Abstract
Objective: To investigate effects of developmental lead exposure on nitric oxide synthase (NOS) activity in different brain regions and on N-methyl-D-aspartate (NMDA) receptor mRNA expression in the hippocampus of rats. On the basis of these observations, we explored possible mechanisms by which lead exposure leads to impaired learning and memorizing abilities in children. Methods: A series of rat animal models exposed to low levels of lead during the developing period was established (drinking water containing 0.025%, 0.05% and 0.075% lead acetate). NOS activities in the hippocampus, the cerebral cortex, the cerebellum and the brain stem were determined with fluorescence measurement and levels of mRNA expression of the NMDA receptor 2A (NR2A) subunit and NMDA receptor 2B (NR2B) subunit in the rat hippocampus were measured with Retro-translation (RT-PCR). Results: There were no differences in the body weight of rat pups between any of the groups at any given time (P>0.05). The blood lead level of Pb-exposed rat pups showed a systematic pattern of change: at 14 d of age, it was lower than that at 7 d of age, then rising to the peak level at 21 d and finally falling to lower levels at 28 d. The hippocampal NOS activities of lead-exposed groups were all lower than that of the control group on the 21st and 28th day (P<0.01). NOS activities in the cerebellum of lead-exposed groups were all lower than that of the control group on the 21st and 28th day (P<0.001) and the NOS activity of the 0.025% group was significantly lower than that of the 0.05% and 0.075% groups on the 28th day (P<0.05). NOS activity in the cerebral cortex of the 0.075% group was significantly lower than that of the control, 0.025% and 0.05% groups on the four day spans (P<0.001). There was no significant difference of NOS activity in the brain stem between any lead-exposed group and the control group on the four day spans. In the 0.05% and the 0.075% groups, the level of NR2A mRNA expression was higher than that in the control group at 7 d and 14 d of age (P<0.05). In the 0.025% group, the level of NR2A was found to be higher than that in the control group at 7 d of age only (P<0.05). No significant differences were found for the levels of NR2B mRNA expression between any of the groups at any given time. Conclusions: NOS activity in the hippocampus, the cerebral cortex and the cerebellum are inhibited by lead exposure. The degree of the inhibitory effect depends on the time span of exposure and the lead concentration. Developmental low-level lead exposure was found to raise the level of NR2A mRNA expression in the hippocampus of rats. Developmental low-level lead exposure does not affect the level of NR2B mRNA expression in the hippocampus.
Keywords: Lead exposure, Rat pups, Nitric oxide synthase (NOS), Fluorescence, Hippocampus, mRNA, Retro-translation (RT-PCR), N-methyl-D-aspartate receptor (NMDAR)
INTRODUCTION
Lead (Pb) is a neurotoxic heavy metal. Children in the developmental stage are particularly susceptible to the toxic effects of lead exposure. Epidemiological investigations have established the relationship between chronic developmental lead exposure and cognitive impairments in young children (Bellinger et al., 1991). The diffusible nitric oxide (NO) is a biological messenger known to be involved in brain development. And the N-methyl-D-aspartate (NMDA) receptor is an excitatory amino acid receptor that plays an important role in learning and memorizing (Collingridge and Singer, 1990). NO is a gaseous substance produced by the nitric oxide synthase (NOS) from L-arginine. As NO is an unstable and reactive compound with a short half-life, the NOS activity is commonly measured reflects the NO levels. Lead affects the NO production through the inhibition of the NOS activity (Chetty et al., 2001). NMDA receptors in the hippocampus region are an important target of lead during the developmental period (Lasley et al., 2001). In this study, an animal model of rats in the developing period was exposed to lead and NOS activities were measured in different brain regions (hippocampus, cerebellum, cerebral cortex, brain stem) in order to investigate the influence of lead on the NOS activity. Semi-quantitative RT-PCR was used to measure the level of mRNA expression of the subunits NR2A and NR2B in the hippocampus, to get better understanding of the influence of lead on the NMDA receptor gene expression and to explore possible mechanisms of the neurotoxic effect of lead exposure that leads to learning and memory impairment in children.
