Abstract
Objective: A series of 4-aryl substituted semicarbazones of levulinic acid (4-oxo pentanoic acid) was designed and synthesized to meet the structural requirements essential for anticonvulsant activity. Methods: All the compounds were evaluated for anticonvulsant activity. Anticonvulsant activity was determined after intraperitoneal (i.p.) administration to mice by maximal electroshock (MES) and subcutaneous metrazol (ScMet) induced seizure methods and minimal motor impairment was determined by rotorod test. Results: A majority of the compounds exhibited significant anticonvulsant activity after intraperitoneal administration. In the present study 4-(4′-fluoro phenyl) levulinic acid semicarbazone emerged as the most active molecule, showing broad spectrum of activity with low neurotoxicity. Unsubstituted levulinic acid semicarbazone was found to be inactive in all the screens. Conclusion: The results obtained validate the hypothesis that presence of an aryl group near the semicarbazone moiety is essential for anticonvulsant activity. The results also indicate that the hydrophilic-hydrophobic site can accommodate hydrophilic groups.
Keywords: Substituted semicarbazones, Anticonvulsant, Levulinic acid
INTRODUCTION
Epilepsy is a common disorder of the central nervous system (CNS). Approximately 0.4%~1% of the population worldwide suffers from this disorder (Ho et al., 2001). The conventional antiepileptic drugs suffer from a range of side effects. Furthermore, the convulsions of 25% of epileptics are inadequately controlled by currently available medications (Craig, 1997). During the past decade several new drugs were approved, e.g., felbamate, fosphenytoin, gabapentin, lamotrigine, vigabatrin and zonisamide (Malawska, 2003). However none of the available antiepileptic drug is ideal as they can be associated with chronic and adverse side effects (McNamara, 2001). Thus the search for new anticonvulsant drugs continues to be an active area of investigation in medicinal chemistry. Aryl semicarbazones have recently acquired an important place as anticonvulsants and can be considered a new class of compounds with anticonvulsant activity (Dimmock and Baker, 1994). It was deduced (Pandeya and Raja, 2002) recently that the requirement for anticonvulsant activity includes (Fig.1):
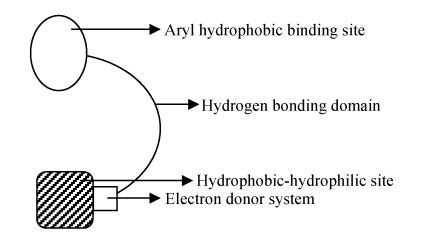
Fig. 1.
Structural requirements for semicarbazones displaying anticonvulsant activity
1. An aryl hydrophobic binding site with halo substituent preferably in the para position.
2. A semicarbazone system containing 2-electron donor system and a hydrogen bonding domain.
3. Another hydrophobic-hydrophilic site controlling the pharmacokinetic properties of the anticonvulsant.
Based on the above model a number of active semicarbazones have been synthesized in our laboratory (Aggarwal and Mishra, 2004). In the present study levulinic acid was selected as the hydrophobic-hydrophilic group, because some well known anticonvulsants possess the carboxyl group in their molecule (Edafiogho and Scott, 1996). Halo substituents selected are F, Cl and Br, because they are known to increase anticonvulsant activity (Pandeya et al., 2000). Other substituents have also been selected for structure activity relationship (SAR) purposes.
MATERIALS AND METHOD
Chemistry
The melting points determined by open capillary method were uncorrected. The purity of the compounds was confirmed by thin layer chromatography (TLC) using silica gel G as stationary phase and benzene as the eluant. NMR spectra were recorded on a Hitachi R-600 high resolution NMR spectrometer, IR spectra were recorded on Perkin Elmer spectrum 2000 FT-IR spectrometer and UV λ max were taken on Cintra 10 UV visible spectrometer and are in accordance with the proposed structure. Estimation nitrogen was within 0.4% of the calculated values.
