Abstract
Objective: To investigate the relationship between the expression of endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF) and angiogenesis in primary astrocytoma. Methods: Thirty-seven primary astrocytomas and 4 astrocytic hyperplasia samples were collected and divided into three groups according to histological grade. The expression of eNOS, VEGF and factor VIII related antigen (FVIIIRAg) were assayed by immunohistochemistry. Microvascular density was assessed by FVIIIRAg immunoreactivity. The intensity of immunoreactivity was graded according to the percentage of positive tumor cells. Results: No eNOS and VEGF were expressed in the astrocytes and vascular endothelium in astrocytic hyperplasia. The expression of eNOS or VEGF was light in low-grade astrocytoma and strong in glioblastoma. eNOS expression in astrocytoma was very positively correlated with VEGF. eNOS and VEGF expression in anaplastic astrocytoma was median in contrast to the low grade astrocytoma and glioblastoma. Lower microvascular density was found in low grade astrocytoma than that in higher grade malignant ones. The expressions of eNOS and VEGF were correlated with microvascular density and tumor malignancy. Conclusion: This finding suggests that eNOS and VEGF may have cooperative effect in tumor angiogenesis and play an important role in the pathogenesis of primary astrocytoma.
Keywords: Endothelial nitric oxide synthase, Vascular endothelial growth factor, Density of microvessels, Primary astrocytoma
INTRODUCTION
Primary astrocytoma is one type of neuroepithelial tumor and can be divided into several grades according to its malignancy. However, the etiology and pathophysiology are unclear and effective therapy is unavailable. Recently, tumor angiogenesis was found to be very important to tumor growth, invasiveness, metastasis and prognosis. Clinical studies found that density of microvessels is closely related to tumor malignancy and prognosis (Bian et al., 2000). Therefore, elucidation of the pathophysiology of tumor angiogenesis will be helpful for developing therapeutic strategies for primary astrocytoma.
Nitric oxide (NO) is an inorganic free radical gas synthesized from L-Arginine by nitric oxide synthase (NOS) and mainly acts as signal and cytotoxic molecule. It has been demonstrated that NO is an important factor in the development of some tumors (Zhang et al., 2003). Three isoforms of NOS have been identified: neural NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS). eNOS mainly exists in endothelial cells. eNOS-derived NO plays a very important role in the regulation of vasodilation and vascular permeability. Excessive expression of eNOS was found in several kinds of tumors and could influence tumor blood supply.
Vascular endothelial growth factor (VEGF) is also crucial to tumor angiogenesis (Zachary and Gliki, 2001). VEGF family is essential for endothelial cell differentiation and for the sprouting of new capillaries from preexisting vessels during development. In vitro studies indicated that VEGF can promote proliferation of endothelial cell and vascular permeability (Zachary and Gliki, 2001). A series of intracellular signal transduction initiated by NO is synthesized by eNOS partially induce the process. eNOS can be activated by VEGF through PI-3K/Akt kinase (Koistinen et al., 2001). NO can up-regulate VEGF expression (Wang et al., 2001). Up to date, however, there is no report about alternation of eNOS in relation to density of microvessels, vascular endothelial growth factor (VEGF), malignancy and prognosis in situ. Therefore, we investigated the relationship between eNOS, VEGF expression and density of microvessels, malignancy and prognosis in human primary astrocytoma.
METHODS
Tissue samples
Thirty-seven samples were obtained from patients with primary astrocytomas in the First Affiliated Hospital of Zhejiang University. Four astrocytic hyperplasia samples were obtained from the patients with other brain lesions (brain injury, cavernous angioma, or arteriovenous malformations) as control. There were 23 male and 14 female patients in the group. The ages of the patients ranged from 4 to 71 years, the mean age was 38 years. The 37 astrocytomas were diagnosed as low-grade astrocytoma in 6 cases, anaplastic astrocytoma in 12 cases, and glioblastoma in 19 cases. The histological grade of the tumors was classified according to the World Health Organization Criteria (Percy et al., 1990) (Astrocytoma was categorized into low-grade astrocytoma, anaplastic astrocytoma and glioblastoma).