MATERIALS AND METHODS
Materials
The Experimental Animal Laboratory of Zhejiang University, School of Medicine provided the thoroughbred SD rats. The lead blood level measurements were done with an atomic absorptiometer (AA700) manufactured by Perkin Elmer Company, USA. The blood lead standard samples were provided by the Wisconsin State Laboratory of Hygiene in the USA. The Nanjing Established Bioengineering Institute produced the kit with the NOS determination reagents. The NR2A, NR2B and β-actin primer were synthesized at Shanghai Bioengineering. DNA scanner (IS 1000 digital image system) was made by Alpha Innotech Corporation. Trizol and PCR marker were bought from Roche and Sigma respectively.
Methods
1. Establishing the animal model: We used 26 healthy mature female SD rats, which were randomly assigned to four groups. There were 8 subjects in the control group that were fed distilled water. The six subjects in each of the other three groups were fed distilled water containing 0.025%, 0.05% and 0.075% lead acetate, respectively. After two weeks, the female rats were mated with male rats at the ratio of 2:1, and after three weeks of pregnancy, the rat pups were delivered. Milk feeding was discontinued on the 21st day after birth (counting the day of birth as zero) and drinking water was fed with lead concentrations similar to that which the adult rats received until the 28th day after birth. On the 7th, 14th, 21st and 28th day after birth, one or two pups were randomly taken from each nest without regard to their sex. A 0.5 ml blood sample was taken by cardiac puncture, EDTA was added for anticoagulation, and the blood samples were then used for the determination of lead blood levels. The pups were decapitated, the hippocampus, cerebellum, cerebral cortex and brain stem were quickly isolated in ice water, washed in 0~4 °C physiological saline, divided and stored at −70 °C.
2. NOS activity determination: NOS activity was measured with the fluorescence method (Han, 1993). The measured sample was compared to the light absorption of a blank control liquid, and the NOS activity was calculated on the basis of the protein. One unit of NOS activity equals a production of 1 nmol NO per minute. The enzyme activity was expressed in units per milligram protein (U/mg protein).
3. PT-PCR: RNA was extracted with Trizol extraction liquid and stored at −70 °C after the determination of purity and concentration. cDNA was synthesised by reverse transcription with OligodT and AMV Rtase. NR2A and NR2B primer were each separately incubated with the internal control β-actin primer to obtain the desired DNA product. Using a 2% agarose gel electrophoresis with a PCR Marker as a molecular weight comparison, the DNA density was measured with a DNA scanner and the density of the subunit band and the density of the internal control β-actin band were determined. The value of the density ratio (subunit/β-actin) represented the level of each subunit mRNA.
4. Statistic analysis was done with the computer program Statistical Package for the Social Sciences (SPSS) 8.0. Body weight, lead blood levels and the results of the RT-PCR in each group were analysed with one-way analysis of variance (ANOVA). The comparison of NOS activities was done with covariance analysis. The ratios of subunit/β-actin were changed from X to lg(X+1) to achieve homogeneity before analysis. For all statistical comparisons, P<0.05 was considered significant. All values were expressed as means±standard deviation (X±SD).
RESULTS
Results from the animal model
1. None of the lead-exposed rat pups showed any neurological, gastroenteral or other symptoms of lead poisoning, such as diarrhoea or seizures, over the course of the experiment. The body weight of the pups did not show any significant difference between any of the groups nor at any age (P>0.05).
2. In all groups of lead exposed rats, the lead blood levels were significantly higher than those in the control group in all measurements (P<0.001). Furthermore, there were significant differences between the lead exposed groups. On the 7th and on the 28th day, the blood lead levels of the 0.05% and the 0.075% groups were significantly higher than those in the 0.025% group (P<0.001). On the 14th day, the blood lead level in the 0.075% group was significantly higher than that in the 0.025% and the 0.05% groups (P<0.05).
3. In all three groups exposed to lead, blood levels showed the same characteristic pattern related to the age of the pups: the lead levels were relatively high on the 7th day, decreased until the 14th day, reached their peak on the 21st day and decreased again until the 28th day after birth (Fig.1). The lead level on the 21st day was significantly higher than that on the 7th day and on the 14th day (P<0.05).
Fig. 1.
