Summary
The alignment of the left-right (LR) body axis relative to the anteroposterior (AP) and dorsoventral (DV) axes is central to the organization of the vertebrate body plan and is controlled by the node/organizer. Somitogenesis plays a key role in embryo morphogenesis as a principal component of AP elongation. How morphogenesis is coupled to axis specification is not well understood. We demonstrate that Wnt3a is required for LR asymmetry. Wnt3a activates the Delta/Notch pathway to regulate perinodal expression of the left determinant, Nodal, while simultaneously controlling the segmentation clock and the molecular oscillations of the Wnt/βcatenin and Notch pathways. We provide evidence that Wnt3a, expressed in the primitive streak and dorsal posterior node, acts as a longe-range signaling molecule, directly regulating target gene expression throughout the node and presomitic mesoderm. Wnt3a may also modulate the symmetry-breaking activity of mechanosensory cilia in the node. Thus Wnt3a links the segmentation clock and AP axis elongation with key left-determining events, suggesting that Wnt3a is an integral component of the trunk organizer.
Introduction
The AP body axis is the first axis to be established during the formation of the mammalian body plan. The LR axis is specified last, and is oriented orthogonally to the pre-existing AP and DV axes. The specification and coordination of all three vertebrate body axes is controlled by a small group of cells known as the Spemann-Mangold organizer (Niehrs, 2004). A transient structure, termed the node, is generally considered to be the murine equivalent of the Spemann-Mangold organizer, however the node first forms at the anterior end of the primitive streak of the gastrulating embryo on embryonic day (E) 7.5, well after AP polarity has been established. The timing of node formation correlates well with LR axis specification, and with the beginning of somitogenesis and the development of the trunk. Somitogenesis generates the segmental structures of the trunk and is a major morphogenetic force driving the elongation of the AP axis. The node plays an important role in trunk development since node ablation results in the loss of LR and DV polarity, retarded somite formation and shortened trunks (Davidson et al., 1999). Thus the node functions as a trunk organizer, coordinating axis determination with trunk elongation.
Members of the transforming growth factor-β (Tgfβ) family, specifically Nodal, Lefty1 and Lefty2, are the first genes to be asymmetrically expressed along the LR axis (Hamada et al., 2002). Nodal is expressed in the periphery of the node, where it functions as the left-determinant (Brennan et al., 2002; Saijoh et al., 2003). Nodal transcription is controlled by the Notch signaling pathway. Activation of Notch receptors by the ligand Delta-like 1 (Dll1), leads to the cleavage and nuclear translocation of the Notch intracellular domain, where it acts as a transcription factor when bound to the DNA-binding protein RBP-J (Schweisguth, 2004). Loss of function mutations in components of the Notch pathway lead to loss of LR asymmetry, and RBP-J binding sites found within the Nodal node-specific enhancer are required for Nodal expression in the node (Krebs et al., 2003; Raya et al., 2003). These data demonstrate that Nodal is a direct target gene of the Notch signaling pathway however the relationship between Notch activity and symmetry-breaking events in the node is not clear.
Cilia emanating from the ventral surface of the node play a crucial role in the breaking of bilateral symmetry (McGrath and Brueckner, 2003). Embryos carrying mutations in genes required for cilia formation or motility display laterality defects (Marszalek et al., 1999; Nonaka et al., 1998, Supp et al., 1999). Motile cilia generate a leftward flow of extraembryonic fluid at the node, termed nodal flow, that is necessary for the generation of LR asymmetry (Nonaka et al., 1998; Okada et al., 1999). Artificial reversal of nodal flow is sufficient to reorient the LR axis (Nonaka et al., 2002) demonstrating that nodal flow is both necessary and sufficient for LR axis specification. These experiments led to the development of the morphogen flow model that proposed that nodal flow, generated by node cilia, set up a morphogen concentration gradient that directs asymmetric gene expression at the node (Nonaka et al., 1998; Okada et al., 1999).
A second population of node cilia, known as mechanosensory cilia, have been proposed to participate in LR determination, largely due to the observation that mutations in the Polycystic kidney disease 2 (Pkd2) gene cause abnormal LR development (Pennekamp et al., 2002). Pkd2 encodes polycystin-2 (PC2), a Ca2+ permeable cation channel expressed in node cilia that is necessary for the generation of asymmetric Ca2+ flux (McGrath et al., 2003). These results led to the development of the two-cilia model for LR initiation in which a centrally-located population of Lrd-containing motile cilia generate nodal flow, while a second population of PC2-expressing nonmotile mechanosensory cilia sense nodal flow on the left side of the node and convert it into an asymmetric Ca2+-dependent signal transduction event (McGrath and Brueckner, 2003; Tabin and Vogan, 2003).
Activation of the Wnt/βcatenin pathway by members of the Wnt family of secreted signaling molecules elevates levels of βcatenin, a transcription cofactor with T cell factor/lymphoid enhancer factor (Tcf/Lef), leading to the activation of target genes (Wnt homepage, http://www.stanford.edu/~rnusse/wntwindow.html). Although it is well-known that Wnts are important molecular components of the vertebrate organizer (Niehrs, 2004), playing critical roles in AP patterning (Yamaguchi, 2001), little is known about the potential roles that Wnts may play in LR determination. Gain of function experiments in the chick embryo have implicated the Wnt/βcatenin pathway in LR patterning (Rodriguez-Esteban et al., 2001), however loss of function mutations have not demonstrated a requirement for Wnts in this process. Interestingly, of the 19 known Wnt genes, Wnt3a is the only one whose expression initiates in the gastrulating mouse embryo at E7.5 (Takada et al., 1994), a stage that correlates with node formation, LR determination and somitogenesis. We hypothesized that Wnt3a may be an important component of the trunk organizer, functioning to coordinate LR axis specification with trunk development.
Materials and Methods
Whole-mount In Situ Hybridization (WISH)
The original cDNA clones described in the literature were used as templates for the generation of cRNA probes. Details are available upon request. WISH was performed as previously described (Wilkinson and Nieto, 1993). Embryos were photographed on a Leica stereoscope or a Zeiss Axiophot compound microscope. Unless indicated otherwise, at least 4 mutant embryos were examined with each probe, and all yielded similar results.
Antibodies
The following reagents were obtained commercially: mouse monoclonal anti-βcatenin (BD Transduction Laboratories), anti-acetylated Tubulin, clone 6-11B-1(Sigma), goat polyclonal anti-PC1 (M-20)(Santa Cruz), Rhodamine-Phalloidin, DAPI, anti-mouse IgG(H+L) goat Alexa-Fluor 488 and anti-goat IgG(H+L) donkey Alexa-Fluor 488 (Molecular Probes), anti-rabbit IgG(H+L) goat Cy3, anti-mouse IgG(H+L) goat Cy3 and Cy5 (Amersham). The YCC anti-PC2 antibody directed against amino acids 687–962 of human PC2 was previously characterized (Cai et al., 1999).
Whole mount immunofluorescence and confocal microscopy
Embryos were fixed with 2% PFA for 20 minutes at room temperature, washed with PBS and stored in 0.1% sodium azide/PBS at 4°C until use. Embryos were permeabilized with 0.1% Triton X-100 and 100 mM Glycine in PBS for 10 minutes at room temperature, blocked with 10% calf serum, 0.1% BSA (Sigma) and 3% normal goat serum (NGS) in TBST (20 mM Tris-HCl, pH 8.0, 150 mM NaCl. 0.05% Tween-20), and then incubated with primary or secondary antibodies diluted in TBST containing 0.1% BSA and 1.5% NGS. All blocking and antibody incubation steps were performed overnight at 4°C followed by multiple TBST washes. For imaging (Zeiss LSM510), embryos were placed in Glass Bottom Culture Dishes (MatTek Corporation) in PBS containing 50% Vectashield mounting medium (Vector Labs), or node regions were dissected and mounted on a slide glass with a spacer in SlowFade Light Antifade Kit (Molecular Probes). Four Wnt3a+/− and four Wnt3a−/− 0-2 somite stage embryos were analysed for expression of the cilia markers PC1, PC2 and acetylated tubulin. PC-positive cilia were quantitated manually.
Mice
To make the BATlacZ mouse, two oligos (TBS-1; 5′-AAT TCA GAA TCA TCA AAG GAC CT-3′ and TBS-2; 5′-AAT TAG GTC CTT TGA TGA TTC TG-3′) containing a Tcf/Lef binding site sequence flanked by EcoRI sites, were annealed and ligated to construct an 8X multimer, and then subcloned into pBluescript to generate a plasmid designated 8X TBS-pBS. A 130bp Xenopus Siamois minimal promoter was amplified by PCR from p01234 (kindly provided by D. Kimelman) using primers xSiamois-1 (5′-CGT GAA TTC TAT TTA TAT TTT TTT CAT-3′) and xSiamois-2 (5′-AGC GGA TCC CTC TGT CTC CCA AAA TG -3′), and then subcloned into the EcoRI/BamHI sites of 8X TBS-pBS. NLS-lacZ from pCS-nβgal was subcloned into the BamHI/XbaI sites of 8X TBS-xSiamois-pBS to generate the BATlacZ transgene. Transgenic mice were generated in the Transgenic Core Facility by pronuclear injection following standard procedures. From the four lines that were generated, the one that most faithfully replicated domains of Wnt signaling was designated as the BATLacZ line. These mice are similar in design to the BATgal mice of Maretto et al. (2003). All animal experiments were performed in accordance with the guidelines established by the NCI-Frederick Animal Care and Use Committee.