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded specimens were used for this study. Sections (4 μm) were deparaffinized in xylene and rehydrated through a graded ethanol series to water after a wash in 0.05 mol/L Tris-buffered saline (TBS) (pH 7.6), immersed in 0.01 mol/L citrate buffer (pH 6.0) and micro-waved two times, for 10 min each time, for antigen retrieval. Slides were then cooled to room temperature and rinsed in TBS for 5 min. Endogenous peroxidase was blocked by a 30-min incubation in TBS containing 3% hydrogen peroxide, at room temperature. After additional washing in TBS, nonspecific binding was blocked with 7.5% normal goat serum at 37 °C temperature for 30 min. The samples were incubated overnight at 4 °C with rabbit polyclonal antibody against human eNOS (1:50 dilution; Boster Biotechnical Corp., Wuhan, China), mouse polyclonal antibody against human VEGF or mouse monoclonal antibody against human factor VIII related antigen (FVIIIRAg) (1:50 dilution; Zhongshan Biotechnical Corp., Beijing, China). Immunostaining for eNOS and VEGF, FVIIIRAg were performed in serial sections. Sections were washed with TBS three times, for 5 min each time, and were then incubated with bioaylated goat anti-mouse IgG and anti-rabbit IgG mouse polyclonal antibody against human VEGF (Zhongshan Biotechnical Corp., Beijing, China) at room temperature for 30 min. After two 5 min rinses with TBS, the sections were treated with peroxidase-conjugated streptavidin (Zhongshan Biotechnical Corp., Beijing, China) for 30 min at 37 °C. Diaminobenzidine/hydrogen peroxide was used as a chromogen, and a hematoxylin counterstain was applied. Sections were dehydrated in alcohol, cleared in xylene, and mounted. The specimen from patient of mammary adenocacinoma was used as a positive control and was confirmed to show the same intensity of immunoreactivity for eNOS or VEGF in each batch of staining. Normal rabbit serum was used as the negative control for eNOS and VEGF.
In the tumor tissue, the positive cell was the yellow or brown-yellow cell stained by three kinds of antibodies. Then, the percentage of positive tumor cells was counted under microscope and was used as labelling index (LI) which is equal to the number of positive cells in the number of counted cells. LI was used to classify the tumor into 4 grades: grade 0 (LI<5%); grade 1 (5%≤LI≤25%); grade 2 (25%<LI≤50%); grade 3 (LI>50%). The intensity of immunoreactivity for eNOS or VEGF in tumor vessels was graded on a scale of grades 1~3: grade 1: slight staining or no detectable staining; grade 2: moderate staining; grade 3: intense staining.
Density of microvessels
Under microscope, endothelial cells were yellow or brown-yellow stained by FVIIIRAg antibodies. So, the density of microvessels (MVD) in tumor tissue was assessed by the number of FVIIIRAg-positive microvessels per microscopic field.
Statistical analyses
SPSS10.0 statistical software was applied and the results were analyzed by Mann-Whitney tests and Spearman rank correlation. For all statistical analyses, a value of P<0.05 was considered to be significant.