Changes in blood lead concentration related to the age of the pups (measured in μg/L)
Effects of lead exposure on NOS activity in each brain region
1. Effect of lead exposure on hippocampal NOS activity
The hippocampal NOS activity of lead-exposed groups were all lower than that of the control group on the 21st and 28th day. The NOS activity of the 0.025% group was significantly lower than that of the 0.075% group on the 21st day, and lower than that of the 0.05% and 0.075% groups on the 28th day (Table 1).
Table 1.
Comparison of NOS activity between groups in hippocampus (X±SD, unit: U/mg protein)
| Group | 7 d (n) | 14 d (n) | 21 d (n) | 28 d (n) |
| Control group | 2.35±0.31 (8) | 2.33±0.20 (7) | 2.36±0.18 (6) | 2.19±0.06 (6) |
| 0.025% group | 1.89±0.44 (8) | 1.95±0.16 (8) | 1.53±0.20 (6)* | 1.12±0.27 (6)* |
| 0.05% group | 1.97±0.12 (6) | 2.16±0.08 (6) | 1.66±0.23 (5)* | 1.80±0.20 (4)*▲ |
| 0.075% group | 2.04±0.82 (6) | 2.16±0.21 (8) | 1.88±0.32 (6)*▲ | 1.66±0.21 (6)*▲ |
Compared with the control group at the same days, P<0.01
Compared with 0.025% group at the same days, P<0.05
2. Effect of lead exposure on NOS activity in the cerebellum of rat pups
NOS activities in the cerebellum of lead-exposed groups were all lower than that of the control group on the 21st and 28th day. The NOS activity of the 0.025% group was significantly lower than that of the 0.05% and 0.075% groups on the 28th day (Table 2).
Table 2.
Comparison of NOS activity between groups in cerebellum (X±SD, unit: U/mg protein)
| Group | 7 d (n) | 14 d (n) | 21 d (n) | 28 d (n) |
| Control group | 1.93±0.19 (7) | 1.43±0.29 (6) | 1.41±0.18 (7) | 1.54±0.50 (8) |
| 0.025% group | 1.81±0.12 (8) | 1.58±0.17 (6) | 0.87±0.24 (5)* | 0.43±0.09 (6)* |
| 0.05% group | 1.74±0.22 (8) | 1.59±0.25 (7) | 0.85±0.09 (5)* | 0.70±0.14 (6)*▲ |
| 0.075% group | 1.85±0.30 (7) | 1.60±0.26 (5) | 0.91±0.18 (6)* | 0.82±0.11 (8)*▲ |
Compared with the control group at the same days, P<0.01
Compared with 0.025% group at the same days, P<0.05
3. Effect of lead exposure on NOS activity in the cerebral cortex of rat pups
NOS activity in the cerebral cortex of the 0.075% group was significantly lower than that of the control, 0.025% and 0.05% groups on the four day spans. And there was no significant difference among the three later groups (Table 3).
Table 3.
Comparison of NOS activity between groups in cerebral cortex (X±SD, unit: U/mg protein)
| Group | 7 d (n) | 14 d (n) | 21 d (n) | 28 d (n) |
| Control group | 2.54±0.31 (7) | 1.64±0.22 (7) | 1.24±0.14 (8) | 1.26±0.07 (7) |
| 0.025% group | 2.42±0.19 (7) | 1.59±0.17 (6) | 1.27±0.12 (6) | 1.21±0.09 (6) |
| 0.05% group | 2.56±0.53 (7) | 1.77±0.19 (6) | 1.24±0.10 (5) | 1.19±0.42 (5) |
| 0.075% group | 1.29±0.14 (7)* | 1.03±0.15 (7)* | 0.69±0.10 (6)* | 0.53±0.09 (6)* |
Compared with the other 3 groups at the same day spans, P<0.001
4. Effect of lead exposure on NOS activity in the brain stem of rat pups
There was no significant difference of NOS activity in the brain stem between any lead-exposed group and the control group on the four day spans (Table 4).
Table 4.
