Abstract
The aim of this study was to explore the protective effect of basic fibroblast growth factor (bFGF) on brain injury following global ischemia reperfusion and its mechanisms. Brain injury following global ischemia was induced by four vessels occlusion and systemic hypotension. Twenty-four rabbits were randomized into three groups: group A, only dissection of vessels; group B, intravenous infusion of normal saline after reperfusion for 6 h; group C, 30 μg/kg bFGF injected intravenously at the onset of reperfusion, then infused with 10 μg/(kg·h) for 6 h. Serum neuron specific enolase (NSE), S-100B, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-8 (IL-8) were measured before ischemia, 30 min after ischemia, 0.5, 1, 3, 6 h after reperfusion. Brain water content was determined and cerebral histopathological damages were compared. NSE and S-100B were increased 1 h after reperfusion and reached their peaks 6 h after reperfusion, but were much higher in group B than those in group C 3, 6 h after reperfusion. In groups B and C, TNF-α was increased after ischemia and IL-1 and IL-8 were increased significantly 0.5 h after reperfusion, then reached their peaks 6 h, 3 h, 6 h after reperfusion respectively. TNF-α and IL-8 at the time points of 1 h and 3 h and IL-1 at 3 h and 6 h in group C were correspondingly lower than those in group B. These indices in group A were nearly unchanged. There were less severe cerebral histopathological damages in group C compared with group B, but no difference in brain water content. It could be concluded that bFGF alleviates brain injury following global ischemia and reperfusion by down-regulating expression of inflammatory factors and inhibiting their activities.
Keywords: Basic fibroblast growth factor, Brain injury, Global ischemia, Neuron specific enolase, S-100B protein, Tumor necrosis factor-α, Interleukin
INTRODUCTION
With increasing public knowledge on cardiopulmonary resuscitation (CPR) and improvements in emergent early response, these have resulted in higher percentage of patients with out-of-hospital cardiac arrest (CA) surviving to admission (Bunch et al., 2004). The hospital discharge rate is only 2%~14%, with most patients being still left with poor neurological outcome (Snyder-Ramos and Bottiger, 2003), due to persistent brain damage. Cerebral resuscitation plays a key role during the CPR process and remains a challenge. Recent studies showed basic fibroblast growth factor (bFGF) had neuro-protective effect against focal ischemic, excitotoxic and traumatic brain injury (Dietrich et al., 1996; Bethel et al., 1997; Himmelseher et al., 1997; Yoshimura et al., 2003). However the effect on global cerebral ischemia-reperfusion injury, following CA and CPR, has not been investigated. By creating an animal model of global ischemia and reperfusion, we investigated effects of bFGF on serum neuron specific enolase (NSE), S-100B, tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), interleukin-8 (IL-8), in order to explore the potential neuroprotection of bFGF in cardiopulmonary resuscitation and the possible mechanisms.
MATERIALS AND METHODS
Animals and model
Cerebral ischemia-reperfusion injury following cardiac arrest was simulated by four vessels occlusion (2 carotids and 2 vertebral arteries) and systemic hypotension (Pomfy and Franko, 1999). Twenty-four healthy rabbits (supplied by Experimental Animal Center, Medical Institute of Zhejiang Province) with mean body weight of 2.6 kg (2.2~3.0 kg), regardless of gender, were randomized into three groups: group A (n=8), subjected to dissection of four vessels without occlusion; group B (n=8), who underwent dissection and occlusion of vessels, infusion of normal saline (NS); group C (n=8), who underwent dissection and occlusion of vessels, infusion of bFGF. The rabbits were anesthetized by slow intravenous injection of Diazepam (4 mg/kg), Haloperidol (0.8 mg/kg) and Fentanyl (16 μg/kg). The femoral artery was cannulated and connected to a transducer. Direct arterial blood pressure together with electrocardiograph (ECG), rectal temperature and partial pressure of end tidal carbon dioxide (P ET,CO2) were monitored using a multiple-parameters monitor (MB-526, USA). Rectal temperature was kept at about 38.5 °C with a heating system. Tracheotomy was performed and the rabbits were intubated, ventilated with an DH-150 animal ventilator (Medical Instrument Factory of Zhejiang University, China). Ventilator settings were adjusted to maintain P ET,CO2 at 40 mmHg. Both common carotids and vertebral arteries were dissected and clamped, while hypotension was induced by exsanguinations via femoral arteries, mean blood pressure was kept at 50%~60% of basal value. After 30 min, the four vessels were unclamped and blood was transfused rapidly. In group B, a bolus of 5 ml NS was given intravenously, followed by a continuous infusion of NS at a rate of 5 ml/(kg·h). In group C, it was replaced by a bolus of 30 μg/kg bFGF (Recombinant bovine basic fibroblast growth factor, 36 micrograms/ampoule, Changchun Changsheng Gene Pharmaceutical Co. Ltd., China), then 10 μg/(kg·h) infused continuously, dissolved in the same volume of NS.
