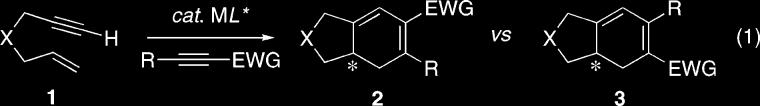
Transition metal-catalyzed [m+n+o] carbocyclization reactions provide powerful methods for the construction of complex polycyclic systems that are generally not accessible through classical pericyclic reactions.1 Although the intermolecular metal-catalyzed [2+2+2] carbocyclization reaction of carbon and heteroatom tethered 1,6-enynes with symmetrical 1,2-disubstituted alkynes has been described, a significant challenge with this process is the ability to regioselectively incorporate unsymmetrical 1,2-disubstituted alkynes.2-6 Furthermore, despite the myriad of metal-catalyzed carbocyclization reactions, the enantioselective version of the metal-catalyzed [2+2+2] carbocyclization of a 1,6-enyne has not been described. In light of these significant challenges, we sought to develop the combined regio- and enantioselective metal-catalyzed [2+2+2] carbocyclization reaction with unsymmetrical 1,2-disubstituted alkynes and thereby provide a new paradigm for this type of transformation. Herein, we now describe the regio- and enantioselective rhodium-catalyzed [2+2+2] carbocyclization of carbon-and heteroatom-tethered 1,6-enynes 1 with unsymmetrical 1,2-disubstituted alkynes to afford the corresponding bicyclohexadienes 2/3 in excellent yield (eq 1).
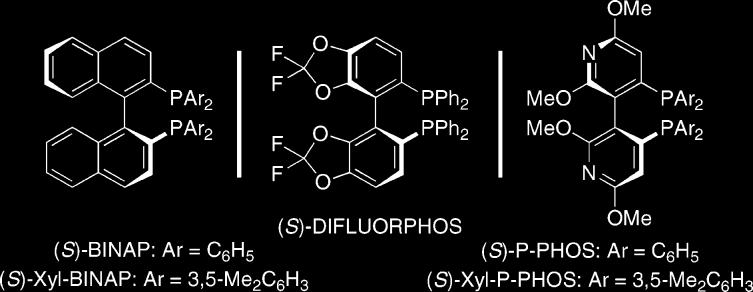
Preliminary studies focused on the development of the regio-and enantioselective version of the rhodium-catalyzed [2+2+2] carbocyclization using the 1,6-enyne 1a as outlined in Table 1. Treatment of 1a with excess methyl phenylpropiolate and the chiral complex derived from AgOTf-modified [RhCl(COD)]2 with (S)-BINAP in benzene at 60 °C, furnished the bicyclohexadienes 2/3 in 27% yield as a 2:1 mixture of regioisomers (entry 1).7,8 Although the overall efficiency and regioselectivity were not particularly encouraging, the major isomer 2a was obtained with high enantioselectivity (86% ee). Previous studies demonstrated that the overall efficiency could be improved dramatically by simply adjusting the nature of the solvent and/or counterion.5c In light of this fact, we probed the effect of coordinating solvents and silver salts with progressively weaker coordinating counterions (entries 2–5). Gratifyingly, the ethereal solvent tetrahydrofuran in combination with the tetrafluoroborate counterion proved optimal in terms of efficiency (entry 5), since these conditions completely suppressed the undesired homo-coupling of enyne1a. Additional optimization focused on the nature of the chiral phosphine ligand to improve and potentially understand the factors that control regioselectivity. Interestingly, switching to (S)-Xyl-BINAP led to significantly improved regioselectivity (entry 5 vs 6). Hence, the more sterically hindered bisphosphine can more effectively discriminate the termini of methyl phenylpropiolate (Ph vs CO2Me). The more π-acidic (S)-DIFLUORPHOS ligand, which has a narrower dihedral angle than (S)-Xyl-BINAP, furnished the product with diminished regioselection, albeit with higher enantioselectivity (entry 7).9 In accord with this observation, the dipyridyl-phosphines CTH-(S)-P-PHOS and (S)-Xyl-P-PHOS ligands, which possesses a dihedral angle similar to that of (S)-DIFLUORPHOS (see Figure 1), afforded excellent enantioselectivity, in which (S)-Xyl-P-PHOS provided the optimum ligand in terms of regioselectivity (entry 9).10 This trend is analogous with the improvement observed for the switch from the (S)-BINAP to (S)-Xyl-BINAP ligand (entry 5 vs 6), presumably due to similar reasoning.