RESULTS
Relationship between eNOS expression and tumor malignancy
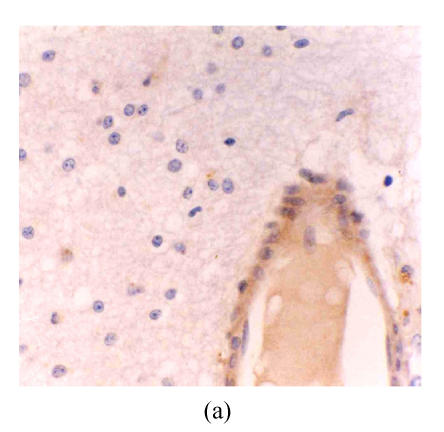
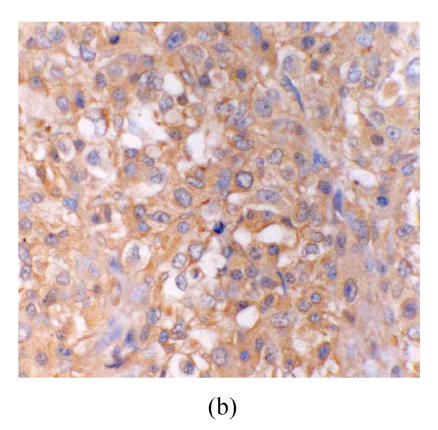
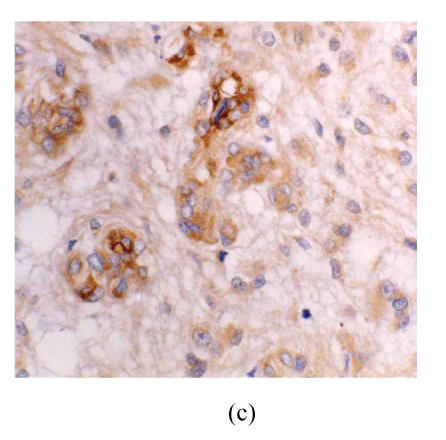
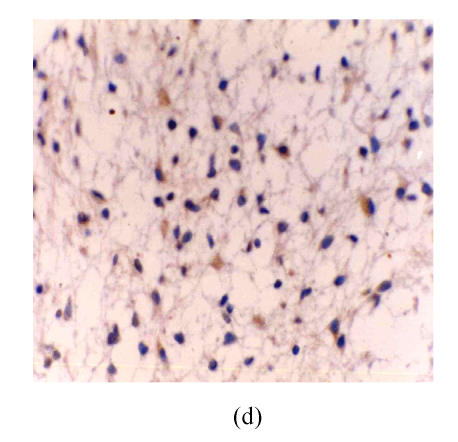
There were no eNOS expression in the astrocytes and vascular endothelium in astrocytic hyperplasia. In low-grade astrocytoma, eNOS LI (eNOS-ir) was low and the tumor cells were mainly lightly stained, but the vascular endothelial cells were stained obviously (Fig.1a). eNOS was strongly expressed in glioblastoma (Fig.1b). The strongest expression was localized in the tumor cells giant around the necrotic region. There were many eNOS positive microvessels and deeply stained endothelial cells, and some glomerulus-like positive vascular structures in the glioblastoma (Fig.1c). eNOS expression in anaplastic astrocytoma (Fig.1d) was median compared to that in the low grade astrocytoma and glioblastoma. The positivity and the stain degree of the tumor cells and endothelial cells varied in the anaplastic astrocytoma. Except for astrocytic hyperplasia and low-grade astrocytoma, there were significant differences between all other groups (P<0.05) (Table 1).
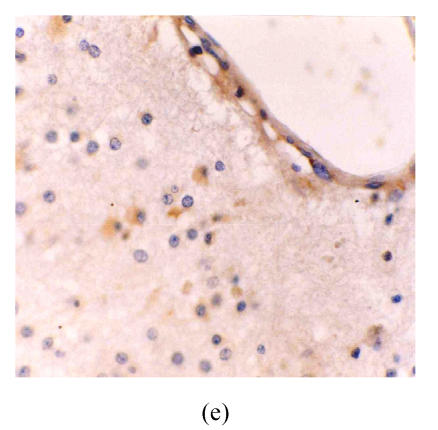
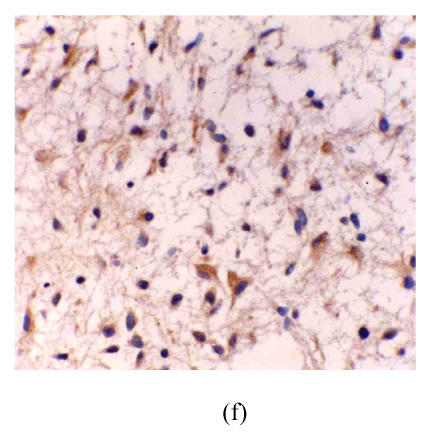
Fig. 1.