Sample preparation and measurements
Three milliliters of blood samples were taken before occlusion of vessels (basal time), at the end of ischemia (0 h), 0.5, 1, 3, 6 h after reperfusion respectively and the same volume of NS was infused after sampling. All blood specimens were processed to serum, centrifugated at 3000 rpm for 10 min at room temperature, then stored in aliquots at −80 °C until batch evaluation. Serum NSE, S-100B, TNF-α, IL-1 and IL-8 were measured by enzyme linked immunosorbant assay on Opsys MR microplate reader (Dynex Inc., USA), according to the operating instructions. Kits for rabbit NSE and S-100B were supplied by Lifekey BioMeditech Inc. (USA), and those for TNF-α, IL-1, IL-8 by Sigma Inc. (USA). The animals were sacrificed by exsanguination 6 h after reperfusion. The right hemispheres were sampled and kept at 100 °C in a drier for 12 h, then the brain water content was determined by the difference between dry and wet weight. The left hemispheres were stored in 10% formalin. Histopathological sections were performed routinely, stained with hematoxylin and eosin (HE).
Statistical analysis
All data were expressed as mean±standard deviation. Differences between groups or different time points were compared using one-way ANOVA and Tukey test (SigmaStat 2.03 software package was used). Correlation between serum NSE and S-100B was analyzed with linear regression. A P value of <0.05 was considered as statistically significant.
RESULTS
General animal characteristics
There were no differences in body weight, sexual ratio, and basal values of rectal temperature, arterial blood pressure and heart rate between the three groups. Compared with group A, arterial blood pressure in groups B and C increased rapidly after occlusion of four vessels, and was maintained at half of the basal value during exsanguination, decreased sequentially after reperfusion, ameliorated gradually after transfusion, then decreased again 1 h after reperfusion. Blood pressure at different time points in group C seemed slightly lower than that in group B, but the differences were not statistically significant.
Changes in serum NSE and S-100B
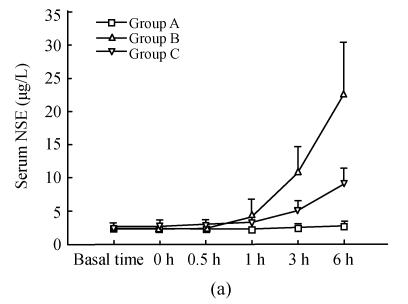
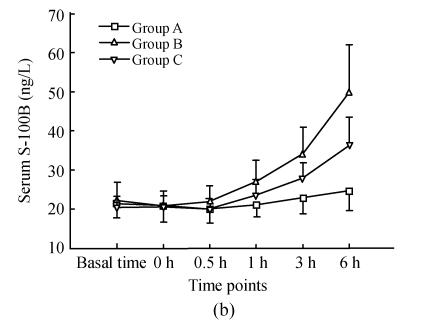
As shown in Fig.1, serum NSE and S-100B in group A at basal time were (2.51±0.67) μg/L, (21.53±3.84) ng/L respectively, remaining unchanged throughout the study. There were no differences between serum NSE and S-100B in groups B and C from those in group A at the first three time points. One hour after reperfusion, serum NSE and S-100B increased, then reached their peaks 6 h after reperfusion. Serum NSE and S-100B in group B were much higher than those in group C 3 h, 6 h after reperfusion. There was significant positive correlation between serum NSE and S-100B (r=0.736, P<0.001).