Comparison of NOS activity between groups in brain stem (X±SD, unit: U/mg protein)
| Group | 7 d (n) | 14 d (n) | 21 d (n) | 28 d (n) |
| Control group | 2.58±0.36 (7) | 2.47±0.71 (6) | 1.69±0.10 (7) | 1.72±0.34 (7) |
| 0.025% group | 2.42±0.29 (7) | 2.15±0.56 (5) | 1.64±0.18 (5) | 1.50±0.18 (7) |
| 0.05% group | 2.85±0.41 (6) | 2.06±0.28 (6) | 1.80±0.16 (6) | 1.54±0.33 (6) |
| 0.075% group | 2.82±0.22 (8) | 2.26±0.21 (6) | 1.71±0.16 (6) | 1.60±0.32 (5) |
Note: There was no significant difference between any 2 groups at the same day span
Results of the RT-PCR
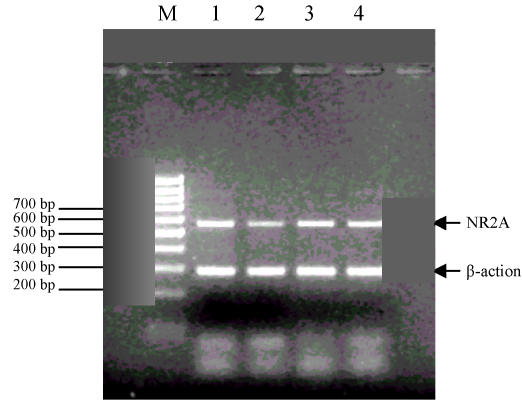
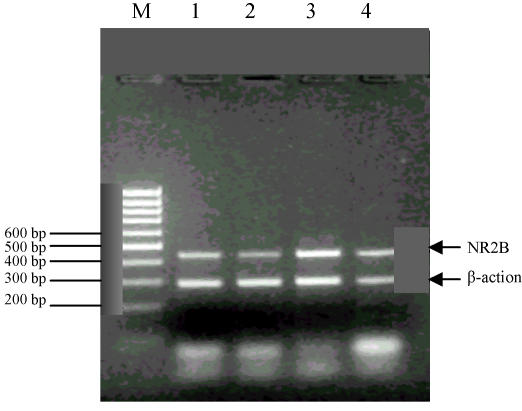
The result of the gel electrophoresis is shown in Fig.2 and Fig.3.
Fig. 2.
NR2A and β-actin simultaneous gel electrophoresis
M: Marker; 1: Control group; 2: 0.025% group; 3: 0.05% group; 4: 0.075% group
Fig. 3.
NR2B and β-actin simultaneous gel electrophoresis
M: Marker; 1: Control group; 2: 0.025% group; 3: 0.05% group; 4: 0.075% group
1. Compared with the control group, the level of NR2A mRNA expression in the 0.05% and the 0.075% group was significantly higher on the 7th and 14th day (P<0.05), while in the 0.025% group this difference was significant on the 7th day only (P<0.05) (Table 5).
Table 5.
Comparison of NR2A mRNA expression in each group (X±SD)
| Group | 7 d (n) | 14 d (n) | 21 d (n) | 28 d (n) |
| Control group | 0.124±0.068 (5) | 0.148±0.027 (5) | 0.223±0.065 (6) | 0.200±0.065 (6) |
| 0.025% group | 0.247±0.092 (4)* | 0.208±0.052 (4) | 0.208±0.093 (5) | 0.265±0.058 (6) |
| 0.050% group | 0.261±0.054 (4)* | 0.250±0.032 (4)* | 0.223±0.073 (5) | 0.254±0.089 (4) |
| 0.075% group | 0.238±0.054 (5)* | 0.227±0.067 (6)* | 0.283±0.133 (6) | 0.245±0.058 (4) |
Compared with the control group at the same day span, P<0.05
2. No statistically significant result was found in the comparison of NR2B mRNA expression between the control group and the different lead-exposed groups at the four different points of measurement (P>0.05) (Table 6).
Table 6.