Results
Wnt3a is expressed in the node and is required for LR axis formation
As a first step towards examining a potential role for Wnt3a in LR determination, we directly compared the expression of Wnt3a with that of the left determinant Nodal by WISH. Wnt3a was symmetrically expressed in the primitive streak and dorsal posterior node, immediately adjacent to Nodal in the ventral node, during all LR determination stages (E7.75-8.5) (Supp. Fig. 1). After the node disappeared (~E8.5), Wnt3a remained on in the streak, and later in the tailbud, during all somitogenesis and axis extension stages (Takada et al., 1994).
Supplementary Figure 1. Wnt3a and Nodal are expressed in adjacent node cells.
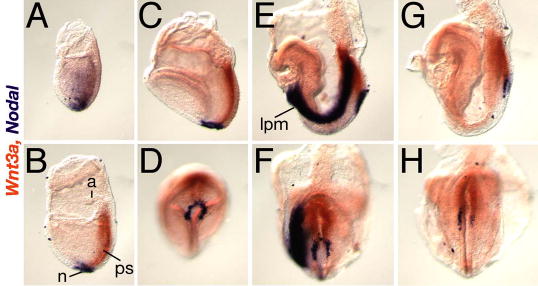
Two color whole-mount in situ hybridization showing Wnt3a (orange) and Nodal (purple) expression in E7.5–8.5 embryos; (A, B, C, E, G) lateral views of left side of embryo, anterior is to the left, (D, F, H) ventral views of the embryos depicted above, posterior end points up. (A) E7.5 early alantoic bud stage; Wnt3a transcripts were first detected in primitive streak ectoderm, prior to node formation, as Nodal expression is down-regulated in embryonic mesoderm and distally restricted to the presumptive node. (B) E7.5 alantoic bud stage, (C, D) E7.75 neural plate stage; note that Wnt3a expression in the ectoderm overlaps Nodal expression in the underlying posterior ventral node endoderm. (E, F) E8.2, 4 somite stage; Nodal is expressed asymmetrically in the node and in the left lateral plate mesoderm (LPM), while Wnt3a continues to be symmetrically expressed in the streak. (G, H) E8.5, 8 somite stage; Nodal expression is undetectable in the left LPM but remains asymmetric in the node, while Wnt3a expression persists in the streak. N, node; ps, primitive streak; a, allantois; lpm, lateral plate mesoderm.
Examination of E8.75–9.5 embryos homozygous for a null allele of Wnt3a (Takada et al., 1994) revealed multiple laterality defects. Although wildtype and Wnt3a+/− hearts invariably looped to the right (Fig. 1A), Wnt3a−/− embryos displayed hearts that looped to the right (45%, n=49)(Fig. 1B), left (31%)(Fig. 1C), or remained in the midline (25%, Supp. Fig. 2B). The direction of axial rotation or embryonic turning was randomized with 47% (n=17) of Wnt3a−/− embryos correctly turning clockwise such that the tail and allantois lay on the right side of the embryo, and 53% turning in the opposite direction (not shown). The heart looping defects were not secondary to earlier defects in cardiogenesis, since several heart markers were expressed normally in the mutants (Supp. Fig. 2 and 3G–J).
Fig. 1. Loss of Wnt3a leads to laterality defects.
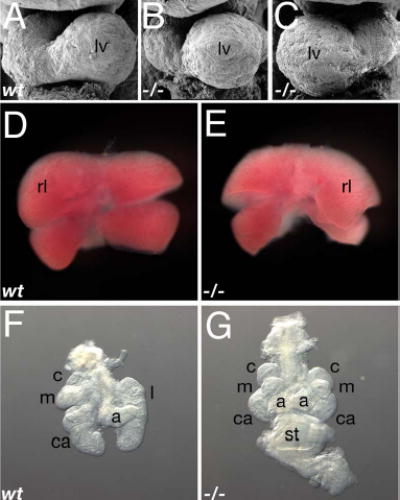
(A–C) SEM micrographs of E9.5 wildtype (A), and Wnt3a−/− (B, C) hearts displayed normal (B) and inverted (situs inversus) looping (C).
(D, E) E11.5 livers. Situs inversus was observed in the asymmetric arrangement of the Wnt3a−/− liver (E), compared to the control (D).
(F, G) E11.5 lungs and stomach. A midline stomach and right pulmonary isomerism was often observed in mutants (G), in contrast to the wildtype lungs (F, stomach not shown). Abbr: lv, left ventricle; rl, right lateral lobe; c, cranial lobe; m, medial lobe; ca, caudal lobe; a, accessory lobe; l, left lobe; st, stomach.
Supplementary Figure 2. Heart looping defects are not due to abnormal cardiac specification.
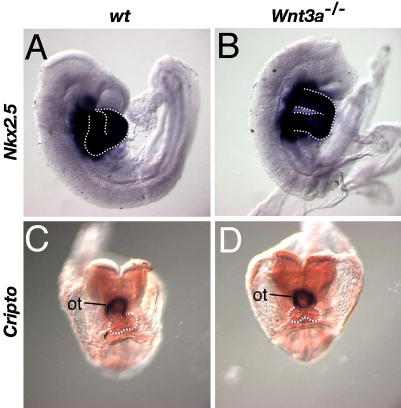
Reduced Wnt/βcatenin signaling is associated with heart formation (Lickert et al., 2002; Marvin et al., 2001). To rule out the possibility that the heart looping defects observed in the Wnt3a mutants were secondary to defects in cardiogenesis, we examined the expression of the heart markers Cripto, and Nkx2.5, the latter of which is required for proper looping morphogenesis (Lyons et al., 1995; Tanaka et al., 1999). WISH was performed on wildtype (A, C) and Wnt3a−/− littermates (B, D) to examine Nkx2.5 expression in the heart at E8.75 (A, B), and Cripto expression in the outflow tract at the 7 somite stage (C, D). Both genes continued to be strongly expressed in hearts undergoing aberrant looping morphogenesis. Note that the mutant Cripto-positive outflow tract remained in the midline at stages (E8.5) when it would normally have undergone a rightward shift in wildtype embryos. The white dashed line outlines the looping ventricles. ot, outflow tract.
Supplementary Figure 3. Node and axial markers are expressed in Wnt3a mutants.
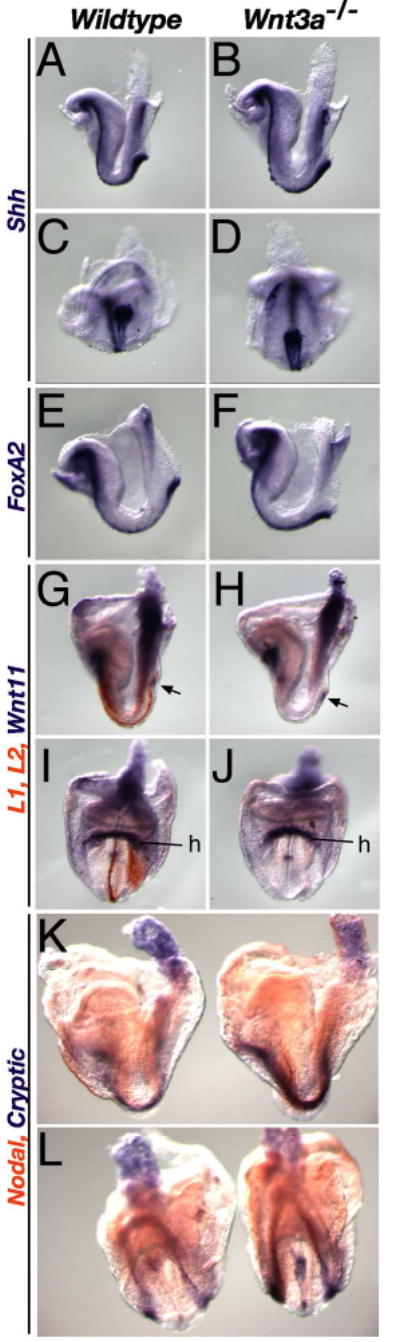
WISH of E8.2–8.5 wildtype (left) and mutant (right) embryos, analyzed for marker gene expression. Markers of the node and axial mesendoderm, such as Shh (A–D) and FoxA2 (E, F), continued to be expressed in 3-4-somite stage Wnt3a−/− embryos (B, D, F). (G–J) Despite the loss of Lefty1 and Lefty2 (orange), normally expressed in the midline PFP and left LPM (G, I), Wnt11 (purple) continued to be expressed in the mutant node (arrow), posterior primitive streak and heart (H, J) as it was in wildtype embryos (G, I). (K, L) Two color WISH analysis of Cryptic (purple) and Nodal (orange) expression. Cryptic was expressed in the midline, including the node, and bilaterally in the LPM of wildtype embryos (left embryos). Cryptic expression in the LPM and node was up-regulated in 6–7 somite stage Wnt3a−/− embryos, suggesting that Wnt3a, directly or indirectly, represses Cryptic transcription. We have previously shown that Brachyury is also expressed in the Wnt3a−/− node and notochord at these stages (Yamaguchi et al., 1999). C, D, L, ventral posterior view; I, J, anterior view; the rest are lateral views; h, heart.
