that more cells in lobule HVI may be involved in CR suppression than CR promotion.
Other studies also have used electrophysiological methods to explore how Purkinje cells in area HVI respond during eyelid conditioning procedures. Gould and Steinmetz (1996) gathered both single- and multiple-unit recording data from cells in area HVI and the anterior interpositus nucleus while animals received forward-paired training, backward-paired training, explicitly unpaired training, or CS-alone presentations. Interestingly, cells in the interpositus nucleus and HVI did not respond uniformly to the various conditioning procedures. Interpositus cells showed learning-related activity (i.e., as CRs developed) exclusively to forward CS–US pairings, whereas changes in Purkinje cell activity were much less specific and could be seen after forward pairing, backward pairing, and even after unpaired CS–US presentations, although responsiveness seemed much greater after forward pairing than after backward pairing. In addition, cells in HVI continued to show evidence of learning-related activity during extinction, long after interpositus cells had ceased responding to the CS-alone presentations. These data suggest that the cells in these two cerebellar regions do not encode CS and US information in the same manner. Indeed, it appears that the interpositus nucleus tracks behavioral responding well, whereas neurons in lobule HVI are highly plastic and change their firing rates in response to a variety of stimulus arrangements (i.e., in both associative and non-associative fashion).
In a series of studies, Bernard Schreurs and his associates used intracellular recording techniques to investigate plasticity of lobule HVI Purkinje cells. Their research was based on the conjoint mossy fiber/climbing fiber activation models described above (e.g., Albus, 1971; Marr, 1969). In their initial study, Schreurs, Sanchez-Andres, and Alkon (1991) trained rabbits using a tone CS and periorbital shock US. About 24 hr after training, slices of lobule HVI were prepared and intracellular recordings from Purkinje cell dendrites were taken. A conditioning-specific increase in the excitability of Purkinje cell dendrites was observed suggesting that the firing properties of these cells had changed after training.
In a second study, Schreurs and Alkon (1993) took intradendritic recordings from Purkinje cells obtained from rabbit cerebellar slices and reported that stimulation of climbing fibers followed by stimulation of parallel fibers produced significant decreases in Purkinje cell responsiveness. This arrangement of climbing fiber and mossy fiber stimulation has been used in many other experiments to produce LTD in cerebellar cortex (e.g., Ekerot & Kano, 1985; Ito, 1989; Linden & Conner, 1991) but in essence constitutes a backward conditioning arrangement for eyeblink conditioning (assuming that the US activates climbing fibers and the CS activates parallel fibers via mossy fiber inputs). Relatively low frequency stimulation of mossy fibers followed by climbing fiber stimulation (which mimics forward CS–US pairing during eyeblink conditioning) also produced a short-term depression effect whereas higher frequency stimulation of mossy fibers produced a more lasting depression. Schreurs and Alkon concluded that although plasticity at the parallel fiber–Purkinje cell synapse could be obtained, the depression or LTD-like effect was not specific to forward CS–US because depression also could be seen with backward and unpaired CS–US presentations.
Similar to the extracellular recordings of Berthier and Moore (1986) and Gould and Steinmetz (1996), Schreurs and Alkon (1993) observed that both increases and decreases in the dendritic excitability of Purkinje cells are seen. Furthermore, increases in Purkinje cell excitability were much more prevalent than decreases. Similar to the in vivo recording experiments, these studies provide intracellular evidence that Purkinje cells in Larsell's lobule HVI are capable of demonstrating plasticity. And, as revealed in extracellular single-unit recordings taken from intact rabbits, some cells in lobule HVI appear not to be forward-pairing specific, and many do not fire in a pattern that promotes CR acquisition and performance. The extracellular and intracellular recording studies provide solid evidence that neurons in lobule HVI change their firing patterns as a result of CS and US presentations, but these changes do not appear to be limited to stimulus conditions that promote CR formation.
Lobule HVI Stimulation Studies
Electrical stimulation delivered to discrete brain regions has proven to be an effective tool for studying brain-behavior relationships. Typically, insulated electrodes with relatively large exposed tips are implanted into brain areas of interest, and microstimulation is delivered in hopes of exciting cells in that brain region. This technique was used to study potential CS and US pathways into the cerebellum for eyeblink conditioning (Mauk, Steinmetz, & Thompson, 1986; Steinmetz, Lavond, & Thompson, 1989; Steinmetz, Rosen, Chapman, Lavond, & Thompson, 1986). In these studies, electrodes were implanted into the pontine nucleus, inferior olive, or both, and electrical stimulation was delivered in lieu of the presentation of peripheral stimuli like tones or air puffs. Rabbits can readily be conditioned when the stimulation is substituted for the peripheral stimuli. The general idea of this method is that if conditioning occurs with the electrical stimulation, then the brain site being stimulated may be a structure in the pathway normally engaged during learning. This technique is not without it problems, however. The spread of current to adjacent structures and pathways is always a possibility, especially when current intensities are high. Also, care must be taken to keep the stimulation parameters within the range of physiological possibility.
In a series of studies, Thompson and his colleagues delivered stimulation in the region of HVI in place of peripheral CSs and/or USs. Swain, Shinkman, Nordholm, and Thompson (1992) implanted stimulating electrodes in the white matter beneath lobule HVI and observed movements of facial and neck muscle when electrical stimulation was delivered. They were able to classically condition these movements by pairing a tone CS with the cortical stimulation US. No conditioning was seen in rabbits that received unpaired CS-US presentations. In a subsequent study, Swain, Shinkman, Thompson, Grethe, and Thompson (1999) showed that lesions of the interpositus nucleus abolished the conditioning produced by the tone CS paired with a cerebellar-cortical-stimulation US. In a third study, conditioned movements were observed when stimulation was delivered to two regions of cerebellar cortex as a CS and US demonstrating that activation of cortical sites in the region of lobule HVI could produce conditioning (Shinkman, Swain, & Thompson, 1996).