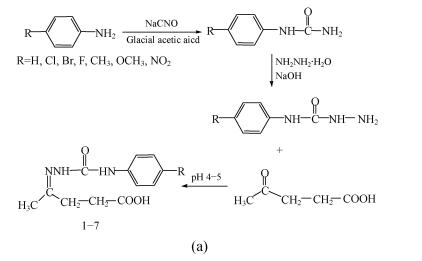
Synthesis of substituted semicarbazones (compounds 1~7, Fig.2a): Different para substituted aryl semicarbazides were prepared by the method of Pandeya et al.(1999). Equimolar quantities of levulinic acid (0.005 mol) and appropriate amount of substituted phenyl semicarbazide (0.005 mol) were dissolved in 20 ml of a mixture of ethanol and water (1:1) and pH of the reaction mixture was adjusted to 4~5 by addition of glacial acetic acid. The mixture was refluxed for 1 h to 2.5 h and then cooled in an ice bath. In some cases the solution was poured on crushed ice to induce crystallization. The resultant precipitates were filtered, dried and recrystallized from aqueous ethanol (95%).
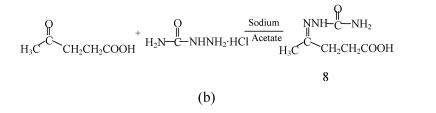
Fig. 2.
Scheme for synthesis of (a) substituted semicarbazones; (b) levulinic acid semicarbazone
Synthesis of levulinic acid semicarbazone (compound 8, Fig.2b): A solution of semicarbazide hydrochloride (0.01 mol) and sodium acetate (0.01 mol) in 20 ml water was added slowly to a stirred solution of levulinic acid (0.01 mol) in 5 ml water. The reaction mixture was stirred at room temperature for 15 min. The precipitate obtained on cooling was filtered and recrystallized from aqueous ethanol (95%) to give the desired product.
Physical characterization of synthesized compounds is given in Table 1.
Table 1.
Physical characterization of synthesized compounds
| Compound | R | Molecular formula | M.P. (°C) | Yield (%) | Rf | Nitrogen estimation |
|
| Calculated | Found | ||||||
| 1 | H | C12H15N3O3 | 222–223 | 58 | 0.41 | 16.85 | 16.81 |
| 2 | Cl | C12H14N3O3Cl | 244–246 | 64 | 0.57 | 14.82 | 14.78 |
| 3 | Br | C12H14N3O3Br | 258–264 | 67 | 0.62 | 12.83 | 12.80 |
| 4 | F | C12H14N3O3F | 166–168 | 48 | 0.65 | 15.77 | 15.82 |
| 5 | CH3 | C13H17N3O3 | 154–155 | 53 | 0.53 | 15.96 | 15.91 |
| 6 | OCH3 | C13H17N3O4 | 216–219 | 66 | 0.72 | 15.04 | 15.09 |
| 7 | NO2 | C12H14N4O5 | 162–164 | 59 | 0.68 | 19.03 | 18.98 |
| 8 | – | C6H11N3O3 | 163 | 74 | 0.47 | 24.26 | 24.19 |
The spectral data of the synthesized compounds were as follows:
1. UV (λ max, nm) 232; IR (KBr, υ cm−1) 3426 (secondary amide NH), 3304 (symmetrical NH), 3025 (broad OH stretching), 1571 (C=N stretch), 1654 (NH-CO-NH), 2904 (Ar-CH stretch), 1900 (C=C stretch); 1HNMR (DMSOd6, δ) 1.98 (s, 3H, -CH3), 2.2~2.63 (m, 4H, -CH2-CH2-), 5.92 (s, 1H, -CONH), 7.2 (br s, 5H, C6H5), 9.10 (s, 1H, =NNH).
2. UV (λ max, nm) 244; IR (KBr, υ cm−1) 3429 (secondary amide NH), 3302 (symmetrical NH), 3027 (broad OH stretching), 1575 (C=N stretch), 1653 (NH-CO-NH), 2907 (Ar-CH stretch), 1900 (C=C stretch); 1HNMR (DMSOd6, δ) 2.1 (s, 3H, -CH3), 2.3~2.62 (m, 4H, -CH2-CH2-), 6.10 (s, 1H, -CONH), 6.9~7.3 (br m, 4H, C6H4), 9.14 (s, 1H, =NNH).
3. UV (λ max, nm) 247; IR (KBr, υ cm−1) 3427 (secondary amide NH), 3304 (symmetrical NH), 3024 (broad OH stretching), 1574 (C=N stretch), 1653 (NH-CO-NH), 2910 (Ar-CH stretch), 1137 (C-Br stretch); 1HNMR (DMSOd6, δ) 2.12 (s, 3H, -CH3), 2.34~2.64 (m, 4H, -CH2-CH2-), 6.12 (s, 1H, -CONH), 7.2~7.5 (br m, 4H, C6H4), 9.1 (s, 1H, =NNH).