To assess laterality defects in later-developing visceral organs, we analyzed Wnt3a−/− embryos at E11.5–12.5. We were unable to examine the full spectrum of laterality defects since mutants lack posterior organs and generally die by E12.5 (Takada et al., 1994). Nevertheless, we were able to assess the laterality of the heart, lungs, liver and stomach. Of the 19 Wnt3a−/− embryos in which multiple organs were assessed, 16 displayed heterotaxy, while the remaining 3 embryos displayed normal situs. Cardiac laterality was assessed in an additional 20 mutants. Dextrocardia (situs inversus) or abnormal midline positioning of the heart was observed in 38.4% of mutants (n=39). Right pulmonary isomerism, ie. 4 lobes on the left and right instead of the single left lobe normally observed in wildtype embryos, was observed 31.6% of the time (n=19; Fig. 1G), while situs ambiguus (2–4 lobes on either side) was observed in an additional 21.1%. Left isomerism was not observed. The liver was particularly sensitive to perturbations in left-right determination as 78.9% (n=19) of mutant embryos displayed abnormalities including situs inversus (10.5%)(Fig. 1E), and situs ambiguus (68.4%). Abnormal midline (Fig. 1G), or right-sided, positioning of the stomach was noted 37.5% of the time (n=16). Thus laterality defects were observed in all of the organs that we were able to assess, indicating that Wnt3a plays an early and critical role in LR determination.
Wnt3a is necessary for asymmetric gene expression
Wnt3a−/− embryos were examined for the expression of the asymmetrically expressed genes Nodal, Lefty1, Lefty2, and Pitx2 at stages prior to the morphological manifestation of LR or AP phenotypes. Nodal transcripts were detected in the ventral node of Wnt3a−/− mutants at presomitic, headfold (E7.75–8) stages however the spatial domain was smaller (Fig. 2B, F) compared to wildtype controls (Fig. 2A, E; Lowe et al., 1996; Collignon et al., 1996). This domain became increasingly restricted to the posterior edge of the mutant node as development proceeded, and was approximately one-third the size of the wildtype domain (cf. Fig. 2H and G) by 2–4 somite stages (Fig. 2D, H, J, N). Nodal mRNA was not detected in the Wnt3a−/− left lateral plate mesoderm (LPM) at these stages (Fig. 2D) when Nodal was normally expressed there in wildtype embryos (Fig. 2C), but was bilaterally expressed in the posterior LPM and streak starting at the 4–5 somite stage (Fig. 2J, N), and remained bilaterally expressed in the LPM (Fig. 2L, P) at stages when Nodal was normally turned off in wt embryos (6–8 somite stages; Fig. 2K, O). The anterior limit of the Nodal LPM expression domain was posteriorized in mutants (arrowhead, Fig. 2L), never extending anteriorly into the heart as in earlier staged wildtype embryos (arrowhead, Fig. 2I).
Fig. 2. Wnt signaling controls LR asymmetric gene expression.
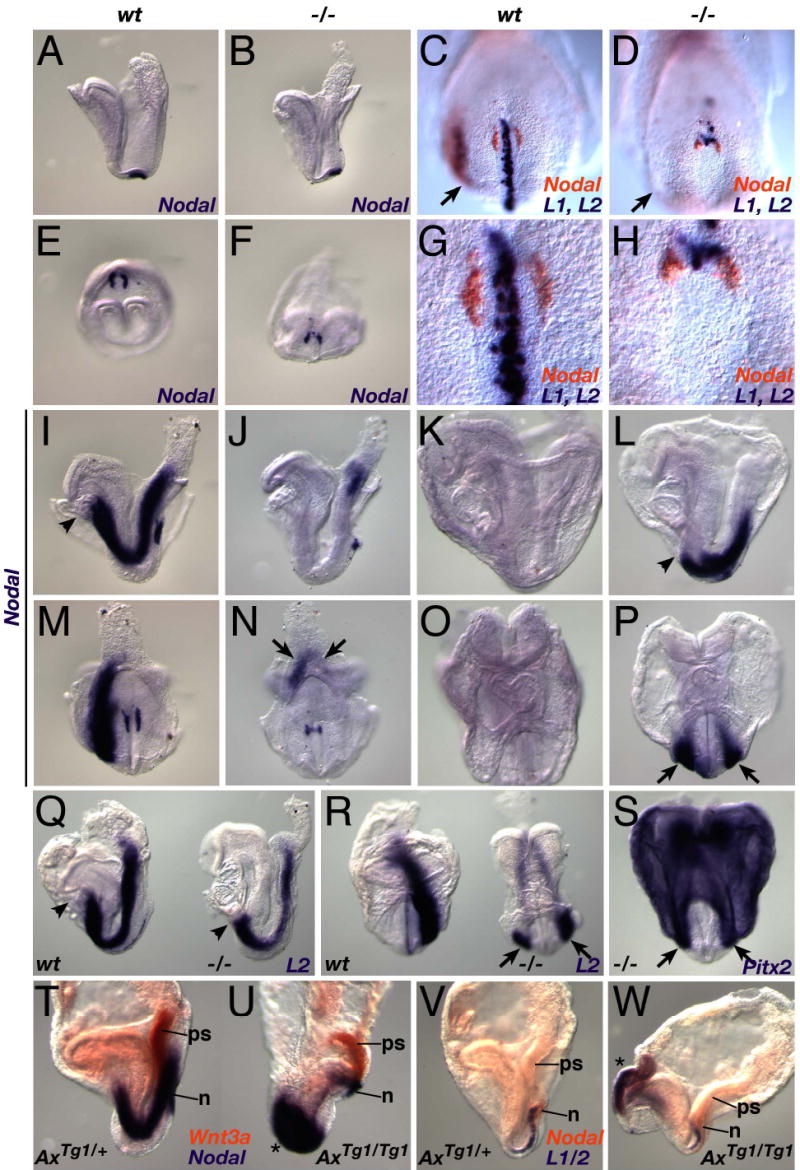
(A–P) WISH analysis of Nodal expression (see text for details). Headfold-stage wildtype (A, E), and Wnt3a−/− (B, F) embryos express Nodal (purple) in the node. Two color WISH showing Nodal (orange), and Lefty1 and Lefty2 expression (purple), in wildtype (C, G) and mutant (D, H) 3 somite stage embryos. (G, H) High power ventral views of the nodes of the wildtype and mutant embryos depicted in (C) and (D). Nodal expression in wildtype 4 somite (I, M) and similarly-staged Wnt3a−/− (J, N) embryos. Arrows in N indicate bilateral Nodal expression in the mutant posterior LPM. Nodal was not expressed in the wildtype LPM after the 6–7 somite stage (K, O), but was bilaterally expressed in the mutant LPM (L, P).
(Q–S) Similar abnormal expression patterns were observed for Lefty2 in 7-somite stage mutants (right embryos in Q and R, cf. to 4-somite wildtype embryos on the left side), and for Pitx2 expression in 6-somite mutants (S).
(T, U) Two color WISH showing Nodal (purple) and Wnt3a (orange) expression in 4- somite wildtype (T) and AxinTg1/Tg1 (U) littermates.
(V, W) Lefty1/2 (purple) and Nodal (orange) expression in 2-somite wildtype (V) and AxinTg1/Tg1 (W) littermates. Asterisks indicate ectopic, bilateral expression of Nodal (U) and Lefty1/2 (W) in anterior domains.
A–D, I–L, Q, T–W, lateral views; E–H, M, N, S, ventral posterior views; O, P, R, anterior views; arrows indicate the LPM; arrowheads, the anterior limit of LPM expression. Abbr.: ps, primitive streak; n, node.
Lefty1 and Lefty2 are required for proper LR patterning, functioning as negative regulators of Nodal (Hamada et al., 2002). Lefty2 is a direct target gene of Nodal, and is normally expressed in the left LPM between 3–6 somite stages (Meno et al., 1998; Fig. 2C, Q, R). Lefty2 expression in Wnt3a−/− embryos mirrored Nodal expression, with expression in the LPM initially delayed, then restricted to the posterior streak (Fig. 2D and not shown), and later bilateral and posteriorized (7 somites, Fig. 2Q, R). Similarly, Pitx2, a bicoid-type homeobox gene expressed in the left LPM and heart (Yoshioka et al., 1998), was delayed, and then bilaterally expressed in mutant posterior LPM (Fig. 2S and data not shown). Lefty1 is asymmetrically expressed in the left prospective floor plate (PFP) in wildtype embryos (Meno et al., 1998; Fig. 2C, G), but was never detected in the mutant PFP, being expressed in only a few individual cells in the posterior node and anterior streak (Fig. 2D, H). Loss of Lefty1 expression is not due to the physical loss of a midline barrier (Hamada et al., 2002) since several markers, including Shh, FoxA2, T, Wnt11, Gdf1, and Cryptic were easily detected in the mutant node, notchord or PFP (Supp. Fig. 3A–L and data not shown). Thus molecular marker analyses demonstrate that a cascade of genes necessary for the generation of LR asymmetry are abnormally expressed in the Wnt3a−/− node and LPM, indicating that Wnt3a functions early in the genetic hierarchy of LR determination.