Although these studies are important in that they show that local stimulation of regions of the cerebellum is sufficient to induce classical conditioning, some interpretation problems exist. Exactly how stimulation of cerebellar cortex results in movement is not clear. To produce movements with the use of cerebellar cortical stimulation, relatively large amounts of current had to be delivered. Because the spread of current would have been relatively large due to the large current intensity, it is possible that the stimulation activated axons projecting to cortical areas, axons of Purkinje cells headed toward the deep cerebellar nuclei, or afferent fibers entering the deep nuclei from pre-cerebellar nuclei. Stimulating Purkinje cells or their axons should inhibit the deep cerebellar nuclei, which runs counter to the response generation shown by many studies in which electrical stimulation of the interpositus nucleus produces robust eyeblinks and other movements.
Rebound excitation of cells in the deep nucleus after cerebellar cortical inhibition is a possible mechanism for activating deep nuclear neurons. Rebound excitation is a rather large-scale excitation of neurons that follows a period of strong inhibition. This phenomenon has been described and proposed as an excitation mechanism in interpositus neurons (after strong inhibition of Purkinje cells) and thus may be important for conditioning (e.g., Aizenman, Manis, & Linden, 1998; Andersson & Hesslow, 1987; Hesslow, 1994; Jahnsen, 1986; Katz, Tracy, & Steinmetz, 2001; McCrea, Bishop, & Kitai, 1977). It also is possible that the cortical stimulation directly activated pre-cerebellar afferents to the interpositus nucleus, exciting neurons in the structure. If this were the case, then the roles of lobule HVI and other areas of cerebellar cortex in conditioning would seem less central.
Combining Brain Inactivation with Unit Recording: Some New Lobule HVI Data
Combining the individual experimental techniques that were described above is a powerful means for exploring the brain correlates of learning. We present here recent data we have collected to illustrate this point, and also to give the reader a relatively in-depth look at how these brain-behavior experiments are conducted. These experiments studied the interactions between Larsell's lobule HVI and the interpositus nucleus during classical eyeblink conditioning. Our strategy was to use permanent chemical lesions (kainic acid) or temporary inactivation (muscimol) in concert with unit recording to assess the relative interdependencies of the interpositus nucleus and lobule HVI during the acquisition and performance of eyeblink CRs. In the studies described, the interpositus nucleus was lesioned or inactivated during or after conditioning, while the activity of Purkinje cells in lobule HVI was recorded. We hypothesized that if the pattern of unit responses in cortex was somehow dependent on normal interpositus nucleus function, lobule HVI Purkinje cell activity should be altered by the lesion or inactivation.
The extent to which learning-related activity in HVI is dependent upon normal interpositus function was first examined by Katz and Steinmetz (1997). In this study, rabbits were conditioned to criterion (75% CRs) before receiving either infusions of kainic acid or vehicle into the interpositus nucleus. The kainic acid destroyed neurons in the interpositus nucleus and, as a result, abolished eyeblink CRs. Post-lesion single-unit recordings then were taken from Purkinje cells in lobule HVI of the lesioned and control rabbits. We found that conditioning-related activity (i.e., learning-related excitatory and inhibitory patterns of action potentials) was still evident in HVI despite the fact that CRs had been abolished by the kainic acid lesion of the interpositus nucleus. At a few recording sites, compared to sham controls, the HVI activity of interpositus-lesioned rabbits appeared to be not as well organized (e.g., timing of firing patterns was disrupted and more units with mixed excitatory and inhibitory patterns of discharge were found). These data demonstrate that the learning-induced plasticity that develops in lobule HVI survives permanent interpositus damage (and the resultant abolition of CRs), thus suggesting that conditioning-related plasticity in cerebellar cortex is maintained somewhat independently of the deep cerebellar nuclei.
Using a similar strategy, we examined the effects of temporarily inactivating the interpositus nucleus on conditioning-related activity in lobule HVI. The advantage of the inactivation approach is that the effects of eliminating interpositus activity can be assessed during both the acquisition and performance stages of learning, given the reversible nature of the method. In a preliminary study (Baker, Tracy, Villarreal, & Steinmetz, 2002), we used temporary inactivation methods in an attempt to replicate the findings of the Katz and Steinmetz (1997) study. Two groups of rabbits were trained for five days then given an additional five days of training during which either muscimol or saline was infused in the interpositus nucleus while lobule HVI recordings were taken. The activity of approximately 140 units was examined during acquisition and retention training. We found that during muscimol infusion, although production of CRs was severely impaired, the pattern of unit responses of muscimol-infused rabbits was very similar to the control rabbits and thus similar to what Katz and Steinmetz (1997) reported after permanent kainic acid lesions. One difference we noted when comparing the Baker et al. (2002) and Katz and Steinmetz (1997) data was that there was no evidence for an increased variability in the timing of the activity of the HVI neurons during the muscimol inactivation.
Here we present new data that provide a more detailed look at how temporary inactivation can be used in conjunction with extracellular single-unit recording to study the function of Lobule HVI. For this study, rabbits were anesthetized with xylazine and ketamine, and during an aseptic surgery procedure they were chronically implanted with a 22-gauge infusion cannula into the left interpositus nucleus and an insulated tungsten microelectrode (3–5 MΩ) into the left Larsell's lobule HVI. After final positions of the cannula and recording electrodes were determined, leads from the electrodes were secured to a standard plug assembly and cemented onto the rabbits' skulls with dental acrylic. Also during surgery, stainless-steel wires were implanted into the musculature surrounding the left eye for subsequent EMG recordings during eyeblink conditioning.