Fig. 1.
Dynamic changes of serum NSE and serum S-100B in three groups (a) Serum NSE at the time points of 3 h, 6 h in group C were significantly less than those in group B (q=6.701, 8.358; both P<0.001); (b) Serum S-100B at the time points of 3 h, 6 h in group C were significantly less than those in group B (q=3.582, 4.379; P=0.049, 0.015)
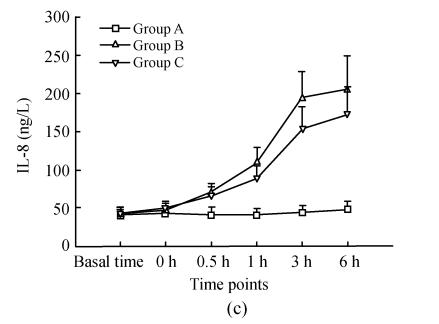
Changes in serum TNF-α, IL-1, IL-8
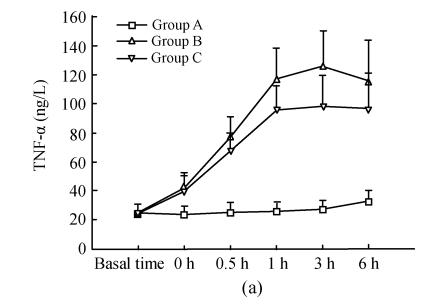
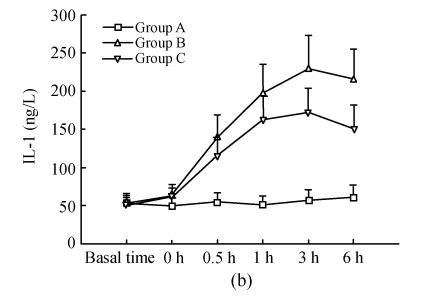
Details are shown in Fig.2. There were no differences for serum TNF-α, IL-1, IL-8 between the three groups at the basal point. Compared with that at basal time point, TNF-α in group B and C increased significantly 30 min after ischemia (0 h) and reached its peak 3 h after reperfusion; these were correspondingly much higher than those in group A; TNF-α at time points of 1 h, 3 h in group B were significantly higher than that in group C, while there were no difference at other time points. IL-1 and IL-8 in groups B and C increased significantly 0.5 h after reperfusion and reached their peaks 3 h, 6 h after reperfusion respectively, and were much higher than those in group A; IL-1 at time points of 3 h, 6 h in group B were significantly higher than those in group C; IL-8 at time points of 1 h, 3 h in group B were significantly higher than those in group C.
Fig. 2.
Dynamic changes of serum TNF-α (a), IL-1 (b), IL-8 (c) in three groups. Compared with that at basal time point, TNF-α in groups B and C increased significantly 30 min after ischemia (0 h) and reached its peak 3 h after reperfusion; these were correspondingly much higher than those in group A; TNF-α at time points of 1 h, 3 h in group B were significantly higher than those in group C (q=3.665, 4.158; P=0.043, 0.021), whereas there was no difference at other time points. IL-1 and IL-8 in group B and C increased significantly 0.5 h after reperfusion and reached their peaks 3 h, 6 h after reperfusion respectively, and were much higher than those in group A; IL-1 at time points of 3 h, 6 h in group B were significantly higher than those in group C (q=5.168, 5.938; P=0.004, 0.001); IL-8 at time points of 1 h, 3 h in group B were significantly higher than those in group C (q=3.852, 4.317; P=0.033, 0.016)
Brain water content
Brain water content was significantly higher in groups B and C (80.19%±0.70%, 80.45%±0.30%) as compared with 78.49%±0.59% in group A (F=6.519, P=0.006; q=3.610, 4.933 and P=0.047, 0.006 respectively). But there was no difference between groups B and C (q=1.322, P=0.625).