If Wnt3a is upstream of left determining genes, then ectopic activation of Wnt signaling should alter their expression. To test this, we examined the expression of left determining genes in embryos lacking Axin, a negative regulator of the Wnt/βcatenin signaling pathway (Zeng et al., 1997). Homozygous AxinTg1 embryos continued to express Wnt3a and Nodal normally in the primitive streak, however Nodal expression in the node was slightly expanded (Fig. 2U and W) and large ectopic domains of symmetrical Nodal (Fig. 2U) and Lefty1/2 (Fig. 2W) expression were observed. Thus, both gain and loss of function alleles of genes in the Wnt/βcatenin signaling pathway lead to aberrant expression of left determining genes.
Cilia are structurally normal but display reduced polycystin-1 (PC1) expression
To determine whether a relationship between Wnt3a and cilia structure or function exists, we first determined whether cilia were present on the Wnt3a−/− node. Scanning electron microscopy (SEM) analysis of mutant nodes at E7.75 revealed the presence of monocilia in the ventral node (Fig. 3B), similar to that observed in wildtype nodes (Fig. 3A). Immunofluorescent labeling of cilia with anti-acetylated tubulin confirmed this, and further showed that the general morphology of the node remained normal (cf. Fig. 3C and D, and data not shown). Quantitation of node cilia in Wnt3a+/− (mean=158+/−13.7, n=4) and Wnt3a−/− (mean=144+/−30.7, n=4) stage-matched embryos revealed no significant differences in total cilia number. The presence of structurally normal cilia indicates that Wnt3a does not lie upstream of genes required for ciliary structure such as Kif3a or Kif3b since these mutants lack cilia (Marszalek et al., 1999; Nonaka et al., 1998; Takeda et al., 1999).
Fig. 3. Examination of cilia in the Wnt3a−/− mutants.
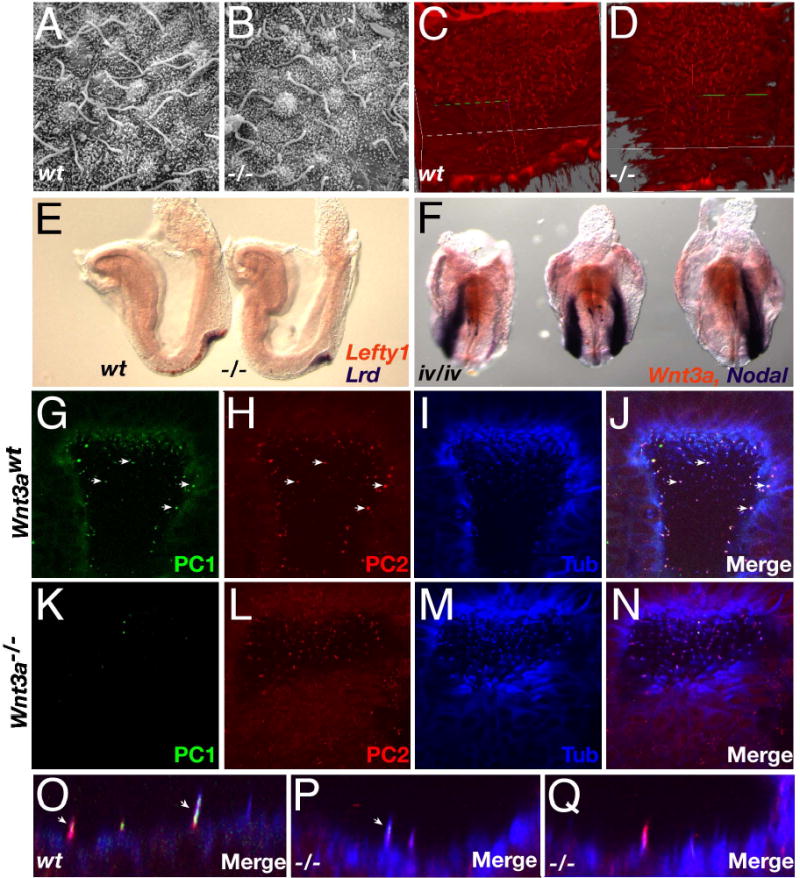
(A, B) High-power SEM micrographs of cilia in wildtype (A) and Wnt3a−/− (B) nodes of headfold stage embryos (~E7.75–8). (C, D) Projection images of node cilia visualized using anti-acetylated tubulin antibodies. (E) Two color WISH analysis of Lefty1 (orange) and Lrd (purple) expression in 3 somite wildtype (left) and Wnt3a−/− (right) embryos. (F) Wnt3a (orange) and Nodal (purple) expression in three iv/iv embryos. (G–Q) Confocal microscopy images of PC1 (G, K), PC2 (H, L), acetylated tubulin (I, M) and the merged images (J, N, O, P, Q) in E7.75 wildtype (G–J, O) and Wnt3a−/− (K–N, P, Q) nodes. The abundance of white spots in global views of the node indicated co-expression of all three markers in individual wildtype cilium (arrows, G–J). In contrast, Wnt3a−/− cilia predominantly appeared as purple spots (N) indicating coexpression of only PC2 and tubulin. Views were selected to present sufficient numbers of labeled cilia and therefore depict slightly different regions of the wildtype and mutant node, however, images are representative of the entire node. Profiles of labeled cilia illustrate the coexpression of PC1 and PC2 in distinct spatial domains in wildtype cilia (arrows, O). Rare mutant cilia coexpressed PC1 and PC2 but these domains were unusually small and not easily detected (arrow, P). Cilia co-expressing PC2 and tubulin were easily detected in the mutant node (Q).
To assess ciliary motility, we examined Wnt3a−/− embryos for the expression of Left-right dynein (Lrd), which encodes a ciliary motor protein. Lrd was easily detected in the mutant node (Fig. 3E, right embryo). Conversely, Wnt3a was normally expressed in inversus viscerum (iv) embryos (which carry a mutation in Lrd (Supp et al., 1997)), including embryos that displayed reversed or bilateral Nodal expression (Fig. 3F). Crosses between Wnt3a and iv did not reveal genetic interactions in transheterozygotes or compound mutants indicating that Wnt3a and iv function in independent genetic pathways (data not shown). In addition, the axonemal dynein heavy chain gene Dnahc5′ (Ibanez-Tallon et al., 2002) was also expressed in the Wnt3a−/− node (not shown). We suggest that ciliary motility was unaffected by the absence of Wnt3a.
The presence of mechanosensory cilia in Wnt3a mutants was evaluated by examining nodes for the expression of polycystin-1 (PC1) and PC2. The Pkd1 gene product, PC1, interacts with PC-2 and is thought to control the gating of PC2 Ca2+ channels (Delmas et al., 2004). While cilia co-expressing PC1, PC2 and acetylated tubulin were easily found in the wildtype node (arrows, Fig. 3J, O), similarly labeled cilia were rarely found in the mutant (Fig. 3N, P, Q). Interestingly, PC1 expression was significantly down-regulated (p<0.0001, Welch’s t-test) in the Wnt3a−/− node cilia (Fig. 3K, N), with only 6.8+/−5.8% (n=4 embryos) of the mutant cilia expressing detectable levels of PC1, compared to the 46.9+/−0.9% (n=4) of cilia that were PC1-positive in Wnt3a+/− embryos (Fig. 3G, J). PC2 was strongly expressed in more than 92% of central and peripheral cilia in both wildtype and Wnt3a−/− nodes (Fig. 3H, L). The rare cilium that co-expressed PC1 and PC2 in Wnt3a−/− nodes expressed PC1 weakly and in a much smaller spatial domain (arrow, Fig. 3P) than in wildtype cilia suggesting that mechanotransduction may be perturbed in the absence of Wnt3a.
Wnt3a signals directly to the node and presomitic mesoderm via βcatenin
Although Wnt3a is expressed in the dorsal posterior node, gene expression in the Wnt3a mutants is perturbed in both the dorsal and ventral node. To determine which tissues respond directly to Wnt signals, we examined two independent transgenic lines that report sites of presumed Wnt/βcatenin activity in vivo. Both the TOPgal and BATlacZ (see Materials and Methods) transgenes were expressed in the node, primitive streak and posterior mesoderm during LR determination stages (Fig. 4A, B, C, E, Supp. Fig. 4A–C; and Merrill et al., 2004). The node was the strongest site of β-galactosidase (βgal) expression in TOPgal embryos at early somite stages (Fig. 4A), with particularly robust expression detected in the ventral node (Fig. 4B).
Fig. 4. Expression of Wnt/βcatenin reporter transgenes in vivo is Wnt3a-dependent.