Rabbits then were assigned to two groups that were given 10 days of training. One group received five days of eyeblink training with muscimol infusions (0.7 nmol in 1 µl saline at 0.1 µl/min over 10 min) into the interpositus nucleus followed by five days of training with no infusions. The second group received 10 days of training with saline infusions into the interpositus nucleus during the first five days. The muscimol group allowed the assessment of the effects on lobule HVI activity of inactivating the interpositus nucleus during the acquisition phase before CRs were established. The saline control group provided comparative HVI recording and behavioral data from animals without interpositus inactivation during acquisition. Rabbits were placed in standard restraint boxes within sound-attenuating chambers and trained using a standard Pavlovian delay procedure. The CS was a 600 ms, 85 dB, 1 kHz tone that co-terminated with a 100 ms, 3 psi corneal air puff US (creating a 500 ms ISI). The intertrial interval was randomized between 20 and 40 s and 120 training trials per day were given. Recordings from lobule HVI were taken on each training day.
The behavioral data that were analyzed included percent CRs, CR amplitude, and CR timing (i.e., onset latency and peak latency). The lobule HVI unit activity was amplified (5000X), filtered (300–3000 Hz) and routed to a neural data-acquisition system (Spike2, CED Ltd) for storage and subsequent offline analysis. Spike separation was accomplished using waveform-matching algorithms that assessed spike amplitude (at least 2X background signal) and action potential shape. Typically, 2–3 spikes per session could be isolated at each recording site. The activities of the separated spikes then were summed across the trial period for each session to form peristimulus time histograms. For analysis purposes, the neural activity was expressed as standard scores (relative to pre-CS baseline activity) so that the activity of lobule HVI neurons of muscimol- and saline-infused rabbits could be compared. This standardization procedure has been described in detail elsewhere (see Katz & Steinmetz, 1997; Lavond & Steinmetz, 2003). After the last session, the rabbits were euthanized, perfused with saline followed by 10% formalin, and their brains removed for histological verification of cannula and electrode placements.
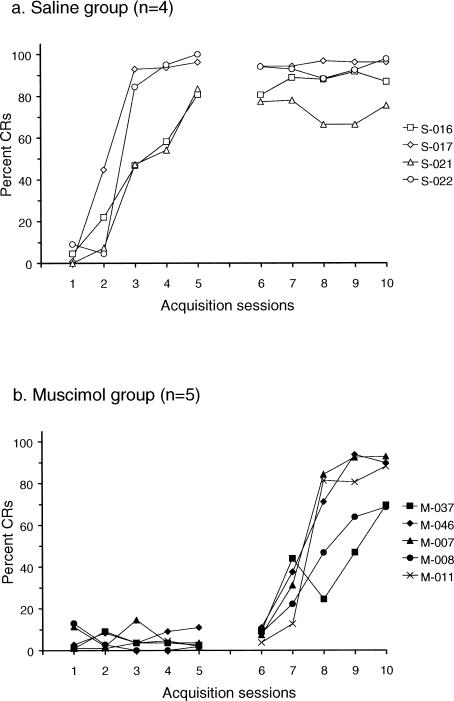
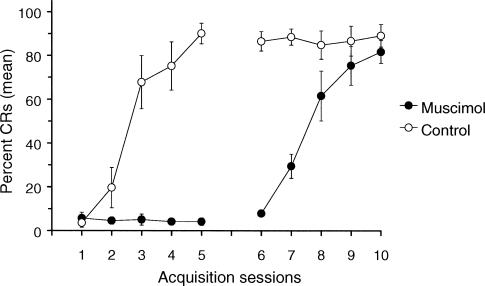
Figures 2 and 3 show the behavioral data from rabbits given five days of training while muscimol was infused into the interpositus nucleus, followed by five days of training with no infusions (n = 5), and control rabbits that were given 5 days of saline infusions (n = 4). Figure 2 shows individual learning curves for the nine rabbits while Figure 3 shows averaged data for the control and muscimol animals. As expected, no eyeblink CRs were evident in any of the muscimol animals during the first five days of training, whereas all the saline animals acquired CRs. ANOVA revealed a significant Group X Session interaction that confirmed the lack of learning in the muscimol-infused group (F(4,28) = 36.05, p = <.0001). During sessions 6–10, CR acquisition was seen in the muscimol group, and the saline group maintained its asymptotic level of conditioning. A significant Group X Session interaction confirmed the learning in the muscimol group during the saline infusion sessions (F(4,28) = 16.96, p < .0001). In fact, performances of the saline group during sessions 1–5 and the muscimol group during session 6–10 were virtually identical (p > .05), indicating that we replicated the interpositus inactivation result reported previously by Krupa et al. (1993): i.e., inactivation of the interpositus nucleus prevented the appearance of eyeblink CRs and, more importantly, no savings of training effects were noted when conditioning was continued after the inactivation sessions. This finding further demonstrates the critical nature of plasticity in the interpositus nucleus during eyeblink conditioning.
Figure 2. Individual learning curves (percent CRs) observed in rabbits given 5 days of training with saline infusions followed by 5 days of training with no infusions (top panel [a]; n = 4) and in rabbits given 5 days of training with muscimol infusions followed by 5 days of no infusions (bottom panel [b]; n = 5).
Figure 3. Mean percent CRs (± S.E.M.) recorded in rabbits given 5 days of training with saline infusions followed by 5 days of training with no infusions (open circles) and in rabbits given 5 days of training with muscimol infusions followed by 5 days of no infusions (filled circles).
Based on a 2 ∶ 1 signal to noise ratio, relatively high spontaneous firing rates (> 15 Hz), and verification of electrode placement in the Purkinje cell layer of lobule HVI, a total of 111 units were isolated from the lobule HVI recordings (62 from muscimol rabbits and 49 from saline rabbits). Given the relatively large size of the spikes, it is assumed that the majority of cells monitored were Purkinje cells, although it is possible that some of the recordings came from granule cells. Similar to previous studies (e.g., Katz & Steinmetz, 1997), we summed the unit activity across a given session and calculated standard scores of activity for 100 ms bins of the CS–US interval to determine if a significant increase or decrease in firing rate occurred between CS and US presentations. The standard scores are essentially difference t-scores that are calculated by finding the differences between mean spikes recorded in a 100-ms CS–US period bin, and a 100-ms period before CS onset, and dividing that difference by the standard deviation of the pre-CS mean (see Katz & Steinmetz, 1997; Lavond & Steinmetz, 2003).