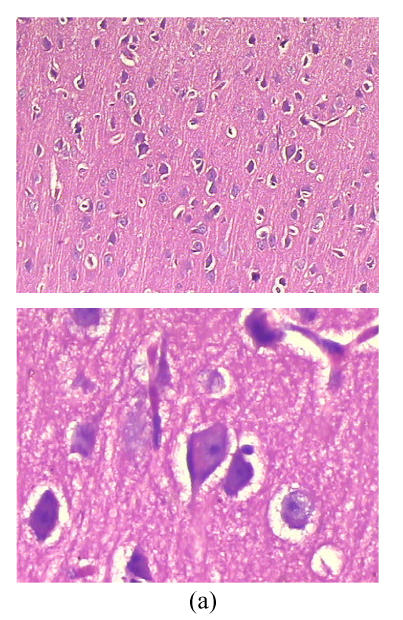
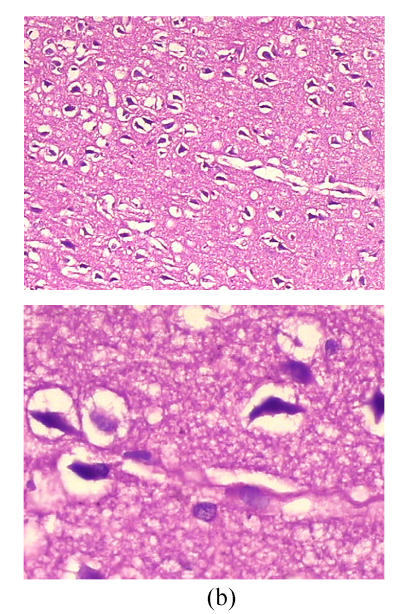
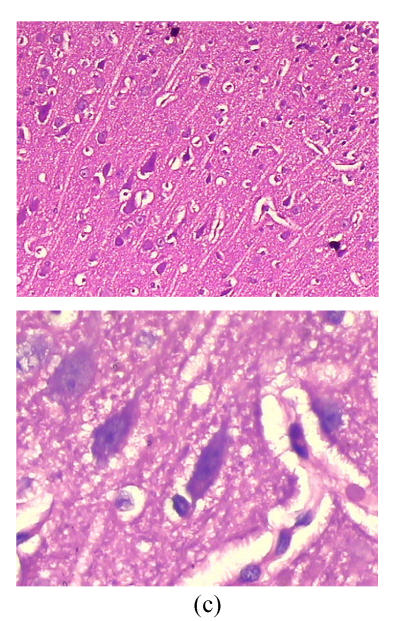
Cerebral histopathological damages
As shown in Fig.3, neurons in group A had normal sizes, clear contour and axons, intact organelles in group B, neurons were shrunken and deeply stained, had unclear structures of organelles and significantly increased gap around neurons and microvessels. Damages of cerebral microscopic structures in group C were less severe than those in group B. Neurons had nearly normal sizes, slightly blurry contour and organelles, had less increased gap around neurons and microvessels.
Fig. 3.
Cerebral histopathologica changes in 3 groups (Stained with HE; Upper, magnification of ×100; Lower, magnification of ×400) (a) In group A, neurons had normal sizes, clear contour and axons, intact organelles; (b) In group B, neurons were shrunken and deeply stained, had unclear structures of organelles and significantly increased gap around neurons and microvessels; (c) In group C, neurons had nearly normal sizes, slightly blurry contour and organelles, less increased gap around neurons and microvessels
DISCUSSION
Complete cerebral ischemia could not be achieved by simplex occlusion of two common carotids and two vertebral arteries in the animals (Furlow, 1982). When this combined with systemic hypotension induced by exsanguinations and reperfusion, changes of blood flow in the entire organism and brain following cardiac arrest and resuscitation are well simulated. So it may be accepted as an ideal animal model for cardiopulmonary cerebral resuscitation. Compared with the lower mean arterial blood pressure (35~40 mmHg) induced during ischemia in one published study (Wang et al., 2001), about 50%~60% of basal values (50~60 mmHg) was maintained in the present experiment. In a preliminary study, we found that animals could hardly survive for more than 3 h with such low blood pressure during ischemia. Furthermore the rate and volume of exsanguination had to be well controlled to ensure a stable animal model.