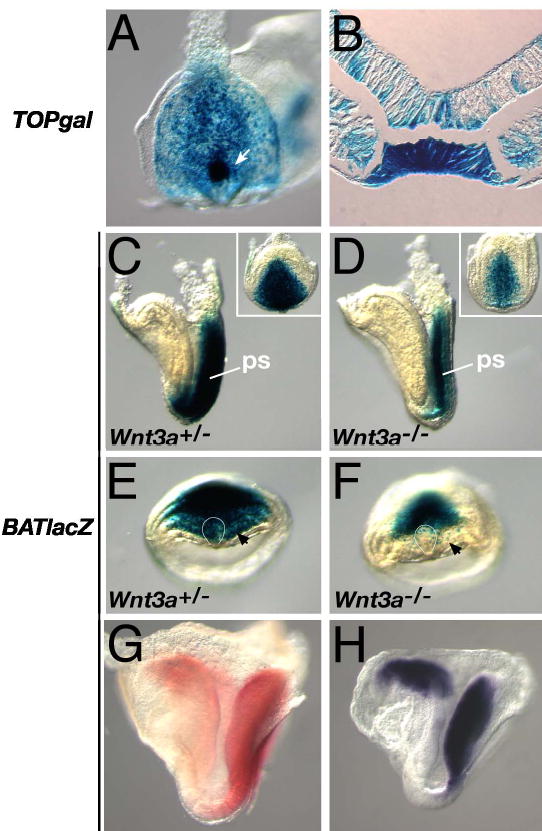
(A) A 5 somite TOPgal embryo showed strong expression in the node (arrow), primitive streak (ps), and posterior mesoderm. (B) Cross-section through the node of embryo shown in (A). (C, E) A headfold stage BATlacZ embryo showing βgal expression in the primitive streak (for posterior view, see inset), anterior psm (black arrow) and the node (curved white line). (D, F) BATlacZ expression was reduced in the Wnt3a−/− streak, and was not expressed in the node (curved white line) and anterior psm (black arrow). (G) βgal activity in 6 somite BATlacZ embryo visualized with Salmon-gal (red). (H) lacZ mRNA expression in a 5 somite BATlacZ embryo visualized by WISH. (A, and insets in C, D) Ventral-posterior views. (E, F) Ventral views, left side of the embryo is on the left. (C, D, G, H) Lateral views, anterior is to the left.
Supplementary Figure 4. TOPgal expression from E7.5–8.5.
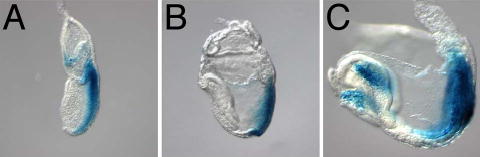
The TOPgal reporter indicates that the canonical Wnt/βcatenin signaling pathway is active in the primitive streak at E7.5, when Wnt3a is first expressed there (A). Expression is easily detected in the node at E7.75 (B), persisting in the node and streak through E8.5 stages (C). Expression is also detected in the mid-hindbrain and heart tube. Staining was not observed in axial derivatives of the node such as the notochord or PFP.
Although the TOPgal and BATlacZ reporters were both expressed in the node and streak, BATlacZ expression was stronger and extended further anteriorly, through the presomitic mesoderm (psm) where Wnt3a functions to regulate segmentation (Aulehla et al., 2003), to reach an anterior limit at the base of the future hindbrain (cf. Fig. 4C and Supp. Fig. 4B). This domain closely paralleled the expression of Wnt8 (Bouillet et al., 1996). This suggested that the BATlacZ transgene was a more sensitive and accurate reporter of Wnt/βcatenin activity than TOPgal since Wnt8 and Wnt3a are co-expressed at these stages and likely signal via βcatenin. We therefore chose to examine BATlacZ expression in the Wnt3a mutants. BATlacZ expression was down-regulated in the E7.75 primitive streak in the absence of Wnt3a (Fig. 4D, inset), compared to controls (Fig. 4C, inset), and was strikingly absent from the node (cf. Fig. 4F with 4E) and anterior psm (arrow, Fig. 4F). This loss of anterior Wnt/βcatenin reporter expression in Wnt3a−/− embryos suggests that Wnt3a, emanating from a posterior, primitive streak source, functions at a distance to directly activate target genes in the node and anterior psm.
Alternatively, the lack of reporter expression in the mutant node and anterior psm could simply be due to down-regulation of the reporter and the consequent reduced levels of the stable βgal protein. To distinguish between these possibilities, we compared the spatial domain of βgal activity in BATlacZ embryos with that of the lacZ mRNA itself. WISH revealed that lacZ mRNA expression (Fig. 4H) was coincident with βgal activity (Fig. 4G) and extends into the anterior psm, indicating that the βgal expression domain is not defined by βgal stability but rather by direct transcriptional activation of the transgene by Wnt/βcatenin signaling. We suggest that Wnt3a is a long-range signaling molecule capable of activating Wnt/βcatenin target genes in node and anterior psm cells at least 15–20 cell diameters away.
The observation that the Wnt/βcatenin reporters were expressed in the node in a Wnt3a-dependent manner strongly indicates that Wnt3a signals in the node via βcatenin. To examine this hypothesis directly, we analysed the expression of βcatenin in the node during LR axis specification. A transient, subtle, asymmetric gradient of cytoplasmic and membrane-associated βcatenin protein was observed in the node in 50% (8/16) of the E7.75 embryos examined (Fig. 5A, B), with elevated levels found on the left side. Asymmetric βcatenin was not observed in Wnt3a−/− embryos at these stages (not shown).
Fig. 5. Asymmetric distribution of canonical Wnt/βcatenin signaling pathway components in the node.
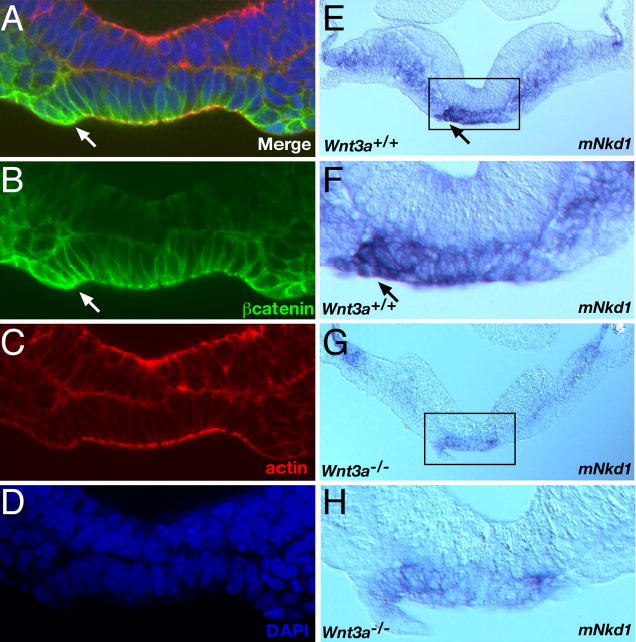
All images are posterior views of cross sections, the left side of the embryo is facing left. (A) Merge of confocal microscopy images of E7.75 wildtype embryo labeled with anti-βcatenin antibody (B), rhodamine phalloidin (C), and DAPI (D). (E–H) Expression of the Wnt/βcatenin target gene mNkd1 in 4 somite stage wildtype (E, F) and Wnt3a−/− embryos. Arrows indicate sites of asymmetric expression in the wildtype node. Boxed regions in E and G are represented as high-power views of the nodes in panels F and H, respectively. Note that all assessments of mNkd1 distribution were performed on whole embryos by WISH (not shown), and subsequently sectioned for confirmation and clarity.
We reasoned that if the transcriptional activator βcatenin was asymmetrically expressed in the node, then target genes of the canonical pathway should also be asymmetrically activated in the node. One such target gene, mNkd1, a mammalian homolog of the Drosophila segment polarity gene naked cuticle (Wharton et al., 2001; Yan et al., 2001), was expressed symmetrically in the primitive streak, psm and node at E7.75, but was asymmetrically distributed in the node by the 2 somite stage. Elevated levels were observed on the left side of the ventral node, while expression in the psm remained symmetric (Fig. 5E, F). Analysis of mNkd1 expression in 3–6 somite stage Wnt3a−/− embryos (n=4) revealed that transcript levels and asymmetric expression were reduced in the node (Fig. 5G, H). Expression in the mutant psm was also down-regulated. Thus Wnt3a signals directly to the psm and ventral node to activate expression of the Wnt/βcatenin target gene mNkd1.
Wnt3a regulates the Dll1/Notch pathway during LR determination and somitogenesis
Since the Dll1/Notch signaling pathway directly controls Nodal expression in the node (Raya et al., 2003; Krebs et al., 2003), we investigated the possibility that the abnormal Nodal expression domain in the Wnt3a mutant node may be due to aberrant Notch signaling. Using Dll1 and Lfng as reporters of Notch activity (Raya et al., 2003), we examined Notch activity in Wnt3a−/− embryos. At E8, Dll1 was expressed in the streak and in psm (Fig. 6A) in a pattern similar to the Wnt reporter (Fig. 4C). Dll1 was expressed in psm cells immediately adjacent to Nodal-expressing peripheral ventral node cells (Fig. 6C). Notably, expression of Dll1 in Wnt3a−/− psm was posteriorized such that Dll1-expressing cells only contacted the posterior-most node (Fig. 6B, D). This domain correlated well with the abnormally small domain of Nodal expression, suggesting that Wnt3a regulates Nodal expression indirectly, via Dll1 and the Notch signaling pathway.
Fig. 6. Components of the Notch pathway participate in a Wnt3a-dependent pathway that controls laterality and somitogenesis.