After calculating five standard scores to quantify the activity for a unit (a standard score for each of the five 100-ms bins that made up the 500-ms CS–US interval for each session), we then classified each unit's response pattern in one of four categories: nonresponsive (no significant change in firing rate was seen); early CS-period units (significant standard scores were found in only the first or second 100-ms bin after CS onset); late CS-period units (significant standard scores were found in the third, fourth or fifth 100-ms bin after CS onset); and early/late CS-period units (changes in firing rate found in both the first or second 100-ms bin and the third, fourth, or fifth 100-ms bin). For ease of presentation, we have combined recording sessions to show four points in conditioning: Days 1–2, 4–5, 6–7, and 9–10.
Table 1 provides a summary of the patterns of activation of lobule HVI neurons observed in the muscimol and control rabbits. Of the total number of cells recorded, 82% showed increases in firing rates during the CS-US interval while 18% showed decreases in firing rates. Table 1 shows that control rabbits demonstrated the pattern of lobule HVI activity seen in previous studies—the presence of relatively little neural activity confined to the early CS period throughout training, but a rather rapid formation of late and early/late period activity as conditioning proceeds. To date, we have made very few chronic recordings after as long as 9 or 10 days of training. The present recordings revealed one potentially interesting new finding: it appears that there is a reduction in conditioning-related activity in lobule HVI when training is extended. Of central importance to this experiment is whether or not conditioning-related activity is seen in rabbits undergoing muscimol infusion into the interpositus nucleus. Inspection of Table 1 provides an answer to this question: Although there appeared to be a relatively large number of non-responsive units on Days 1 and 2, by Days 4 and 5 a substantial number of units were responding in both the early and latter portions of the CS-period even though no CRs were executed. In addition, there was a decrease in the number of non-responsive units by Days 4 and 5. Past studies (e.g., Berthier & Moore, 1986; Gould & Steinmetz, 1996; Katz & Steinmetz, 1997) have shown that many of the units that increase their spiking during the latter portion of the CS-US interval often do so in close relationship with the onset of the CR. Therefore, these units are good candidates for making contributions to the execution of the CR. This pattern was somewhat stable over the final five days of training when muscimol infusion was suspended. There were some differences between the groups in response patterns. The number of nonresponsive units was greater in muscimol rabbits than saline rabbits across training, as was the number of early CS period neurons. And, unlike control rabbits, a decrease in muscimol rabbits' learning-related activity was not seen in very late stages of training.
Table 1. Percentage of units recorded from lobule HVI neurons in muscimol and control rabbits that showed activation or inhibition in the early CS period, late CS period, both early and late CS periods, or were nonresponsive.
| Days 1 and 2 |
Days 4 and 5 |
Days 6 and 7 |
Days 9 and 10 |
|||||
| Muscimol | Control | Muscimol | Control | Muscimol | Control | Muscimol | Control | |
| Early CS period activity | 16 | 8 | 21 | 0 | 13 | 0 | 5 | 0 |
| Late CS period activity | 11 | 8 | 0 | 0 | 13 | 13 | 14 | 0 |
| Early/late CS period activity | 26 | 61 | 58 | 90 | 74 | 56 | 52 | 50 |
| Non-responsive | 47 | 23 | 21 | 10 | 0 | 31 | 29 | 50 |
| Total cells recorded | 19 | 13 | 14 | 10 | 8 | 16 | 21 | 10 |
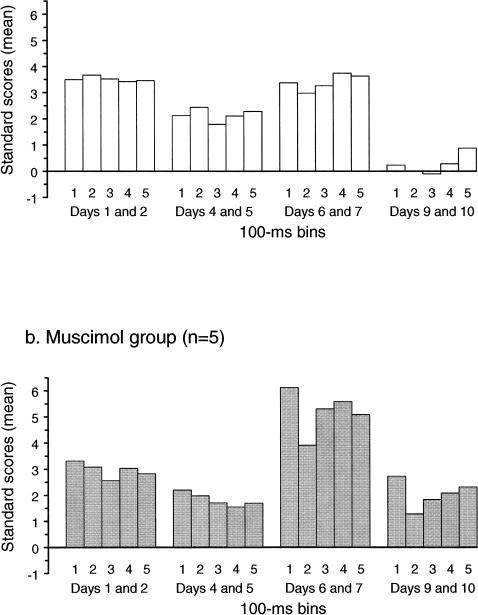
Figure 4 provides a summary of average standard scores for the two groups calculated for the four phases of conditioning. Statistical analyses of these data provide a verification of the patterns of unit responding shown in Table 1. Excitatory and inhibitory cells were included in these averages to provide a summary of the activity of the population of lobule HVI neurons from which recordings were made. In essence, this figure shows the mean within-trial pattern of activity present across training sessions.
Figure 4. Standard scores of unit activity recorded in control rabbits (panel a) and muscimol rabbits (panel b).
For the two groups, the sets of five bars represent four phases of training (days 1–2, days 4–5, days 6–7 and days 9–10). For each set of bars, the activity in consecutive five 100-ms bins provides a summary of unit firing during the 500-ms CS–US interval.
Analyses of variances conducted on these data showed no group differences when activity patterns during the first five days of training were compared (ps > .05); both groups showed CS-period activation at similar levels during the first phase of conditioning. Significant differences in lobule HVI activity were seen when the second five days of training were compared. A significant Group X Bin interaction was found on days 6 and 7 [F(4,88) = 2.72, p < .05] due to a significantly higher activation during the first 100-ms bin in rabbits that had received muscimol during the first five days of training. Significantly lower levels of responding were seen in control rabbits on Days 9 and 10 [F(1,29) = 10.49, p < .05] reflecting the loss of CS- period activity in the control rabbits.