Neuron-specific enolase (NSE) is an isoenzyme of the glycplytic enzyme enolase (2-phospho-D-glycerate hydrolase), a dimeric enzyme composed of 2 gamma-subunits with a molecular weight of 78 kDa and biological half-life of approximately 24 h (Tiainen et al., 2003). It is nearly exclusively located in neurons, neuroendocrine cells (APUD cells) and neuroendocrine tumors, including small-cell lung cancer (Tiainen et al., 2003). S-100B is a dimeric acidic calcium-binding protein with a molecular weight of 21 kDa and biological half-life of 0.5 h (Jonsson et al., 2000), and is found especially in high concentrations in astroglial cells that are prevalent in the white matter of the brain (Mussack et al., 2002). When the brain suffers from different insults leading to impaired cellular structure and blood-brain-barrier integrity, NSE and S-100B are released quite rapidly into the cerebrospinal fluid and then into the circulation. Increased serum levels are proportionally related to the extent of cellular brain injury (Martens et al., 1998). So serum NSE and S-100B are sensitive and quantitative markers of parenchymal brain injury due to ischemia stroke, intracerebral hemorrhage, trauma and cardiopulmonary surgery (Mabe et al., 1991; Skogseid et al., 1992; Schaarschmidt et al., 1994; Isgro et al., 1997; Missler et al., 1997; Elting et al., 2000; Jonsson et al., 2001). Cardiac arrest leads to a period of temporary systemic and global cerebral ischemia, while the following cardiopulmonary resuscitation causes reperfusion injury, mediated by a series of inflammatory responses (Jean et al., 1998). As a consequence, the observed significantly elevated NSE and S-100B levels in the blood served as predictors of neurological outcome in several previous studies (Martens et al., 1998; Schoerkhuber et al., 1999; Rosen et al., 2001; Mussack et al., 2002; Tiainen et al., 2003).
In the present study, serum NSE and S-100B began to increase 1 h after reperfusion, continued to rise and reached their peaks 6 h later in groups B and C. The good correlation between the two indices (r=0.736) indicated increases of serum NSE and S-100B occurred very early after global cerebral ischemia-reperfusion injury. In group C treated with basic fibroblast growth factor, changes of blood pressure, heart rate and body temperature were similar to those in group B. But serum NSE and S-100B levels were lower at corresponding time points in group C, and with less severe cerebral histopathological damages. So it could be deduced that bFGF alleviated global cerebral ischemia-reperfusion injury, which was in accordance with previous findings showing that bFGF protected the brain from different insults. Himmelseher et al.(1997) found that bFGF increased cell survival and supported axonal re-elongations in adult hippocampal neurons in vitro when applied after axotomy. In cats undergoing left middle cerebral artery (MCA) occlusion for 4 h, intravenous bFGF at a rate of 5, 50, 250 μg/kg per hour decreased injury volume of ipsilateral cerebral cortex (Bethel et al., 1997). A continuous intravenous infusion of 45 μg/(kg·h) bFGF for 3 h, beginning 30 min after fluid-percussion brain injury, reduced both the number of damaged neurons and the contusion volume in rats (Dietrich et al., 1996). Brain water content was not decreased by bFGF in the present study, with results different from those of Liu and Zhu (1998) who found that bFGF minimized brain water content in rat following forebrain ischemia-reperfusion, which could probably be attributable to different animal models employed. In this study, the test animals underwent complete global cerebral ischemia and longer time of reperfusion, which led to much severer brain injury.