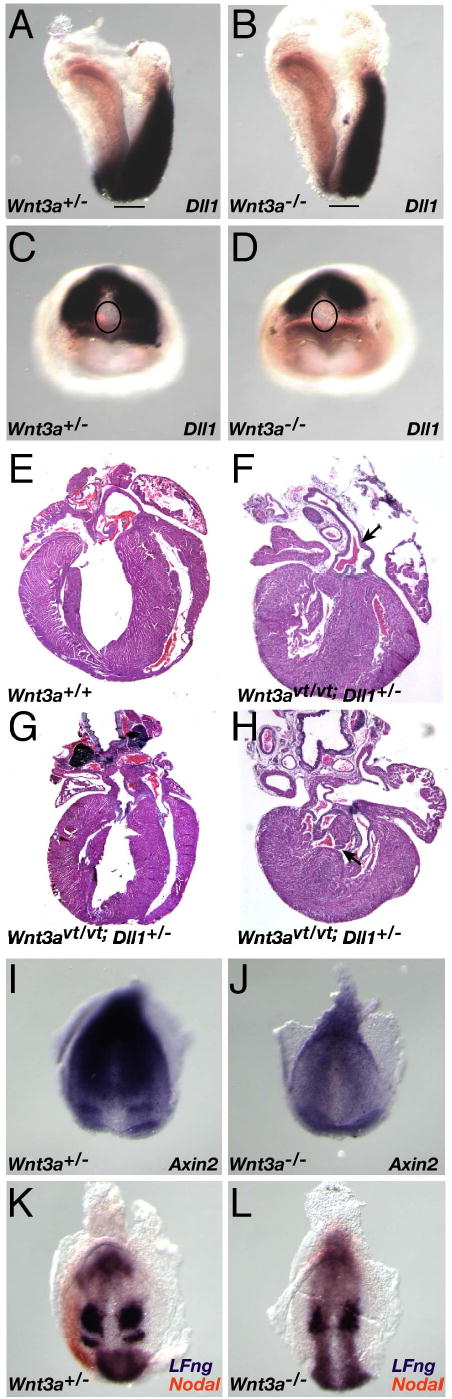
(A–D) WISH analysis of Dll1 expression in E8 wildtype (A, C) and Wnt3a−/− (B, D) embryos. Note that Dll1 expression in the psm surrounds the wildtype node (C), but only abuts the posterior-most node in a Wnt3a−/− embryo (D). The lines in A and B, and ovals in C and D, indicate the location of the node. (E–H) Histological sections of wildtype neonatal heart (E), and three examples of cardiac laterality defects in compound Wnt3avt; Dll1 mutants, including PTA (arrow, F), TGA (G) and VSD (arrow, H). Axin2 expression in the streak, psm, and in an anterior stripe (presomite 0) in wildtype 1-somite embryos (I), was down-regulated in Wnt3a−/− mutants and no psm stripes were observed (J). Two color WISH on 4 somite stage embryos illustrating cycling LFng (purple), and Nodal expression (orange) in the wildtype left LPM. Dynamic Lfng expression was not observed in the absence of Wnt3a, as only a single psm stripe was observed in the Wnt3a mutants (L). Nodal was not expressed in the mutant LPM at these stages.
To examine whether Dll1 might function in the same genetic pathway as Wnt3a, we crossed vestigial tail (vt) mice, carrying a hypomorphic allele of Wnt3a (Greco et al., 1996), with mice carrying a targeted allele of Dll1 (Hrabe de Angelis et al., 1997). Analysis of the progeny of Wnt3avt/+; Dll1+/− animals crossed to Wnt3avt/vt indicated that animals of the expected genotypes were born at Mendelian frequencies but reduced viability of Wnt3avt/+; Dll1+/− and Wnt3avt/vt; Dll1+/− compound mutants was observed by weaning stages (Supp. Table 1). Examination of offspring at postnatal stages revealed that cardiac abnormalities were the likely cause of the reduced viability. 14.8% of Wnt3avt/+; Dll1+/− neonatal hearts (n=27; Table 1) displayed situs ambiguus or atrial or ventricular septation defects, and 41.7% of Wnt3avt/vt; Dll1+/− hearts displayed persistent truncus arteriosis (PTA) (Fig. 6F), transposition of the great arteries (TGA) (Fig. 6G), ventricular septation defects (VSD) (Fig. 6H), or other abnormalities consistent with cardiac laterality defects (Maclean and Dunwoodie, 2004). No other visceral laterality defects were observed. These results suggest that Wnt3a and Dll1 participate in a common genetic pathway to regulate cardiac laterality.
Supplementary Table 1.
Offspring from a Wnt3avt/vt;Dll1+/− X Wnt3avt/vt;Dll1+/+
| Genotype | Wnt3avt/+;Dll1+/− | Wnt3avt/+;Dll1+/+ | Wnt3avt/vt;Dll1+/− | Wnt3avt/vt;Dll1+/+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Birth | 3 days | 3 wks | Birth | 3 days | 3 wks | Birth | 3 days | 3 wks | Birth | 3 days | 3 wks |
| Offspringa | 27 | 27 | 22 | 24 | 24 | 24 | 24 | 14 | 14 | 26 | 26 | 26 |
| Frequency (%) | 26.7 | 21.8 | 23.8 | 23.8 | 13.9 | 25.7 | ||||||
| Expected | 25 | 25 | 25 | 25 | ||||||||
| Frequency (%) | ||||||||||||
n=101
Table 1.
Laterality Defects in Neonatal Offspring from Wnt3avt/+/Dll1+/− X Wnt3avt/vt/Dll1+/+ Cross
| Phenotypic Trait | Wnt3avt/+/Dll1+/− | Wnt3avt/+/Dll1+/+ | Wnt3avt/vt/Dll1+/− | Wnt3avt/vt/Dll1+/+ |
|---|---|---|---|---|
| n=27 | n=24 | n=24 | n=26 | |
| situs solitis | 85.2% | 100% | 58.3% | 100% |
| situs inversus or situs ambiguus | 3.7% | 0% | 0% | 0% |
| atrial or ventricular septa defects | 11.1% | 0% | 29.1% | 0% |
| Transposition of the great arteries | 0% | 0% | 4.2% | |
| Common truncus arteriosis | 0% | 0% | 4.2% | 0% |
| stenosis of pulmonary artery | 0% | 0% | 4.2% | 0% |
Analysis of the Wnt3avt allele has shown that Wnt3a and the Dll1/Notch pathways play important roles in somitogenesis at tailbud stages (E9.5 onwards), functioning as integral components of the segmentation clock (Aulehla and Herrmann, 2004; Dubrulle and Pourquie, 2004). Consideration of the temporal and spatial proximity of LR determination (at E7.75–8 in the node) and the onset of somitogenesis (at E8 in the adjacent psm), and the common Wnt3a/Dll1 molecular components, suggests an intimate relationship between LR determination and somitogenesis. This relationship is strengthened by our demonstration that Wnt3a is required for activation of a Wnt/βcatenin reporter in both the node and psm (Fig. 4F). To explore this further, we examined the expression of segmentation clock genes in Wnt3a−/− mutants at these earlier stages. Axin2 is a direct Wnt/βcatenin target gene and negative regulator of the Wnt pathway (Jho et al., 2002; Lustig et al., 2002) and is expressed at E9.5 in a graded, oscillating manner in the tailbud, and in a single stripe in the anterior psm (Aulehla et al., 2003). At E7.75, Axin2 is expressed symmetrically in the wildtype node, streak, and posterior mesoderm (not shown), and an additional stripe of expression in the anterior psm becomes detectable at early somite stages (Fig. 6I). Axin2 expression is downregulated in stage-matched Wnt3a−/− embryos, and the stripe is no longer detectable in the mutant anterior psm (Fig. 6J).
Lunatic fringe (Lfng) expression oscillates in the psm and is dependent upon Notch signaling (Barrantes et al., 1999) and Wnt3a (Aulehla et al., 2003) at tailbud stages. At early somitogenesis and LR determination stages when Nodal is expressed in the left LPM, Lfng is expressed in a dynamic manner that can manifest in a diffuse, patch in the posterior primitive streak, and in the psm as two stripes adjacent, and anterior, to the node (Fig. 6K). Interestingly, LFng is also expressed in the node periphery, overlapping with Nodal expression in the node (Fig. 6K and not shown). Wnt3a−/− mutants (0–7 somites, n=7) displayed only a single abnormally shaped stripe of Lfng expression posterior to the node and no expression was detected in the node periphery (Fig. 6L). More than one set of stripes was never observed suggesting that dynamic, oscillating, Lfng expression did not occur in the absence of Wnt3a. Together, the Axin2 and Lfng expression patterns indicate that the segmentation clock does not function properly at early somitogenesis stages in the absence of Wnt3a. Wnt3a appears to play dual roles at these stages, signaling to the node and psm to regulate LR determination and somitogenesis.
Discussion
We have identified Wnt3a as an important new component of the molecular LR determination pathway. Wnt3a plays an early role in this process by regulating the expression of the Wnt target gene Dll1 in the psm, which in turn activates the expression of the left determinant, Nodal, at the psm/node boundary, and regulates somitogenesis in the psm itself (Hrabe de Angelis et al., 1997). Despite the fact that Wnt3a transcription is limited to the primitive streak and posterior node, we demonstrate that Wnt3a protein can signal over long distances to directly stimulate gene expression in the node and anterior psm. We confirm and extend the findings of Aulehla et al. (2003) by showing that Wnt3a is required for the oscillating expression of the Wnt target gene Axin2, as well as the Notch target Lfng, in the psm at the onset of somitogenesis. We have also presented evidence that Wnt3a may regulate the function of mechanosensory cilia in the node. Thus Wnt3a regulates multiple target genes to simultaneously control LR determination and segmentation.