We conclude from these data that eyeblink conditioning-related plasticity in lobule HVI can be established independently of activity in the interpositus nucleus—conditioning-related activity in lobule HVI was evident in spite of the fact that CR acquisition and interpositus nucleus activity was blocked by muscimol infusions into the interpositus nucleus. Interestingly, our data indicate a relatively high level of early CS-period activation during the first two days of training without muscimol, suggesting that the training with muscimol infusions into the deep cerebellar nuclei may affect subsequent processing of the conditioning stimuli. This effect appears to be transient, however, as CS-period lobule HVI activity decreased with additional training.
Larsell's Lobule HVI and Eyeblink Conditioning: What Can We Conclude from Data Generated through the Use of Convergent Behavioral Neuroscience Techniques?
What can we conclude from the last 20 years or so of research on the role of lobule HVI in eyeblink conditioning? There are few, if any, who would disagree with the statement that lobule HVI is engaged and is involved in eyeblink conditioning. Disagreement centers on the extent of lobule HVI's involvement. Specifically, is lobule HVI the sole site of plasticity that underlies eyeblink conditioning or is plasticity formed in lobule HVI along with other regions of cortex and/or the deep cerebellar nuclei?
The permanent lesion data remain inconclusive as reports on the effects of lobule HVI removal run the gamut from complete abolition, to partial effects, to little or no effect. The difficulty in achieving consistent findings may lie in the approach—lesion sizes have varied greatly from study to study, and it is very difficult to determine if underlying white matter and parts of the deep nuclei (as well as other cortical areas) are included in the lesion. Further, variations in the basic delay conditioning procedure may be an important factor, certainly worth exploring further, including the choice of stimulus modality used as the CS (e.g., light versus tone), the length of the ISI, the choice of response measurement system employed (e.g., EMG versus use of mechanical transducers), and the number of training sessions given. And, assessing the physiological response of interpositus neurons after lobule HVI removal or inactivation has yet to be done. Further studies using temporary chemical or pharmacological inactivation methods would seem warranted, as the results to date have been relatively consistent. This approach also has the advantage of allowing for an assessment of cellular mechanisms involved in the learning. For example, AMPA receptor involvement has been assessed using CNQX infusions, while general inactivation (leaving AMPA transmission intact) has been explored using muscimol (Attwell et al., 1999; Attwell et al., 2001; Attwell et al., 2002).
Microstimulation of cerebellar cortex has provided some data concerning the involvement of lobule HVI in conditioning. For example, Hesslow and his colleagues have shown that stimulation delivered on the surface of lobule HVI above the appropriate longitudinal zone can elicit eyeblinks, indicating that this region of cortex is connected eventually to eyeblink musculature (e.g., Hesslow, Svensson, & Ivarsson, 1999). The anatomical tract-tracing studies that have been conducted support the connectivity between lobule HVI and cranial motor nerve nuclei. Also, research from the Thompson laboratory has shown that stimulating the white matter below lobule HVI produces discrete eyeblinks (e.g., Swain et al., 1992). These studies suggest that neurons in lobule HVI are “wired” in a fashion that could participate in eyeblink conditioning. However, not all laboratories have been successful in eliciting eyeblinks with lobule HVI stimulation, including our own laboratory, when relatively high impedance, small-tipped stimulating electrodes are used, restricting the region of stimulation. In addition, because Purkinje cell output to the deep nucleus is solely inhibitory, it is unclear exactly what mechanism produces the eyeblinks that are seen (i.e., are axons entering the interpositus nucleus activated, or does a rebound from inhibition effect activate deep nuclear neurons?).
Unit recording studies have consistently revealed neurons in lobule HVI that display firing patterns that change with learning and also correlate highly with CR execution (as well as encoding the CS and the US used in training). These data strongly implicate lobule HVI in eyeblink conditioning. However, some complications in these data exist. Of note, recording studies have consistently found more cells that increase their firing rate with conditioning than cells that decrease their firing rate. Because Purkinje cells inhibit their target neurons, the inhibitory cells are thought to be the cells that contribute to activation of the CR, yet they appear to be relatively fewer in number. One possibility is that both populations contribute to the execution of the CR; excitatory Purkinje cells suppress behavioral responding while inhibitory Purkinje cells activate behavioral responses during a given trial, thus accounting for initial response inhibition, eyelid closure, and subsequent eyelid opening. In addition, it is possible that the population of excitatory cells inhibit deep nuclear cells that are involved in non-eyeblink response systems, in essence honing accuracy of the discrete response. All of these possibilities are testable.
Finally, our most recent studies that coupled lobule HVI recordings with temporary inactivation of the interpositus nucleus have shown that plasticity in cerebellar cortex forms, and is maintained, independently from the deep cerebellar nuclei. Conditioning-related activity in the cerebellum could be seen during acquisition training even though the acquisition of CRs was blocked by muscimol infusions. And, similar to previous studies, the majority of units we recorded showed increases in spiking during the CS–US interval. Although lobule HVI unit activity seems largely unaffected by inactivation of the interpositus nucleus, we do not know if inactivation of the cortex would have negligible affects on the formation of activity in the deep nuclei. Some models do predict an effect (e.g., Medina & Mauk, 1999). Also, this approach has not been used yet to study relationships between the anterior lobe of cerebellar cortex and the deep cerebellar nuclei. These two areas of studies are ongoing in our laboratory.
The Neural Substrates of Eyeblink Conditioning: Integrating the Circuitry
This article has concentrated, for the most part, on summarizing the results of experiments that were designed to explore the role of lobule HVI in eyeblink conditioning, but it is important to remember that this simple form of learning and memory appears not to be based on plasticity established in a single brain area. Rather, the neural substrates of eyeblink conditioning are better described as a network of interconnected brain areas that each encode important features of the conditioning process. The rudiments of this network were shown in Figure 1.