4. UV (λ max, nm) 245; IR (KBr, υ cm−1) 3429 (secondary amide NH), 3307 (symmetrical NH), 3034 (broad OH stretching), 1575 (C=N stretch), 1656 (NH-CO-NH), 2908 (Ar-CH stretch), 1223 (C-F stretch); 1HNMR (DMSOd6, δ) 2.10 (s, 3H, -CH3), 2.38~2.64 (m, 4H, -CH2-CH2-), 6.14 (s, 1H, -CONH), 7.4~7.8 (br m, 4H, C6H4), 9.14 (s, 1H, =NNH).
5. UV (λ max, nm) 242; IR (KBr, υ cm−1) 3432 (secondary amide NH), 3302 (symmetrical NH), 3022 (broad OH stretching), 1578 (C=N stretch), 1662 (NH-CO-NH), 2904 (Ar-CH stretch); 1HNMR (DMSOd6, δ) 2.04 (s, 3H, -CH3), 2.24 (s, 3H, Ar-CH3) 2.38~2.64 (m, 4H, -CH2-CH2-), 6.08 (s, 1H, -CONH), 6.9~7.3 (br m, 4H, C6H4), 9.14 (s, 1H, =NNH).
6. UV (λ max, nm) 246; IR (KBr, υ cm−1) 3434 (secondary amide NH), 3298 (symmetrical NH), 3024 (broad OH stretching), 1576 (C=N), 1664 (NH-CO-NH), 2906 (Ar-CH stretch); 1HNMR (DMSOd6, δ) 2.12 (s, 3H, -CH3), 2.32~2.58 (m, 4H, -CH2-CH2-), 3.94 (s, 3H, Ar-OCH3) 6.12 (s, 1H, -CONH), 7.1~7.5 (br m, 4H, C6H4), 9.16 (s, 1H, =NNH).
7. UV (λ max, nm) 236, 374; IR (KBr, υ cm−1) 3438 (secondary amide NH), 3308 (symmetrical NH), 3023 (broad OH stretching), 1578 (C=N stretch), 1657 (NH-CO-NH), 2906 (Ar-CH stretch), 1342 (NO2 stretch); 1HNMR (DMSOd6, δ) 2.10 (s, 3H, -CH3), 2.34~2.68 (m, 4H, -CH2-CH2-), 6.14 (s, 1H, -CONH), 7.5~7.9 (br m, 4H, C6H4), 9.18 (s, 1H, =NNH).
8. UV (λ max, nm) 223; IR (KBr, υ cm−1) 3474 (amide NH), 3309 (symmetrical NH stretch), 2927 (broad OH stretch), 2914 (Ar-CH stretch), 1894 (C=C stretch), 1687 (C=O stretch, amide), 1613 (amide NH bend), 1575 (C=N stretch); 1HNMR (DMSOd6, δ) 2.14 (s, 3H, -CH3), 2.36~2.62 (m, 4H, -CH2-CH2-), 5.4 (s, 2H, -CONH2), 9.2 (s, 1H, =NNH).
Pharmacology
All the compounds were screened by maximal electroshock (MES) test and subcutaneous metrazol (ScMet) test for anticonvulsant activity. The neurotoxicity (NT) was measured by the rotorod test. The results are summarized in Table 2.
Table 2.
Anticonvulsant evaluation of compounds in the MES, ScMet and neurotoxicity screenings after intraperitoneal injection in mice
| Compound | Intraperitoneal injection in mice |
|||||
| MES screening |
ScMet screening |
NT screening |
||||
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | |
| 1 | – | – | 300 | – | 300 | – |
| 2 | – | – | 300 | – | – | – |
| 3 | – | – | 300 | – | – | – |
| 4 | 100 | – | 300 | – | 300 | – |
| 5 | – | – | – | – | – | – |
| 6 | 300 | – | 300 | – | – | – |
| 7 | 100* | – | – | – | 100* | – |
| 8 | – | – | – | – | 300 | – |
| Phenytoin | 30 | 100 | – | – | 100 | 100 |
| Carbamazepine | 30 | – | 100 | – | 100 | 300 |
The figures in the table indicate the dose in mg/kg at which bioactivity was observed in a majority of the animals. The (–) sign indicates absence of activity at the maximum dose administered.