Inflammatory response plays a main causative role in cerebral ischemia-reperfusion injury, in which a variety of inflammatory mediators are involved. Increased levels of TNF-α are observed early in cerebral ischemia and reperfusion (Liu et al., 1994; Buttini et al., 1996). The sources of TNF-α under these conditions include activated macrophages/monocytes, neurons, astrocytes and microglias. TNF-α recruits and activates leukocytes, leading to increased leukocyte-endothelial adhesion (Fabry et al., 1992; Liu et al., 1994), which progressively induces expression of other cytokines, including IL-1, IL-6, IL-8 and so on, thus forming a positive feedback loop further exacerbating brain injury. Levels of TNF-α, IL-1 and IL-8 are positively correlated proportionably to neurons damage, brain edema and infarction extension, and can serve as good indicators of cerebral ischemia-reperfusion injury (Yamasaki et al., 1995; Barone et al., 1997; Yang et al., 1997). Elevated serum IL-8 and TNF-α levels comprise the worse outcome in patients following out-of-hospital cardiac arrest (Ito et al., 2001). Blocking or countering their activities lead to decreased neutrophil infiltration, blood-brain barrier breakdown, infarct size and extent of injury (Kadoya et al., 1995; Matsumoto et al., 1997; Yamasaki et al., 1997; Yang et al., 1997). In the present study, serum TNF-α was increased 30 min after ischemia, continued to rise after reperfusion, concomitant with increased serum IL-1 and IL-8. In those treated with bFGF, serum TNF-α, IL-1 and IL-8 were significantly decreased 1, 3, 6 h after reperfusion, compared with those in group B. It may indicate that bFGF probably exerts its neuroprotective effect by interfering with the expression and functions of these cytokines. In rats undergoing superior mesenteric artery occlusion (45 min) and reperfusion (3 d), an intravenous administration of 4 μg bFGF at the onset of reperfusion reduced serum tumor necrosis factor-alpha and free radicals, while protecting gut and liver against ischemia and reperfusion injury (Fu et al., 1997). bFGF could suppress apoptosis of L929 cells induced by TNF-α, which was mediated by activation of the MAPK (mitogen activated protein kinases) intracellular signaling pathways (Gardner and Johnson, 1996). bFGF also reduced IL-1 secretion (Bodo et al., 2000), and down-regulated expression of vascular cell adhesion molecules stimulated by TNF-α and IL-1, resulting in markedly decreased levels of leukocyte recruitment and adhesion (Kikuchi, 2000; Tromp et al., 2000), subsequently decreasing tissue injury.
CONCLUSION
The present study suggests that basic fibroblast growth factor alleviates brain injury following global ischemia reperfusion. And this is probably achieved by down-regulating expression of inflammatory factors and countering their activities to reduce inflammatory injury during reperfusion. It shows promising application in patients with cardiac arrest and deserves further investigations.
References
- 1.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28(6):1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 2.Bethel A, Kirsch JR, Koehler RC, Finklestein SP, Traystman RJ. Intravenous basic fibroblast growth factor decreases brain injury resulting from focal ischemia in cats. Stroke. 1997;28(3):609–615. doi: 10.1161/01.str.28.3.609. [DOI] [PubMed] [Google Scholar]
- 3.Bodo M, Baroni T, Carinci F, Becchetti E, Conte C, Bellucci C, Pezzetti F, Calvitti M, Bellocchio S, Stabellini G, et al. Interleukin secretion, proteoglycan and procollagen alpha (1) gene expression in Crouzon fibroblasts treated with basic fibroblast growth factor. Cytokine. 2000;12(8):1280–1283. doi: 10.1006/cyto.1999.0730. [DOI] [PubMed] [Google Scholar]
- 4.Bunch TJ, West CP, Packer DL, Panutich MS, White RD. Admission predictors of in-hospital mortality and subsequent long-term outcome in survivors of ventricular fibrillation out-of-hospital cardiac arrest: A population-based study. Cardiology. 2004;102(1):41–47. doi: 10.1159/000077003. [DOI] [PubMed] [Google Scholar]