Wnt signaling and organ laterality
Although posterior organs did not develop in Wnt3a mutants due to a requirement for Wnt3a for posterior development (Takada et al., 1994), laterality phenotypes in anterior viscera such as the heart, lungs and liver were assessed. The majority of E11.5–12.5 Wnt3a−/− embryos were heterotaxic, ie. at least one organ displayed laterality defects. The laterality phenotypes were not secondary to the posterior truncation phenotype since aberrant gene expression in the node was observed well before AP phenotypes emerged. In fact, Dll1 and Nodal are two of the earliest known genes to be affected by the Wnt3a mutation and display aberrant expression prior to somitogenesis, arguing that Wnt3a, signaling via its target gene Dll1, regulates LR determination first, and somitogenesis and AP elongation second.
The process of cardiac looping determines the relative positions of the heart chambers and their connections with the aorta and pulmonary artery. Alterations in the direction of cardiac looping lead to alignment defects that result in a range of cardiovascular abnormalities such as TGA, PTA, double outlet right ventricle (DORV), and atrioventricular septal defects (AVSD) (Maclean and Dunwoodie, 2004). Several of these anomalies were observed in the Wnt3avt;Dll1 compound mutants, consistent with Wnt3a and Dll1 functioning in a common genetic pathway to regulate cardiac laterality.
The aberrant expression of Nodal in Wnt3a−/− embryos presents an opportunity to examine the importance of the timing and asymmetric nature of Nodal and Lefty signaling for organ laterality. Since Nodal is required in the node to activate Lefty1 expression in the dorsal node and Nodal expression in the LPM (Brennan et al., 2002), we suggest that the reduced levels of Nodal in the Wnt3a−/− node are insufficient to activate Lefty1 or Nodal at the 2 somite stage when they are normally activated, but are sufficient to account for the delayed Nodal expression observed in the LPM. Lefty1 was never detected in the mutant PFP indicating that the midline barrier to Nodal diffusion was absent, and providing an explanation for the bilateral expression of Nodal in the >4 somite-stage LPM. Interestingly, left pulmonary isomerism was not observed in Wnt3a mutants as would be predicted from the Lefty1−/− phenotype (Meno et al., 1998). Instead, the Wnt3a mutants more closely resembled Cryptic mutants which lack Nodal expression in the LPM and display randomized situs and right isomerism (Yan et al., 1999). Since Nodal is not expressed bilaterally in the Wnt3a−/− LPM until the 5 somite stage, these phenotypes suggest that asymmetric Nodal expression in the LPM must be established between the 2–4 somite stages (a 6 hour window) to establish proper LR asymmetry in anterior organs. Bilateral Nodal expression in the LPM after the 5 somite stage appears to be insufficient to induce left isomerisms in any of the organs examined, however it should be noted that Nodal expression in the LPM never extended anteriorly into the heart, as it did in wildtype embryos.
Wnts and polycystins in LR determination
The membrane receptor PC1 colocalizes with PC2 in renal mechanosensory cilia where it senses mechanical bending of the primary cilium induced by fluid flow, transducing it into a chemical Ca2+ flux by activating PC2 (Nauli et al., 2003). Kidney cells lacking PC1 form cilia but do not display Ca2+ influx when stimulated by fluid flow (Nauli et al., 2003) or activating antibodies (Delmas et al., 2004). Our observation that PC1 is coexpressed with PC2 in node mechanosensory cilia suggests that a similar regulatory relationship between PC1 and PC2 exists in node cilia. Embryos lacking Wnt3a display structurally normal node cilia that robustly express PC2 but display reduced levels of PC1. These results predict that Ca2+ asymmetry will be perturbed in the Wnt3a−/− node despite the presence of PC2, and this will be addressed in future experiments. Since PC1 and PC2 activity appear to be mutually dependent, and mutations in either Pkd1 or Pkd2 result in identical polycystic kidney disease phenotypes (Delmas, 2004), it seems likely that Pkd1 mutants will also display laterality defects and a loss of Ca2+ asymmetry. The cardiovascular defects observed in embryos homozygous for a targeted allele of Pkd1 (Boulter et al., 2001) are consistent with a role for Pkd1 in the regulation of cardiac laterality.
Despite reports in the literature that PKD1 is a direct target gene of Wnt/βcatenin signaling (Rodova et al., 2002), our results suggest otherwise. Examination of 5kb of the mouse Pkd1 promoter revealed five consensus Tcf1 binding sites, however activation of the Wnt/βcatenin pathway did not activate Pkd1 promoter luciferase reporter constructs in transient transfections in vitro (data not shown). Furthermore, mutational analysis showed that the Tcf sites were not necessary for basal expression. It is unclear how Wnt3a indirectly regulates ciliary PC1 expression however it is tempting to speculate that the mechanism involves Inversin, another ciliary protein required for proper LR determination (Watanabe et al., 2003), that has recently been shown to bind Dishevelled and regulate Wnt signaling (Simons et al, 2005).
Wnt3a signaling and target gene expression in the node
Although much of the LR phenotype observed in Wnt3a mutants can be directly attributed to the aberrant expression of Dll1 in the psm, and consequently of Nodal in the node, our data indicates that Wnt3a also directly regulates gene expression in the ventral node. Two independent Wnt/βcatenin reporters, as well as the Wnt/βcatenin target genes mNkd1and Axin2, were expressed there. Interestingly, mNkd1 expression was asymmetric in the ventral node. The significance of this asymmetric expression, and the mechanisms underlying it, are presently unclear. Given that Wnt3a is symmetrically expressed in the streak and node, one possible mechanism, interpreted in the context of the morphogen flow model, is that the Wnt3a ligand itself becomes asymmetrically distributed at the ventral node surface by cilia-generated nodal flow. This hypothesis would require that Wnt3a, expressed by the dorsal epiblast, is able to traverse the ventral node epithelium to reach the apical surface of the node where the cilia are located. This is unlikely to occur since the transverse movement of secreted molecules across an epithelial tissue is blocked by the tight junctions of the polarized epithelium. More importantly, this postulate is not supported by our data demonstrating that neither of the Wnt/βcatenin reporters, nor Axin2, were asymmetrically expressed in the node. Perhaps a more likely scenario is one in which mNkd1 is symmetrically activated in the node by Wnt3a, but mNkd1 mRNA becomes graded due to asymmetric localization or decay. Although mNkd1 has also been shown to exhibit oscillatory gene expression in the psm (Ishikawa et al., 2004), its function remains unclear since animals lacking mNkd1 do not display embryonic phenotypes (Li et al., 2005).
Wnt3a is a major component of the trunk organizer
Embryological studies performed primarily in amphibians, fish, and chick have demonstrated that the Spemann-Mangold organizer is a dynamic structure that can be subdivided into head, trunk, and tail organizers based on their distinct cell subpopulations and differing inductive capacities (Niehrs, 2004). In the mouse, evidence for the distinction of all three organizers remains relatively scant (Robb and Tam, 2004), however a strong argument can be made for trunk organizer activity residing in the node: 1) transplantation experiments demonstrate that the node is sufficient to induce patterned, ectopic trunks, but not heads (Beddington, 1994; Tam et al., 1997), 2) surgical ablation studies show that the node is necessary for DV and LR asymmetry, and proper segmentation and AP elongation of the prospective trunk, but is not required for AP polarity (Davidson et al., 1999). The timing of node formation, which occurs after AP polarity and head structures have been specified but before LR determination and trunk development, is also consistent with the node functioning as a trunk organizer.
Our demonstration that Wnt3a is expressed in the node and is required for LR determination and segmentation, coupled with previous studies demonstrating that Wnt3a is required in a dose-dependent manner for the formation of the entire posterior trunk and tail (Greco et al., 1996; Takada et al., 1994), suggests that Wnt3a is a major component of the trunk organizer. We present a model for how Wnt3a could function in this capacity (Fig. 7). A source of Wnt3a is established at E7.5 in the primitive streak and node progenitors at the posterior end of the gastrulating embryo. Wnt3a specifies mesoderm fates in the streak by directly regulating Brachyury transcription (Galceran et al., 2001; Yamaguchi et al., 1999). Wnt3a also regulates Dll1 expression in the psm directly, and indirectly via Brachyury (Galceran et al., 2004; Hofmann et al., 2004). Dll1 expression in the psm stimulates Notch activity at the psm/node boundary, to activate Nodal transcription in the node periphery (Krebs et al., 2003; Raya et al., 2003). Activation of Nodal in the lateral aspects of the node establishes an axis of Nodal expression that is perpendicular to the AP axis, leading to the orthogonal orientation of the LR axis. Elevated Notch activity also activates LFng in the node periphery, which could serve to restrict Nodal to the node periphery by inhibiting Notch in a negative feedback loop (Dale et al., 2003).
Fig. 7. Wnt3a functions as a trunk organizer.