Observation taken after 0.25 h
Anticonvulsant screening: Anticonvulsant evaluation of semicarbazones was undertaken by following the National Institute of Health (NIH). Anticonvulsant Drug Development (ADD) Program protocol (Krall et al., 1978; Porter et al., 1985). Male albino mice (18~25 g) and male albino rats (100~125 g) were used as experimental animals. The semicarbazones were suspended in 0.5% methyl cellulose/water mixture. All the compounds were administered i.p. in doses of 30, 100 and 300 mg/kg to one to four animals. Some selected compounds were examined for oral activity in rats.
Neurotoxicity screening: Minimal motor impairment was measured in mice by the rotorod test. The mice were trained to stay on an accelerating 3.2 cm diameter rotorod rotating at 10 revolutions per minute. Previously trained mice were given test compounds intraperitoneally in doses of 30, 100, and 300 mg/kg. Neurotoxicity was indicated by the inability of the animal to maintain equilibrium on the rod for at least one minute in each of the three trials.
RESULTS
Preliminary anticonvulsant evaluation of all the synthesized compounds was obtained by testing procedures described in National Institute of Neurological Disorders and Stroke, NIH, Bethesada, MD, USA, for Anticonvulsant Screening Project (ASP). Compounds giving protection in the MES test may prove to be useful in treating generalized tonic-clonic and complex partial seizures, while activity in the ScMet screening is deemed to denote the agents of value in treating seizures (Krall et al., 1978). Neurotoxicity in mice may be measured by the rotorod test. These procedures were implemented in the present study. All the compounds were screened in these tests in doses of 30, 100, 300 mg/kg by intraperitoneal injection. The results of these screenings are summarized in Table 2. The data reveal that 70% of the compounds were active in the ScMet screening as compared to 43% in the MES test. Thus the compounds exhibit some ScMet selectivity. The majority of the compounds showed activity after 0.5 h. Compound 7 showed activity after 0.25 h. Thus indicating that the synthesized compounds are rapid acting anticonvulsants. Compounds 2, 3, 5 and 6 did not exhibit neurotoxicity at the highest administered dose. All other compounds except 7, showed neurotoxicity at 300 mg/kg. Compound 7 showed neurotoxicity at 100 mg/kg. Unsubstituted levulinic acid semicarbazone (compound 8) showed no activity in all in the screenings.
Compounds 3, 4 and 7 were further evaluated by the MES test by oral administration in rats (Table 3). Compound 3 gave 25% protection at 1 h and 50% protection at 0.5 h. Compound 4 gave 25% protection at 1 h. Compound 7 showed no activity at the dose given. These compounds exhibited no acute neurotoxicity upon oral administration.
Table 3.
Evaluation of compounds 3, 4 and 7 in the MES test by oral administration (30 mg/kg) in ratsa
| Time (h) | 3b |
4 |
7 |
|||
| MES | TOX | MES | TOX | MES | TOXc | |
| 0.25 | 1/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 0.5 | 2/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| 1.0 | 1/4 | 0/4 | 1/4 | 0/4 | 0/4 | 0/4 |
| 2.0 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
Number of animals protected/number of animals used
Evaluated at 50 mg/kg
TOX: Toxicity
DISCUSSION AND CONCLUSION
A number of clinically active anticonvulsants contain a nitrogen hetero atomic system bearing one or two phenyl rings and at least one carbonyl group in their structure. Many investigations indicated that at least one aryl group, one or two-electron donor atom and/or an NH group in a special spatial arrangement seem to be essential for anticonvulsant activity (Camerman and Camerman, 1980). In the present series of compounds, 4-aryl substituted semicarbazones of levulinic acid were designed and synthesized to meet structural requirements essential for anticonvulsant activity. The results obtained showed that the majority of the compounds exhibited anticonvulsant activity. Thus the results validated the four binding site hypothesis for semicarbazones. The results also indicated that the compounds showed some ScMet selectivity which might be due to the presence of the carboxyl group in the molecule. In the present study 4-F phenyl substituted semicarbazone emerged as the most active compound, showing broad spectrum of activity with low neurotoxicity. Inactivity of levulinic acid semicarbazone in both the screenings, validated the hypothesis that an aryl group near the semicarbazono moiety is essential for activity. Our earlier work on substituted semicarbazones of lipophilic carbonyl molecules also yielded compounds with excellent anticonvulsant activity (Aggarwal and Mishra, 2004). In the present study substituted semicarbazones of a hydrophilic molecule like levulinic acid yielded compounds with anticonvulsant activity. Thus it can be concluded that the hydrophilic-hydrophobic site in anticonvulsant semicarbazones can accommodate hydrophilic as well as lipophilic groups. These new facts might be useful in the future development of semicarbazones as novel anticonvulsants.