Table 1.
Optimization of Intermolecular Rhodium-Catalyzed [2+2+2] Carbocyclization Reactiona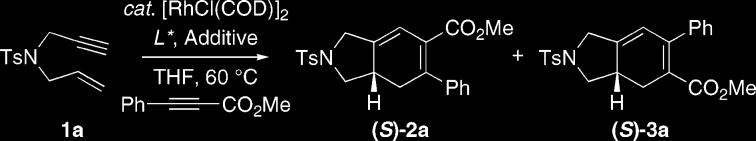
| entry | solvent | additive | ligand (L*) | yield (%)b | rs (2a:3a)c | ee of 2a (%)d,e |
|---|---|---|---|---|---|---|
| 1 | PhH | AgOTf | (S)-BINAP | 27 | 2:1 | 86 |
| 2 | MeCN | “ | “ | 0 | – | – |
| 3 | THF | “ | “ | 68 | 3:1 | 92 |
| 4 | “ | AgSbF6 | “ | 82 | 3:1 | 89 |
| 5 | “ | AgBF4 | “ | 95 | 3:1 | 92 |
| 6 | “ | “ | (S)-Xyl-BINAP | 93 | 8:1 | 88 |
| 7 | “ | “ | (S)-DIFLUORPHOS | 73 | 4:1 | 97 |
| 8 | “ | “ | (S)-P-PHOS | 75 | 5:1 | 97 |
| 9 | THF | AgBF4 | (S)-Xyl-P-PHOS | 98 | 10:1 | 97 |
All reactions were carried out on a 0.25 mmol reaction scale utilizing the chiral complex derived from 5 mol % of [RhCl(COD)]2 and 12 mol % of the bidentate phosphine ligand, further modified with 20 mol % of silver salt and methyl phenylpropiolate (3 equiv) under an atmosphere of argon.11
Isolated yields.
Regioselectivity was determined by 400 MHz 1H NMR on the crude reaction mixtures.
Enantiomeric excess of the major regioisomer 2a was determined by chiral HPLC analysis.
The regioselectivity and absolute configuration of (S)-2a were established by NOESY and X-ray crystallography, respectively.
Figure 1.
Chiral ligands used in the optimization studies.
Table 2 summarizes the application of the optimized reaction conditions (Table 1, entry 9) to the various carbon- and heteroatomtethered 1,6-enynes using an array of methyl para-substituted arylpropiolates. Interestingly, the carbocyclization reaction is highly enantioselective regardless of the nature of the enyne tether and/or the aryl substituent, whereas the yield and/or regioselectivity are influenced by these parameters. For example, although all the enynes undergo regioselective carbocyclizations, the nature of the tether has a profound influence on the level of regiocontrol (O ≫ NTs > C(CO2Me)2). Similarly, the overall efficiency and regioselectivity can be directly related to the electronic nature of the aryl substituents. This trend is particularly prominent with carbon tethers (entries 7–10), whereas regioselectivity and efficiency are somewhat affected in the nitrogen (entries 2–5) and oxygen tethers (entries 12–15), respectively. Overall, this work now provides access to previously unknown enantiomerically enriched bicyclohexadienes that are useful synthons for target-directed synthesis.
Table 2.