- 5.Buttini M, Appel K, Sauter A, Gebicke-Haerter PJ, Boddeke HW. Expression of tumor necrosis factor alpha after focal cerebral ischemia in the rat. Neuroscience. 1996;71(1):1–16. doi: 10.1016/0306-4522(95)00414-9. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich WD, Alonso O, Busto R, Finklestein SP. Posttreatment with intravenous basic fibroblast growth factor reduces histopathological damage following fluid-percussion brain injury in rats. J Neurotrauma. 1996;13(6):309–316. doi: 10.1089/neu.1996.13.309. [DOI] [PubMed] [Google Scholar]
- 7.Elting JW, de Jager AE, Teelken AW, Schaaf MJ, Maurits NM, van der Naalt J, Sibinga CT, Sulter GA, de Keyser J. Comparison of serum S-100 protein levels following stroke and traumatic brain injury. J Neuro Sci. 2000;181(1-2):104–110. doi: 10.1016/s0022-510x(00)00442-1. [DOI] [PubMed] [Google Scholar]
- 8.Fabry Z, Waldschmidt MM, Hendrickson D, Keiner J, Love-Homan L, Takei F, Hart MN. Adhesion molecules on murine brain microvascular endothelial cells: Expression and regulation of ICAM-1 and Lgp 55. J Neuroimmunol. 1992;36(1):1–11. doi: 10.1016/0165-5728(92)90026-h. [DOI] [PubMed] [Google Scholar]
- 9.Fu X, Sheng Z, Wang Y, Ye Y, Xu M, Sun T, Zhou B. Basic fibroblast growth factor reduces the gut and liver morphologic and functional injuries after ischemia and reperfusion. J Trauma. 1997;42(6):1080–1085. doi: 10.1097/00005373-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Furlow TW. Cerebral ischemia produced by four-vessel occlusion in the rat: A quantitative evaluation of cerebral blood flow. Stroke. 1982;13(6):852–855. doi: 10.1161/01.str.13.6.852. [DOI] [PubMed] [Google Scholar]
- 11.Gardner AM, Johnson GL. Fibroblast growth factor-2 suppression of tumor necrosis factor alpha-mediated apoptosis requires Ras and the activation of mitogen-activated protein kinase. J Biol Chem. 1996;271(24):14560–14566. doi: 10.1074/jbc.271.24.14560. [DOI] [PubMed] [Google Scholar]
- 12.Himmelseher S, Pfenninger E, Georgieff M. Effects of basic fibroblast growth factor on hippocampal neurons after axonal injury. J Trauma. 1997;42(4):659–664. doi: 10.1097/00005373-199704000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Isgro F, Schmidt C, Pohl P, Saggau W. A predictive parameter in patients with brain related complications after cardiac surgery? Eur J Cardiothorac Surg. 1997;11(4):640–644. doi: 10.1016/s1010-7940(96)01089-5. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Saitoh D, Fukuzuka K, Kiyozumi T, Kawakami M, Sakamoto T, Okada Y. Significance of elevated serum interleukin-8 in patients resuscitated after cardiopulmonary arrest. Resuscitation. 2001;51(1):47–53. doi: 10.1016/s0300-9572(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 15.Jean WC, Spellman SR, Nussbaum ES, Low WC. Reperfusion injury after focal cerebral ischemia: The role of inflammation and the therapeutic horizon. Neurosurgery. 1998;43(6):1382–1396. doi: 10.1097/00006123-199812000-00076. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson H, Johnsson P, Hoglund P, Alling C, Blomquist S. Elimination of S100B and renal function after cardiac surgery. J Cardiothorac Vasc Anesth. 2000;14(6):698–701. doi: 10.1053/jcan.2000.18444. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson H, Johnsson P, Birch-Iensen M, Alling C, Westaby S, Blomquist S. S100B as a predictor of size and outcome of stroke after cardiac surgery. Ann Thorac Surg. 2001;71(5):1433–1437. doi: 10.1016/s0003-4975(00)02612-6. [DOI] [PubMed] [Google Scholar]
- 18.Kadoya C, Domino EF, Yang GY, Stern JD, Betz AL. Preischemic but not postischemic zinc protoporphyrin treatment reduces infarct size and edema accumulation after temporary focal cerebral ischemia in rats. Stroke. 1995;26(6):1035–1038. doi: 10.1161/01.str.26.6.1035. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi H. Differential influences of bFGF and VEGF on the expression of vascular cell adhesion molecule-1 on human umbilical vein endothelial cells. Nihon Rinsho Meneki Gakkai Kaishi. 2000;23(1):12–21. doi: 10.2177/jsci.23.12. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Zhu XZ. Basic fibroblast growth factor protected forebrain against ischemia-reperfusion damage in rats. Acta Pharmacologica Sinica. 1998;19(6):527–530. (in Chinese) [PubMed] [Google Scholar]