Comparison of NR2B mRNA expression in each group (X±SD)
| Group | 7 d (n) | 14 d (n) | 21 d (n) | 28 d (n) |
| Control group | 0.260±0.080 (6) | 0.196±0.058 (4) | 0.196±0.051 (6) | 0.232±0.010 (4) |
| 0.025% group | 0.276±0.043 (4) | 0.249±0.053 (5) | 0.218±0.106 (6) | 0.275±0.061 (6) |
| 0.050% group | 0.272±0.054 (4) | 0.226±0.031 (5) | 0.203±0.086 (5) | 0.322±0.103 (4) |
| 0.075% group | 0.268±0.055 (6) | 0.216±0.062 (6) | 0.221±0.099 (5) | 0.232±0.081 (6) |
Note: There was no significant difference between any 2 groups at the same day span
DISCUSSION
Following the development of modern transportation and industry, the environmental lead pollution is getting ever more severe and the situation with regard to the lead poisoning of children is serious. The nervous system of children is in a stage of rapid development and maturation and is thus particularly susceptible to the toxic effects of lead exposure. Once the blood lead concentration exceeds 0.483 μmol/L (100 μg/L) in children, learning and memory abilities can be impaired even though noticeable clinical symptoms may not be present. In recent years, much research has been done on the neurotoxic mechanism of lead. It was found that lead inhibits NOS and NMDA receptor activity in the central nervous system and thus influences learning and memory functions (Zhang et al., 2002; Chetty et al., 2001). Yet those results have not yet been finally verified. No studies on the selective influence of low-level lead exposure on the NOS activity in different brain regions or on the dosage-dependency of that inhibitory effect have been reported so far. Furthermore, it is still unclear whether lead has an influence on the expression of NMDA-R mRNA in different subunits and whether there is a connection between the effects and different stages of development.
In this study, we successfully established a rat animal model for low-level lead exposure over different stages of development. Apart from the 0.075% group, in which the blood lead concentration reached 500–600 μg/L on the 21st and 28th day, all other measurements ranged from 200 to 400 μg/L, a level that is comparable to the present diagnostic standard of lead poisoning in Chinese children. In the process of lead exposure, no gastroenteral, neurological or other symptoms of lead poisoning were observed in the maternal rats nor in the pups. The body weight between the different groups did not show significant differences, either, so that our animal model represents a model of subclinical lead poisoning.
The blood lead concentration in all experimental groups followed a characteristic pattern. It was relatively high on the 7th day, slightly lower on the 14th day, markedly elevated on the 21st day and showed a slight decrease again on the 28th day. This result is comparable to that reported by Guilarte and McGlothan (1998). As the renal capacity of lead excretion is relatively low within the first week after birth, blood lead levels may be high on the 7th day and then decrease as the renal excretion functions mature until the 14th day. Starting from the 19th day, the rat pups learn to take in food by themselves, while the milk feeding has not yet been discontinued. Thus, on the 21st day after birth the pups are exposed to lead from the mother’s milk as well as from water, so that the blood lead concentration was found to be markedly elevated at that point of measurement. And as the 28th day approaches, the small bowl of the pups matures and gradually absorbs less lead until a level similar to adult rats is reached, thus resulting in decreasing blood lead concentrations.
In this study, we found that the NOS activity in hippocampus and cerebellum was not affected by lead exposure on the 7th and 14th day. But on the 21st and 28th day, the NOS activity in the hippocampus and cerebellum of lead-exposed groups was significantly lower than that of the control one. These data suggested that the inhibition of low level lead exposure on the NOS activity in hippocampus and cerebellum depended on the exposure time. This was consistent with a previous study (Chetty et al., 2001). On the other hand, the inhibition of lead exposure on the NOS activity in hippocampus and cerebellum of the 0.05% and 0.075% groups was not so great and evident as that of the 0.025% group. It needs to be further investigated whether there is a compensation mechanism that will be activated when the lead level of hippocampus and cerebellum is too high to seriously inhibit NOS activity.
NOS activity in the cerebral cortex of the 0.075% group significantly decreased as compared with the control group at all four day spans (P<0.05). But the other lower level lead-exposed groups were not affected. This indicated that the lead inhibition on NOS activity in the cerebral cortex related to the exposure level of lead. The decrease of NOS activity in cerebral cortex was consistent with the report that lead exposure decreased the number of NOS positive neurons in the cerebral cortex.
There was no significantly difference of NOS activity in the brain stem between any lead-exposed group and the control group on the four day spans (P>0.05). This indicated that NOS activity in the brain stem might not be significantly affected by lead exposure during developmental period. The influence of low level lead exposure on the NOS activity in the brain stem needs further study.