Scope of the Regio- and Enantioselective Rhodium-Catalyzed [2+2+2] Carbocyclization Reaction (eq 1; R = p-FG-C6H4, EWG = CO2Me)a
| entry | 1,6-enyne 1 X = | alkyne FG = | yield (%)b | rs (2:3)c | ee of 2 (%)d | ||
|---|---|---|---|---|---|---|---|
| 1 | TsN | a | H | 98 | a | 10:1 | 97 |
| 2 | “ | “ | OMe | 84 | b | 14:1 | 97 |
| 3 | “ | “ | Me | 95 | c | 11:1 | 97 |
| 4 | “ | “ | F | 87 | d | 10:1 | 97 |
| 5 | “ | “ | CF3 | 86 | e | 10:1 | 98 |
| 6 | C(CO2Me)2 | b | H | 88 | f | 9:1 | ≥99 |
| 7 | “ | “ | OMe | 85 | g | 10:1 | 98 |
| 8 | “ | “ | Me | 80 | h | 9:1 | 95 |
| 9 | “ | “ | F | 74 | i | 7:1 | 98 |
| 10 | “ | “ | CF3 | 65 | j | 5:1 | 98 |
| 11 | O | c | H | 86 | k | ≥19:1 | ≥99 |
| 12 | “ | “ | OMe | 95 | l | ≥19:1 | 98 |
| 13 | “ | “ | Me | 87 | m | ≥19:1 | 98 |
| 14 | “ | “ | F | 75 | n | 17:1 | ≥99 |
| 15 | “ | “ | CF3 | 72 | o | 17:1 | 97 |
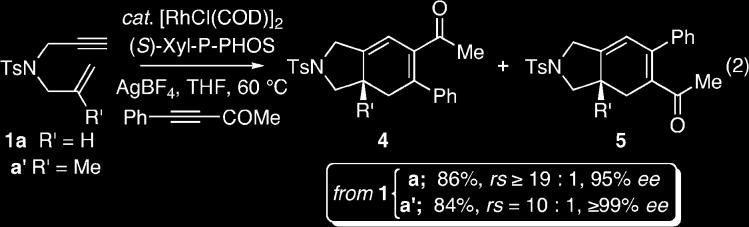
To further demonstrate the scope of this transformation, we elected to examine an alternative electron-withdrawing group within the alkyne. Treatment of the 1,6-enyne 1a under the optimized reaction conditions with 4-phenyl-3-butyn-2-one furnished the bicyclohexadienes 4a/5a (R′ = H) in 86% yield, with ≥ 19:1 regioselectivity and 95% ee for 4a (eq 2).12 Additionally, we envisioned the application of this methodology to a substituted 1,6-enyne 1a′ (R′ = Me) would facilitate the enantioselective introduction of a quaternary carbon stereogenic center, which would be a particularly attractive feature of this methodology.13 Gratifyingly, treatment of 1a′ under the optimized carbocyclization conditions with 4-phenyl-3-butyn-2-one furnished the quaternary substituted bicyclic azacycles 4a′/5a′ (R′ = Me) in 84% yield, with 10:1 regioselectivity and ≥ 99% ee for 4a′.12
In conclusion, we have developed the first regio- and enantioselective crossed intermolecular rhodium-catalyzed [2+2+2] carbocyclization of carbon- and heteroatom-tethered 1,6-enynes with unsymmetrical 1,2-disubstituted alkynes. This study clearly delineates the specific ligand requirements for obtaining excellent regio-and enantioselectivity. Furthermore, the ability to utilize various electron-withdrawing groups, and to introduce quaternary carbon stereogenic centers, provides the level of versatility necessary for its application to target-directed synthesis. Additional studies on the development and application of this novel methodology to the total synthesis of natural products are currently underway.14
Supplementary Material
Acknowledgment
We sincerely thank National Institutes of Health (GM58877) for generous financial support. We also thank Johnson and Johnson for a Focused Giving Award, and Pfizer Pharmaceuticals for the Creativity in Organic Chemistry Award (P.A.E.).
Footnotes
Supporting Information Available: Spectral data for 2a–o and 4a/a′ and X-ray crystallographic analysis of (S)-2a (where X = NTs, R = C6H5, and EWG = CO2Me). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Aubert C, Buisine O, Malacria M. Chem. Rev. 2002;102:813. doi: 10.1021/cr980054f. For recent reviews on metal-catalyzed carbocyclization reactions, see: [DOI] [PubMed] [Google Scholar]; (b) Nakamura I, Yamamoto Y. Chem. Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- 2.(a) Malacria M, Aubert C, Renaud JL. In: Science of Synthesis: Houben-Weyl Methods for Molecular Transformations. Lautens M, Trost BM, editors. Vol. 1. Georg Thieme Verlag; New York: 2001. pp. 439–530. For recent reviews on metal-mediated [2+2+2] carbocyclizations, see: [Google Scholar]; (b) Varela J, Saá C. Chem. Rev. 2003;103:3787. doi: 10.1021/cr030677f. [DOI] [PubMed] [Google Scholar]; (c) Fujiwara M, Ojima I. In: Modern Rhodium-Catalyzed Organic Reactions. Evans PA, editor. 2005. pp. 129–150. Chapter 7. and pertinent references therein. [Google Scholar]
- 3.(a) Grigg R, Scott R, Stevenson P. J. Chem. Soc., Perkin Trans. 1988;1:1357. For pioneering work on the rhodium-catalyzed intermolecular [2+2+2] carbocylization using tethered 1,6-diynes and 1,6-enynes, see: [Google Scholar]; (b) Grigg R, Scott R, Stevenson P. J. Chem. Soc., Perkin Trans. 1988;1:1365. [Google Scholar]
- 4.Oh CH, Sung HR, Jung SH, Lim YM. Tetrahedron Lett. 2001;42:5493. For a recent example of a rhodium-catalyzed [2+2+2] dimerization of 1,6-enynes, see: [Google Scholar]
- 5.(a) Trost BM, Tanoury GJ.J. Am. Chem. Soc 19871094753.For examples of an intermolecular metal-catalyzed [2+2+2] carbocyclization with various 1,6-enynes using symmetrical alkynes, see:Pd: [Google Scholar]; (b) Kezuka S, Okado T, Niou E, Takeuchi R.Org. Lett 200571711Ir: [DOI] [PubMed] [Google Scholar]; (c) Evans PA, Sawyer JR, Lai KW, Huffman JC.Chem. Commun 20053971Rh: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C-A, King JA, Jr., Vollhardt KPC. J. Chem. Soc., Chem. Commun. 1981:53. For an example of an intermolecular cobalt-mediated [2+2+2] carbocyclization with various 1,6-enynes using an unsymmetrical alkyne, see: [Google Scholar]
- 7.The in situ activation of [RhCl(COD)]2 with AgBF4 provides superior results as compared to the [Rh(COD)2]BF4 precatalyst in terms of chemical yield.