- 21.Liu T, Clark RK, McDonnell PC, Young PR, White RF, Barone FC, Feuerstein GZ. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994;25(7):1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- 22.Mabe H, Suzuki S, Mase M, Umemura A, Nagai H. Serum neuron-specific enolase levels after subarachnoid hemorrhage. Surg Neurol. 1991;36(3):170–174. doi: 10.1016/0090-3019(91)90108-l. [DOI] [PubMed] [Google Scholar]
- 23.Martens P, Raabe A, Johnsson P. Serum S-100 and neuron-specific enolase for prediction of regaining consciousness after global cerebral ischemia. Stroke. 1998;29(11):2363–2366. doi: 10.1161/01.str.29.11.2363. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto T, Ikeda K, Mukaida N, Harada A, Matsumoto Y, Yamashita J, Matsushima K. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest. 1997;77(2):119–125. [PubMed] [Google Scholar]
- 25.Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28(10):1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- 26.Mussack T, Biberthaler P, Kanz KG, Wiedemann E, Gippner-Steppert C, Mutschler W, Jochum M. Serum S-100B and interleukin-8 as predictive markers for comparative neurologic outcome analysis of patients after cardiac arrest and severe traumatic brain injury. Crit Care Med. 2002;30(12):2669–2674. doi: 10.1097/00003246-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Pomfy M, Franko J. Validation of a four-vessel occlusion model for transient global cerebral ischemia in dogs. J Hirnforsch. 1999;39(4):465–471. [PubMed] [Google Scholar]
- 28.Rosen H, Sunnerhagen KS, Herlitz J, Blomstrand C, Rosengren L. Serum levels of the brain-derived proteins S-100 and NSE predict long-term outcome after cardiac arrest. Resuscitation. 2001;49(2):183–191. doi: 10.1016/s0300-9572(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 29.Schaarschmidt H, Prange HW, Reiber H. Neuron-specific enolase concentrations in blood as a prognostic parameter in cerebrovascular diseases. Stroke. 1994;25(3):558–565. doi: 10.1161/01.str.25.3.558. [DOI] [PubMed] [Google Scholar]
- 30.Schoerkhuber W, Kittler H, Sterz F, Behringer W, Holzer M, Frossard M, Spitzauer S, Laggner AN. Time course of serum neuron-specific enolase. A predictor of neurological outcome in patients resuscitated from cardiac arrest. Stroke. 1999;30(8):1598–1603. doi: 10.1161/01.str.30.8.1598. [DOI] [PubMed] [Google Scholar]
- 31.Skogseid IM, Nordby HK, Urdal P, Paus E, Lilleaas F. Increased serum creatine kinase BB and neuron specific enolase following head injury indicates brain damage. Acta Neurochir (Wien) 1992;115(3-4):106–111. doi: 10.1007/BF01406367. [DOI] [PubMed] [Google Scholar]
- 32.Snyder-Ramos SA, Bottiger BW. Molecular markers of brain damage–clinical and ethical implications with particular focus on cardiac arrest. Restor Neurol Neurosci. 2003;21(3-4):123–139. [PubMed] [Google Scholar]
- 33.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 34.Tromp SC, Oude Egbrink MG, Dings RP, van Velzen S, Slaaf DW, Hillen HF, Tangelder GJ, Reneman RS, Griffioen AW. Tumor angiogenesis factors reduce leukocyte adhesion in vivo. Int Immunol. 2000;12(5):671–676. doi: 10.1093/intimm/12.5.671. [DOI] [PubMed] [Google Scholar]
- 35.Wang XB, Kuang X, Wu HX, Non N. Delayed protective effect of adenosine A1 receptors on brain following preconditioning ischemia in rabbits. Chin J Anaeth (Chin) 2001;21(1):40–43. [Google Scholar]
- 36.Yamasaki Y, Matsuo Y, Matsuura N, Onodera H, Itoyama Y, Kogure K. Transient increase of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family, in ischemic brain areas after focal ischemia in rats. Stroke. 1995;26(2):318–322. doi: 10.1161/01.str.26.2.318. [DOI] [PubMed] [Google Scholar]
- 37.Yamasaki Y, Matsuo Y, Zagorski J, Matsuura N, Onodera H, Itoyama Y, Kogure K. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997;759(1):103–111. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- 38.Yang GY, Zhao YJ, Davidson BL, Betz AL. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751(2):181–188. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Qiu J, Harada J, Waeber C, Breakefield XO, Moskowitz MA. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112(8):1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]