The hippocampus is a central neuronal structure for learning and memory. The human cerebral cortex receives information processed by the hippocampus and other regions of the brain. The cerebral cortex plays an important role in the storage of information. Like the hippocampus and the cerebellum, the cerebral cortex may also be affected by lead exposure. Not only does experimental data provide evidence for the damage done to cerebral neurons, clinical observations also show that lead exposure can lead to impaired cognitive functions with loss of attention, impaired coordination abilities and other symptoms. Furthermore, structural abnormalities in the cerebellum can result from lead exposure and influence long-term depression (LTD). The results of this study show that low levels of lead have certain inhibitory effect on the NOS activity in different brain regions of rats in the developing period. This inhibitory effect was found to be selective for different regions and was shown to depend on the time of exposure and/or the concentration of lead the subjects were exposed to. The decrease of NOS activity explained the lead-mediated cognitive deficits because NO regulates long-term potentiation (LTP) and other neurophysiological events in the developing nervous system (O’Dell et al., 1991).
In this work, we investigated the mRNA expression of the NMDA receptor subunits NR2A and NR2B and the influence that lead exposure has on this expression. Compared with the normal control group, the level of NR2A expression was significantly higher in the 0.05% and 0.075% groups on the 7th and the 14th day of age. In the 0.025% group, a significant difference was found on the 7th day only. No significant results for NR2A mRNA expression were found at all other points of measurements. The level of NR2B mRNA expression did not differ between the control and the experimental groups. This shows that low-level lead exposure during the developmental period can increase the mRNA expression for the NR2A subunit of the NMDA receptor in the hippocampus of rat pups. This effect was particularly noticeable at an early stage of development (7th day, 14th day). The same level of lead exposure did not affect the mRNA expression for the NR2B subunit of the NMDA receptor.
The normal physiological and pharmacological function of the NMDA receptor depends on its proportional composition of all subunits (Monyer et al., 1992). The increased expression of NR2A mRNA in the hippocampus may lead to a change in the number or in the structure of NMDA receptors and eventually could influence the LTP conductance for which the NMDA receptor is the physiological basis (Zhang et al., 2002). The hippocampus LTP, in turn, is known to be one of the important neurological factors in learning and memory functions. The main results derived from this study direct attention on the first two weeks after the birth of the pups. These two weeks after birth have been characterized as the key period for the development of the function of the hippocampus, of learning and memory in mammals (Altmann et al., 1993). Therefore, we conclude that the influence on NR2A mRNA expression may be one toxicological mechanism through which lead affects learning and memory. This mechanism needs to be further investigated.
Footnotes
Project (No. 021103009) supported by the Science and Technology Bureau of Zhejiang Province, China
References
- 1.Altmann L, Weinsberg F, Sveinsson K. Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol Lett. 1993;66(1):105–112. doi: 10.1016/0378-4274(93)90085-c. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Watemaux C. Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics. 1991;87:219–227. [PubMed] [Google Scholar]
- 3.Chetty CS, Reddy GR, Murthy KS, Johnson J, Sajwan K, Desaiah D. Perinatal lead exposure alters the expression of neuronal nitric oxide synthase in rat brain. Int J Toxicol. 2001;20(3):113–120. doi: 10.1080/109158101317097692. [DOI] [PubMed] [Google Scholar]
- 4.Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. TIPS. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- 5.Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Research. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- 6.Han JS. Neuro-Science Compendium. Beijing, China: Beijing Medical University and Xiehe Medical University United Publishing Company; 1993. pp. 32–38. (in Chinese) [Google Scholar]
- 7.Lasley SM, Green MC, Gilbert ME. Rat hippocampal NMDA receptor binding as a function of chronic lead exposure level. Neurotoxicol Teratol. 2001;23(2):185–189. doi: 10.1016/s0892-0362(01)00116-7. [DOI] [PubMed] [Google Scholar]
- 8.Monyer H, Sprengel R, Shoepfer R. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 9.O’Dell TJ, Hawkins RD, Kandel ER. Tests of the roles of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci USA. 1991;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang XY, Lin AP, Ruan DY, Liu J. Effect of developmental lead exposure on the expression of specific NMDA receptor subunit mRNAs in the hippocampus of neonatal rats by digoxigenin-labeled in situ hybridization histochemistry. Neurotoxicol Teratol. 2002;24(2):149–160. doi: 10.1016/s0892-0362(01)00210-0. [DOI] [PubMed] [Google Scholar]