Acknowledgments
The authors would like to thank J.P. Stables and other members of the Anticonvulsant Drug Development Program, USA, for their extraordinary assistance in anticonvulsant evaluation. We would also like to thank Dr. Gurmeet Singh, University of Delhi, for spectral & elemental analysis and Dr. S.N. Pandeya, Banaras Hindu University, for helpful discussions. Thanks are also due to Prof. N.K. Jain, Head, Department of Pharmaceutical Sciences, Dr. Harisingh Gour University, Sagar, M.P. India, for providing laboratory facilities.
References
- 1.Aggarwal N, Mishra P. Synthesis of 4-aryl substituted semicarbazones of some terpenes as novel anticonvulsants. J Pharm Pharmaceut Sci. 2004;7(2):260–264. [PubMed] [Google Scholar]
- 2.Camerman A, Camerman N. Stereochemical Similarities in Chemically Different Antiepileptic Drugs. In: Glaser GH, Penry JK, Woodbury DM, editors. Antiepileptic Drugs: Mechanism of Action. New York: Raven Press; 1980. pp. 223–231. [PubMed] [Google Scholar]
- 3.Craig CR. Anticonvulsant Drugs. In: Craig CR, Stitzel RE, editors. Modern Pharmacology with Clinical Application. 5th Edition. New York: Little Brown and Company; 1997. pp. 391–405. [Google Scholar]
- 4.Dimmock JR, Baker GB. Anticonvulsant activities of 4-bromobenzaldehyde semicarbazone. Epilepsia. 1994;35(3):648–655. doi: 10.1111/j.1528-1157.1994.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 5.Edafiogho IO, Scott KR. Anticonvulsants. In: Wolff ME, editor. Burger’s Medicinal Chemistry and Drug Discovery. 5th Edition. Vol. 3. New York: John Wiley and Sons; 1996. pp. 175–260. [Google Scholar]
- 6.Ho B, Crider AM, Stables JP. Synthesis and structure activity relationships of potential anticonvulsants based on 2-piperidine carboxylic acid and related pharmacophores. Eur J Med Chem. 2001;36:265–286. doi: 10.1016/s0223-5234(00)01206-x. [DOI] [PubMed] [Google Scholar]
- 7.Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Anticonvulsant drug development: Anticonvulsant drug screening. Epilepsia. 1978;19:409–428. doi: 10.1111/j.1528-1157.1978.tb04507.x. [DOI] [PubMed] [Google Scholar]
- 8.Malawska B. Application of pharmacophore models for the design and synthesis of new anticonvulsant drugs. Mini Rev Med Chem. 2003;3:159–165. doi: 10.2174/1389557033488088. [DOI] [PubMed] [Google Scholar]
- 9.McNamara JO. Drugs Effective in the Therapy of the Epilepsies. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gilman’s, the Pharmacological Basis of Therapeutics. 10th Edition. New York: The McGraw-Hill; 2001. pp. 521–547. [Google Scholar]
- 10.Pandeya SN, Raja AS. Synthesis of isatin semicarbazones as novel anticonvulsants–role of hydrogen bonding. J Pharm Pharmaceut Sci. 2002;5(3):266–271. [PubMed] [Google Scholar]
- 11.Pandeya SN, Aggarwal N, Jain JS. Evaluation of semicarbazones for anticonvulsant and sedative hypnotic properties. Pharmazie. 1999;54:300–302. [PubMed] [Google Scholar]
- 12.Pandeya SN, Mishra V, Ponnilavarsan I, Stables JP. Anticonvulsant activity of p-chloro phenyl substituted aryl semicarbazones–the role of primary terminal amino group. Pol J pharmacol. 2000;52:283–290. [PubMed] [Google Scholar]
- 13.Porter RJ, Hessie BJ, Cereghino JJ, Gladding GD, Kupferberg HJ, Scoville B, White BG. Advances in the clinical development of antiepileptic drugs. Fed Proc. 1985;44(10):2645–2649. [PubMed] [Google Scholar]