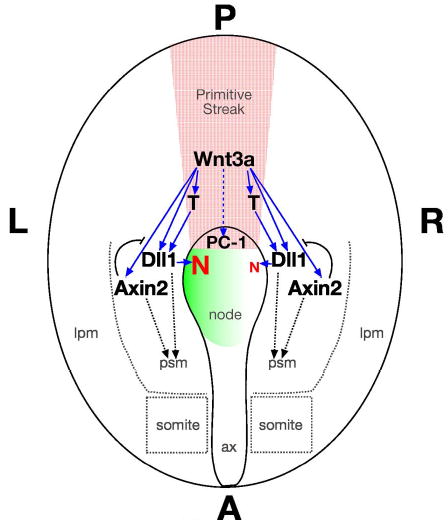
The diagram depicts the ventral view of an E8 embryo. Wnt3a (red stippling) is expressed in the streak and node where it directly activates (solid blue arrows) T (Brachyury), Dll1, and Axin2 via the Wnt/βcatenin pathway. Please see the text for details. Abbr.: red N, Nodal; solid blue arrow, direct gene regulation; dashed blue arrow, indirect regulation; green gradient, left-sided Ca2+ flux; curved black line, negative feedback loop. Ax, axial mesendoderm.
The indirect regulation of PC1 expression in the node by Wnt3a may regulate the ability of mechanosensory cilia to interpret symmetry-breaking leftward nodal flow and generate asymmetric Ca2+ flux. Since alterations in local Ca2+ concentrations can affect the affinity of Dll1 for its Notch receptor (Raya et al., 2004), then it’s conceivable that Wnt3a could regulate the generation of Nodal asymmetry in the node by asymmetrically elevating Ca2+ levels on the left side of the node, thereby enhancing Dll1-Notch interactions, and Nodal expression, on the left side. Concomitantly, Wnt3a activates Axin2 in the psm to initiate a Wnt-centered oscillating feedback loop, and Dll1 activates LFng to initiate the Notch-centered oscillating feedback loop (Aulehla and Herrmann, 2004). Together, the two oscillating loops constitute the segmentation clock that controls continued elongation of the trunk and tail along the AP axis. Wnt3a also regulates AP patterning in the trunk and tail by influencing Cdx1 and Hox gene expression (Lohnes, 2003). We propose that Wnt3a functions in the trunk organizer to coordinate patterning and morphogenesis along multiple body axes.
Our work implicates βcatenin as the primary transducer of canonical Wnt signals that coordinately regulate LR specification and segmentation. The use of conditional βcatenin alleles and Cre drivers that are expressed in the node or psm will help address the specific roles that βcatenin plays in the node during LR determination, and in the psm in the regulation of oscillating gene expression during somitogenesis.
Acknowledgments
We thank D. Epstein, F. Costantini, M. Kuehn, M. Shen, H. Hamada, J. Brennan, D. Kimelman, K. Wharton, M. Brueckner, J. Yost, S. Somlo and A. Gossler for providing reagents and mice. We thank our colleagues at the NCI-Frederick Imaging Facility (S. Lockett, K. Nagashima), and the Transgenic Core Facility (L. Feigenbaum) for their assistance, and C. Stewart, M. Lewandoski, and N. Jenkins for comments on the manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Aulehla A, Herrmann BG. Segmentation in vertebrates: clock and gradient finally joined. Genes Dev. 2004;18:2060–2067. doi: 10.1101/gad.1217404. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, Kanzler B, Herrmann BG. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Barrantes IB, Elia AJ, Wunsch K, Hrabe de Angelis MH, Mak TW, Rossant J, Conlon RA, Gossler A, de la Pompa JL. Interaction between Notch signalling and Lunatic fringe during somite boundary formation in the mouse. Curr Biol. 1999;9:470–480. doi: 10.1016/s0960-9822(99)80212-7. [DOI] [PubMed] [Google Scholar]
- Beddington RS. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Oulad-Abdelghani M, Ward SJ, Bronner S, Chambon P, Dolle P. A new mouse member of the Wnt gene family, mWnt-8, is expressed during early embryogenesis and is ectopically induced by retinoic acid. Mech Dev. 1996;58:141–152. doi: 10.1016/s0925-4773(96)00569-2. [DOI] [PubMed] [Google Scholar]
- Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16:2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- Collignon J, Varlet I, Robertson EJ. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, Pourquie O. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421:275–278. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- Davidson BP, Kinder SJ, Steiner K, Schoenwolf GC, Tam PP. Impact of node ablation on the morphogenesis of the body axis and the lateral asymmetry of the mouse embryo during early organogenesis. Dev Biol. 1999;211:11–26. doi: 10.1006/dbio.1999.9276. [DOI] [PubMed] [Google Scholar]
- Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118:145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. Faseb J. 2004;18:740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. Coupling segmentation to axis formation. Development. 2004;131:5783–5793. doi: 10.1242/dev.01519. [DOI] [PubMed] [Google Scholar]
- Galceran J, Hsu SC, Grosschedl R. Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression. Proc Natl Acad Sci U S A. 2001;98:8668–8673. doi: 10.1073/pnas.151258098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J, Sustmann C, Hsu SC, Folberth S, Grosschedl R. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 2004;18:2718–2723. doi: 10.1101/gad.1249504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat Rev Genet. 2002;3:103–113. doi: 10.1038/nrg732. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Schuster-Gossler K, Watabe-Rudolph M, Aulehla A, Herrmann BG, Gossler A. WNT signaling, in synergy with T/TBX6, controls Notch signaling by regulating Dll1 expression in the presomitic mesoderm of mouse embryos. Genes Dev. 2004;18:2712–2717. doi: 10.1101/gad.1248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabe de Angelis M, McIntyre J, 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- Ibanez-Tallon I, Gorokhova S, Heintz N. Loss of function of axonemal dynein Mdnah5 causes primary ciliary dyskinesia and hydrocephalus. Hum Mol Genet. 2002;11:715–721. doi: 10.1093/hmg/11.6.715. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Kitajima S, Takahashi Y, Kokubo H, Kanno J, Inoue T, Saga Y. Mouse Nkd1, a Wnt antagonist, exhibits oscillatory gene expression in the PSM under the control of Notch signaling. Mech Dev. 2004;121:1443–1453. doi: 10.1016/j.mod.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125:4225–4234. doi: 10.1242/dev.125.21.4225. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev. 2003;17:1207–1212. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ishikawa T, Miyoshi H, Oshima M, Taketo MM. A targeted mutation of Nkd1 impairs mouse spermatogenesis. J Biol Chem. 2005;280:2831–2839. doi: 10.1074/jbc.M405680200. [DOI] [PubMed] [Google Scholar]
- Lohnes D. The Cdx1 homeodomain protein: an integrator of posterior signaling in the mouse. Bioessays. 2003;25:971–980. doi: 10.1002/bies.10340. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–161. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean K, Dunwoodie SL. Breaking symmetry: a clinical overview of left-right patterning. Clin Genet. 2004;65:441–457. doi: 10.1111/j.0009-9163.2004.00258.x. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–392. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Meno C, Shimono A, Saijoh Y, Yashiro K, Mochida K, Ohishi S, Noji S, Kondoh H, Hamada H. lefty-1 is required for left-right determination as a regulator of lefty-2 and nodal. Cell. 1998;94:287–297. doi: 10.1016/s0092-8674(00)81472-5. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–434. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, Morita M, Raya RM, Dubova I, Bessa JG, et al. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev. 2003;17:1213–1218. doi: 10.1101/gad.1084403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- Robb L, Tam PP. Gastrula organiser and embryonic patterning in the mouse. Semin Cell Dev Biol. 2004;15:543–554. doi: 10.1016/j.semcdb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Rodova M, Islam MR, Maser RL, Calvet JP. The polycystic kidney disease-1 promoter is a target of the beta-catenin/T-cell factor pathway. J Biol Chem. 2002;277:29577–29583. doi: 10.1074/jbc.M203570200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Esteban C, Capdevila J, Kawakami Y, Izpisua Belmonte JC. Wnt signaling and PKA control Nodal expression and left-right determination in the chick embryo. Development. 2001;128:3189–3195. doi: 10.1242/dev.128.16.3189. [DOI] [PubMed] [Google Scholar]
- Saijoh Y, Oki S, Ohishi S, Hamada H. Left-right patterning of the mouse lateral plate requires nodal produced in the node. Dev Biol. 2003;256:160–172. doi: 10.1016/s0012-1606(02)00121-5. [DOI] [PubMed] [Google Scholar]
- Schweisguth F. Notch signaling activity. Curr Biol. 2004;14:R129–138. [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development. 1999;126:5495–5504. doi: 10.1242/dev.126.23.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Steiner KA, Zhou SX, Quinlan GA. Lineage and functional analyses of the mouse organizer. Cold Spring Harb Symp Quant Biol. 1997;62:135–144. [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Jr, Zimmermann G, Rousset R, Scott MP. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev Biol. 2001;234:93–106. doi: 10.1006/dbio.2001.0238. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:R713–724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wallingford JB, Sun TQ, Nelson AM, Sakanaka C, Reinhard C, Harland RM, Fantl WJ, Williams LT. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc Natl Acad Sci U S A. 2001;98:3802–3807. doi: 10.1073/pnas.071041898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Meno C, Koshiba K, Sugihara M, Itoh H, Ishimaru Y, Inoue T, Ohuchi H, Semina EV, Murray JC, et al. Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell. 1998;94:299–305. doi: 10.1016/s0092-8674(00)81473-7. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]