Our current working model is that plasticity that underlies the classically conditioned eyeblink response is formed in lobule HVI and the dentate/interpositus nucleus. Also, discrete regions of the anterior lobe of cerebellar cortex contain neurons that appear to be very important for eyeblink conditioning (e.g., Medina, Nores, Ohyama, & Mauk, 2000). Our inactivation/recording data presented above suggest that cortex and the deep nuclei establish plasticity independently of one another. However, we again note that studies inactivating cortex while recording in the interpositus nucleus have not been completed, that would provide evidence against the possibility that interpositus plasticity is somehow dependent on cerebellar cortical plasticity.
What are the respective roles of the cerebellar cortex and the interpositus nucleus in conditioning? For now, we assume that excitability changes in the deep nucleus are critical for driving activity in brainstem nuclei that generate the behavioral CR by activating eyeblink musculature. However, without cortical input we believe that the activation is weak, at best, and not particularly effective. Our model contends that neurons in cerebellar cortex (lobule HVI and the anterior lobe) provide important modulating input to interpositus neurons, that adds appropriate gain to the response as well as the precise response timing that is characteristic of eyeblink conditioning. Again, all of these ideas are testable and are the focus of our current research efforts. We must also keep in mind that higher brain areas like the cerebral cortex, hippocampus, striatum, and amygdala appear to play roles in the conditioning process. How these areas influence the brainstem and cerebellar circuitry shown in Figure 1 (and vice versa) has gone largely unstudied. It seems likely, however, that these and other brain areas are importantly engaged during eyeblink conditioning, especially when stimulus and response demands are more complex, such as during discrimination/reversal learning and trace conditioning.
One central point that we want to emphasize is that the combined use of behavioral and neuroscience approaches to study learning have complemented each other. That is, the use of the eyeblink conditioning paradigm has advanced our understanding of the brain substrates of the behavior, and the use of the neuroscience approach has advanced our understanding of learning. The research presented in this paper provides a number of examples of how variations of the conditioning procedure produce new insights into the working of the brain, such as when shifts in the ISI are used to perturb CR timing, and changes in patterns of neuronal activity are noted. Backward conditioning is an example of how the study of the neurobiology of eyeblink conditioning has aided our understanding of the behavioral processes involved. Normally, when the US precedes the CS during eyeblink conditioning, no CR is established to the CS even though the stimuli are presented closely in time. The connectivity of the cerebellar and brainstem circuitry that is proposed to be involved in the conditioning may provide an explanation for why backward eyeblink conditioning is not obtained. It appears that mossy fiber input from the pontine nuclei (the CS) must arrive in the interpositus nucleus before climbing fiber input from the inferior olive (the US) for plasticity to occur (Gould & Steinmetz, 1996). It appears that both forward and backward pairing can produce plasticity in the cerebellar cortex, but the forward procedure is much more effective at producing cellular excitability changes (Gould & Steinmetz, 1996). Thus, it seems that the pattern of synaptic connectivity between the brainstem and the cerebellum limits the order of stimuli that can produce behavioral learning. In this case, the knowledge of the neural correlates of eyeblink conditioning has offered an explanation for the behavioral phenomenon that is observed.
In closing, we make the following observations. When some of us began working in this area, we naively thought that the neural substrates of eyeblink conditioning would be quite simple—perhaps involving plasticity of a single brain system and more akin to the conceptualizations of Pavlov who thought of conditioning as the simple linkage of a CS neural center with a US neural center (Pavlov, 1927). This is certainly not the case, even for simple delay conditioning. The circuit involved in conditioning is relatively complex, involving multiple sites of plasticity and feedback (and perhaps feed-forward) connections. It also should be noted that this neuroscience/behavioral approach has proven valuable for studying more complex learning and memory systems. For example, our understanding of the neural substrates of fear conditioning has been advanced significantly over the last decade or so when these same basic neuroscience tools were used to study brain–behavior correlates (see Fanselow, 2001, for review). Indeed, it is our view that more significant advances in our understanding of learning will come when rigorous behavioral analyses are combined with neuroscientific analyses.
Acknowledgments
This research was supported by NIMH grant MH62013 to Joseph Steinmetz.
References
- Aizenman C.D, Manis P.B, Linden D.J. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Albus J.S. A theory of cerebellar functions. Mathematical Biosciences. 1971;10:25–61. [Google Scholar]
- Andersson G, Hesslow G. Activity of Purkinje cells and interpositus neurons during and after periods of high frequency climbing fibre activation in the cat. Experimental Brain Research. 1987;67:533–542. doi: 10.1007/BF00247286. [DOI] [PubMed] [Google Scholar]
- Attwell P.J, Cooke S.F, Yeo C.H. Cerebellar function in consolidation of a motor memory. Neuron. 2002;34(6):1011–20. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Attwell P.J, Rahman S, Ivarsson M, Yeo C.H. Cerebellar cortical AMPA-kainate receptor blockade prevents performance of classically conditioned nictitating membrane responses. Journal of Neuroscience. 1999;19:RC45. doi: 10.1523/JNEUROSCI.19-24-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell P.J, Rahman S, Yeo C.H. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. Journal of Neuroscience. 2001;21:5715–5722. doi: 10.1523/JNEUROSCI.21-15-05715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K.B, Tracy J, Villarreal R.P, Steinmetz J.E. Orlando, Florida: 2002. Inactivation of interpositus nucleus of cerebellum during eyeblink conditioning causes conditioned response (CR) impairment but not loss of learning-related activity in HVI region of cerebellar cortex. Poster presented at the Society for Neuroscience Meeting, [Google Scholar]