- 8.Shimizu H, Nagasaki I, Saito T. Tetrahedron. 2005;61:5405. For an excellent review on the development of chiral bisphosphine ligands, see: and pertinent references therein. [Google Scholar]
- 9.Jeulin S, Duprat de Paule S, Ratovelomanana-Vidal V, Genêt J-P, Champion N, Dellis P. Angew. Chem., Int. Ed. 2004;43:320. doi: 10.1002/anie.200352453. [DOI] [PubMed] [Google Scholar]
- 10.(a) Pai C-C, Lin C-W, Lin C-C, Chen C-C, Chan ASC, Wong WT. J. Am. Chem. Soc. 2000;122:11513. [Google Scholar]; (b) Wu J, Chen H, Kwok W, Guo R, Zhou Z, Yeung C, Chan ASC. J. Org. Chem. 2002;67:7908. doi: 10.1021/jo026168f. [DOI] [PubMed] [Google Scholar]
- 11.Representative Experimental Procedure: [RhCl(COD)]2 (6.2 mg, 5 mol %) and AgBF4 (9.7 mg, 20 mol %) were suspended in anhydrous THF (1.0 mL) and stirred at room temperature under an atmosphere of argon for ca. 10 min. (S)-Xyl-P-PHOS (22.7 mg, 12 mol %) in anhydrous THF (3.0 mL) was then added to the yellow suspension, and the mixture was stirred at room temperature for an additional ca. 30 min. Methyl phenylpropiolate (120.1 mg, 0.75 mmol) was added in one portion, followed by addition of 1,6-enyne 1a (62.3 mg, 0.25 mmol) in anhydrous THF (2.0 mL) via syringe pump over ca. 2 h at 60 °C, followed by an additional ca. 30 min (TLC control). The reaction mixture was allowed to cool to room temperature, and the resultant mixture was filtered through a short pad of silica gel (eluting with 50% ethyl acetate/hexanes) and concentrated in vacuo to afford the crude product. Purification by flash chromatography (silica gel, eluting with a 10–30% ethyl acetate/hexanes gradient) afforded the bicyclohexadienes 2a/3a (94.1 mg, 98%) as a white solid, with 10:1 regioselectivity and 97% ee.
- 12.The absolute configuration was made by analogy with (S)-2a, which was determined by X-ray crystallography.
- 13.(a) Christoffers J, Mann A. Angew. Chem., Int. Ed. 2001;40:4591. doi: 10.1002/1521-3773(20011217)40:24<4591::aid-anie4591>3.0.co;2-v. For recent reviews on the enantioselective construction of quaternary carbon stereocenters, see: [DOI] [PubMed] [Google Scholar]; (b) Denissova I, Barriault L. Tetrahedron. 2003;59:10105. and pertinent references therein. [Google Scholar]
- 14.The application of this methodology to alkyl-substituted alkynes also provides excellent enantioselectivity, albeit with diminished regioselectivity. Hence, conjugated alkynes (where R = aryl or vinyl) are crucial for achieving useful levels of regiocontrol. For example, for 1a, where EWG = CO2Me and R = Me (rs = 4:1, 97% ee, 90%) whereas R = C([unk]CH2)Me (rs = 10:1, 97% ee, 66%).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.