- Berger T.W, Thompson R.F. Neuronal plasticity in the limbic system during classical conditioning of the rabbit nictitating membrane response. I. The hippocampus. Brain Research. 1978;145:323–346. doi: 10.1016/0006-8993(78)90866-1. [DOI] [PubMed] [Google Scholar]
- Berthier N.E, Moore J.W. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Experimental Brain Research. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- Brodal A. The cerebellum of the rabbit. Journal of Comparative Neurology. 1940;72:63–81. [Google Scholar]
- Brodal A, Jansen J. The ponto-cerebellar projection in the rabbit and cat. Journal of Comparative Neurology. 1946;84:31–118. doi: 10.1002/cne.900840105. [DOI] [PubMed] [Google Scholar]
- Chen L, Bao S, Lockard J.M, Kim J.J, Thompson R.F. Impaired classical eyeblink conditioning in cerebellar lesioned and Purkinje cell degeneration (pcd) mutant mice. Journal of Neuroscience. 1996;16:2829–2838. doi: 10.1523/JNEUROSCI.16-08-02829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.E, Lavond D.G. Neural unit activity in the trigeminal complex with interpositus or red nucleus inactivation during classical eyeblink conditioning. Behavioral Neuroscience. 1996;110:1–9. doi: 10.1037//0735-7044.110.1.13. [DOI] [PubMed] [Google Scholar]
- Clark R.E, Zhang A.A, Lavond D.G. Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior. Behavioral Neuroscience. 1992;106:879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- Ekerot C.F, Kano M. Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Research. 1985;342:357–360. doi: 10.1016/0006-8993(85)91136-9. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S. Toward a neurobiology of functional behavior systems: Contrasting Pavlovian emotional and motor learning. In: Steinmetz J.E, Gluck M.A, Solomon P.R, editors. Model systems and the neurobiology of associative learning: A festschrift in honor of Richard F. Thompson. Mahwah, NJ: Erlbaum; 2001. pp. 379–393. [Google Scholar]
- Garcia K.S, Mauk M.D, Weidemann G, Kehoe E.J. Covariation of alternative measures of responding in rabbit (Oryctalagus cuniculus) eyeblink conditioning during acquisition training and tone generalization. Behavioral Neuroscience. 2003;117:292–303. doi: 10.1037/0735-7044.117.2.292. [DOI] [PubMed] [Google Scholar]
- Gormezano I. Classical conditioning. In: Sidowski J.B, editor. Experiment methods and instrumentation in psychology. New York: McGraw-Hill; 1966. pp. 385–420. [Google Scholar]
- Gormezano I, Kehoe E.J, Marshall B.S. Twenty years of classical conditioning with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Gould T.J, Steinmetz J.E. Changes in rabbit cerebellar cortical and interpositus nucleus activity during acquisition, extinction, and backward classical eyelid conditioning. Neurobiology of Learning & Memory. 1996;65:17–34. doi: 10.1006/nlme.1996.0003. [DOI] [PubMed] [Google Scholar]
- Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. Journal of Physiology (London) 1994;476:245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Hirano T. Depression and potentiation of the synaptic transmission between a granule cell and a Purkinje cell in rat cerebellar culture. Neuroscience Letters. 1990;119:141–144. doi: 10.1016/0304-3940(90)90818-t. [DOI] [PubMed] [Google Scholar]
- Ito M. Long-term depression. Annual Review Of Neuroscience. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- Jahnsen H. Electrophysiological characteristics of neurons in the guinea-pig deep cerebellar nuclei in vitro. Journal of Physiology (London) 1986;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell H, Ekerot C.-F. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- Katz D.B, Steinmetz J.E. Single-unit evidence for eye-blink conditioning in cerebellar cortex is altered, but not eliminated, by interpositus nucleus lesions. Learning & Memory. 1997;4:88–104. doi: 10.1101/lm.4.1.88. [DOI] [PubMed] [Google Scholar]
- Katz D.B, Tracy J.A, Steinmetz J.E. Rabbit classical eyeblink conditioning is altered by brief cerebellar cortical stimulation. Physiology & Behavior. 2001;72:499–510. doi: 10.1016/s0031-9384(00)00444-3. [DOI] [PubMed] [Google Scholar]
- Krupa D.J, Thompson J.K, Thompson R.F. Localization of a memory trace in the mammalian brain. Science. 1993;260:898–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Lavond D.G, Kanzawa S.A. Inside the black box. In: Steinmetz J.E, Gluck M.A, Solomon P.R, editors. Model systems and the neurobiology of associative learning: A festschrift in honor of Richard F. Thompson. Mahwah, NJ: Erlbaum; 2001. pp. 245–269. [Google Scholar]
- Lavond D.G, Steinmetz J.E. Handbook of classical conditioning. Boston: Kluwer; 2003. [Google Scholar]
- Lavond D.G, Steinmetz J.E, Yokaitis M.H, Thompson R.F. Reacquisition of classical conditioning after removal of cerebellar cortex. Experimental Brain Research. 1987;67:569–593. doi: 10.1007/BF00247289. [DOI] [PubMed] [Google Scholar]
- Linden D.J, Conner J.A. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science. 1991;254:1656–1659. doi: 10.1126/science.1721243. [DOI] [PubMed] [Google Scholar]
- Lisberger S.G, Pavelko T.A, Bronte-Stewart H.M, Stone L.S. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. Journal of Neurophysiology. 1994;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. Journal of Physiology. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk M.D, Donegan N.H. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learning & Memory. 1997;3:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- Mauk M.D, Thompson R.F. Retention of classically conditioned eyelid responses following acute decerebration. Brain Research. 1987;403:89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Mauk M.D, Steinmetz J.E, Thompson R.F. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences (USA) 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D.A, Lavond D.G, Thompson R.F. Concomitant classical conditioning of the rabbit nictitating membrane and eyelid responses: Correlations and implications. Physiology and Behavior. 1982;28:769–775. doi: 10.1016/0031-9384(82)90192-5. [DOI] [PubMed] [Google Scholar]
- McCormick D.A, Thompson R.F. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- McCrea R.A, Bishop G.A, Kitai S.T. Electrophysiological and horseradish peroxidase studies of precerebellar afferents to the nucleus interpositus anterior: II. Mossy fiber system. Brain Research. 1977;122:215–228. doi: 10.1016/0006-8993(77)90290-6. [DOI] [PubMed] [Google Scholar]
- Medina J.F, Mauk M.D. Simulations of cerebellar motor learning: Computational analysis of plasticity at the mossy fiber to deep nucleus synapse. Journal of Neuroscience. 1999;19:7140–7151. doi: 10.1523/JNEUROSCI.19-16-07140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J.F, Nores W.L, Ohyama T, Mauk M.D. Mechanisms of cerebellar learning suggested by eyelid conditioning. Current Opinion in Neurobiology. 2000;10:717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- Moore J.W, editor. A neuroscientist' s guide to classical conditioning. New York: Springer; 2002. [Google Scholar]
- Moyer J.R, Jr, Deyo R.A, Disterhoft J.F. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Oakley D.A, Russell I.S. Neocortical lesions and Pavlovian conditioning. Physiology & Behavior. 1972;8:915–926. doi: 10.1016/0031-9384(72)90305-8. [DOI] [PubMed] [Google Scholar]
- Olds J, Disterhoft J.F, Segal M, Kornblith C.L, Hirsh R. Learning centers of the rat brain mapped by measuring latencies of conditioned unit responses. Journal of Neurophysiology. 1972;35:202–219. doi: 10.1152/jn.1972.35.2.202. [DOI] [PubMed] [Google Scholar]
- Pavlov I.P. Conditioned reflexes. Oxford: Oxford University Press; 1927. [Google Scholar]
- Perrett S.P, Ruiz B.P, Mauk M.D. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz L.W, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus). Journal of Comparative & Physiological Psychology. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Schreurs B.G, Alkon D.L. Rabbit cerebellar slice analysis of long-term depression and its role in classical conditioning. Brain Research. 1993;631:235–240. doi: 10.1016/0006-8993(93)91540-9. [DOI] [PubMed] [Google Scholar]
- Schreurs B.G, Sanchez-Andres J.V, Alkon D.L. Learning-specific differences in Purkinje-cell dendrites of lobule HVI (Lobulus simplex): Intracellular recording in a rabbit cerebellar slice. Brain Research. 1991;548:18–22. doi: 10.1016/0006-8993(91)91100-f. [DOI] [PubMed] [Google Scholar]
- Sears L.L, Steinmetz J.E. Acquisition of classically conditioned-related activity in the hippocampus is affected by lesions of the cerebellar interpositus nucleus. Behavioral Neuroscience. 1990;104:681–692. doi: 10.1037//0735-7044.104.5.681. [DOI] [PubMed] [Google Scholar]
- Shinkman P.G, Swain R.A, Thompson R.F. Classical conditioning with electrical stimulation of cerebellum as both conditioned and unconditioned stimulus. Behavioral Neuroscience. 1996;110:914–921. doi: 10.1037//0735-7044.110.5.914. [DOI] [PubMed] [Google Scholar]
- Skelton R.W. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behavioral Neuroscience. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Solomon P.R, Vander Schaaf E.R, Thompson R.F, Weisz D.J. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Stanton M.E, Freeman J.H, Jr, Skelton R.W. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E. Brain substrates of classical eyeblink conditioning: A highly localized but also distributed system. Behavioural Brain Research. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E, Kim J, Thompson R.F. Biological models of associative learning. In: Gallagher M, Nelson R, editors. Handbook of psychology: Vol. 3. Biological psychology. New York: Wiley; 2002. pp. 499–541. [Google Scholar]
- Steinmetz J.E, Lavond D.G, Thompson R.F. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E, Logan C.G, Rosen D.J, Thompson J.K, Lavond D.G, Thompson R.F. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences (USA) 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz J.E, Logue S.F, Steinmetz S.S. Rabbit classically conditioned eyelid responses do not reappear after interpositus nucleus lesion and extensive post-lesion training. Behavioural Brain Research. 1992;51:103–114. doi: 10.1016/s0166-4328(05)80317-1. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E, Rosen D.J, Chapman P.F, Lavond D.G, Thompson R.F. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E, Sengelaub D.R. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. I. Anatomical evidence for direct projections from the pontine nuclei to the cerebellar interpositus nucleus. Behavioral & Neural Biology. 1992;57:103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Swain R.A, Shinkman P.G, Nordholm A.F, Thompson R.F. Cerebellar stimulation as an unconditioned stimulus in classical conditioning. Behavioral Neuroscience. 1992;106:739–750. doi: 10.1037//0735-7044.106.5.739. [DOI] [PubMed] [Google Scholar]
- Swain R.A, Shinkman P.G, Thompson J.K, Grethe J.S, Thompson R.F. Essential neuronal pathways for reflex and conditioned response initiation in an intracerebellar stimulation paradigm and the impact of unconditioned stimulus preexposure on learning rate. Neurobiology of Learning & Memory. 1999;71:167–193. doi: 10.1006/nlme.1998.3872. [DOI] [PubMed] [Google Scholar]
- Yeo C.H, Hardiman M.J, Glickstein M. Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behavioural Brain Research. 1984;13:261–266. doi: 10.1016/0166-4328(84)90168-2. [DOI] [PubMed] [Google Scholar]
- Yeo C.H, Hardiman M.J, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Experimental Brain Research. 1985a;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- Yeo C.H, Hardiman M.J, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. III. Connections of cerebellar lobule HVI. Experimental Brain Research. 1985b;60:114–126. doi: 10.1007/BF00237024. [DOI] [PubMed] [Google Scholar]
- Young R.A, Cegavske C.F, Thompson R.F. Tone-induced changes in excitability of abducens motoneurons and of the reflex path of nictitating membrane response in rabbit (Oryctolagus cuniculus). Journal of Comparative and Physiological Psychology. 1976;90:424–434. doi: 10.1037/h0077219. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ni H, Harper R.M. A miniaturized cryoprobe for functional neuronal blockade in freely-moving animals. Journal of Neuroscience. 1986;16:79–87. doi: 10.1016/0165-0270(86)90010-5. [DOI] [PubMed] [Google Scholar]