(a) Low grade astrocytoma: Light and few expressions of eNOS in tumor cell, but comparatively strong in the vascular endothelial cells (immunohistochemical staining ×400); (b) Glioblastoma: eNOS strongly expressed in tumor cells (immunohistochemical staining ×400); (c) Glioblastoma: Glomerulus-like eNOS positive vascular structure (immunohistochemical staining ×400); (d) Anaplastic astrocytoma: Median expression of eNOS in tumor cells (immunohistochemical staining ×400); (e) Low grade astrocytoma: Light expression of VEGF in a few tumor cells, but obvious in the vascular endothelial cells (immunohistochemical staining ×400); (f) Anaplastic astrocytoma: Median expression of VEGF in tumor cells (immunohistochemical staining ×400)
Table 1.
eNOS-ir, VEGF-ir, MVD in astrocytic hyperplasia and astrocytoma
| Grade | No. | eNOS-ir (%) | VEGF-ir (%) | MVD (mf−1) |
| H | 4 | 0.00 | 0.00 | 8.25±1.71 |
| LA | 6 | 3.07±2.24* | 4.43±2.33** | 11.83±3.50 |
| AA | 12 | 31.35±19.17** | 23.85±13.34** | 20.87±4.57* |
| GBM | 19 | 71.20±13.98** | 58.63±15.02** | 34.72±9.56** |
| Total | 41 | 42.61±32.13 | 34.80±26.76 | 24.73±12.35 |
Note: Analyzed with Mann-Whitney tests
Compared with previous groups, P<0.05
Compared with previous groups, P<0.01
eNOS-ir: Labelling index of eNOS; VEGF-ir: Labelling index of VEGF; MVD: Microvascular density; H: Astrocytic hyperplasia; LA: Low-grade astrocytoma; AA: Anaplastic astrocytoma; GBM: Glioblastoma; mf−1: per microscopic field
Relationship between VEGF expression and tumor malignancy
The astrocytes and vascular endothelium present in astrocytic hyperplasia were not stained. The distribution of VEGF was in accordance with eNOS in astrocytoma. There were not stained or a few lightly stained tumor cells in low-grade astrocytoma, but the endothelial cells in the bigger vessels were stained obviously (Fig.1e). In anaplastic astrocytoma and glioblastoma, the tumor cells were stained darker (Fig.1f) and the labelling index of tumor cells (VEGF-ir) became higher when the astrocytoma became more and more malignant. There was also significant difference between all groups (P<0.01) (Table 1).
Relationship between MVD and tumor malignancy
There was no FVIIIRAg in the tumor cells. Relatively lower MVD was shown in low-grade astrocytoma compared to that of more malignant ones (Table 1). Most vessels found in astrocytic hyperplasia and low-grade astrocytomas were bigger vessels. In contrast, there were a lot of microvessels in glioblastoma.
Relationship between eNOS, VEGF expression and MVD
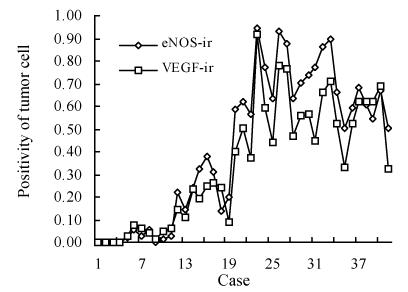
The laws governing eNOS expression have some correlation with VEGF and MVD in astrocytoma and astrocytic hyperplasia (Fig.2). MVD and the positivity of eNOS and VEGF increased with higher histological grade, eNOS-ir, VEGF-ir and MVD were higher in glioblastoma than in anaplastic and low-grade astrocytoma. eNOS, VEGF and MVD positively correlated with each other.
Fig. 2.
The obvious positive correlation of the expression of eNOS and VEGF
eNOS-ir: Labelling index of eNOS; VEGF-ir: Labelling index of VEGF; Cases 1–4: Astrocytic hyperplasia; Cases 5–10: Low-grade astrocytoma; Cases 11–22: Anaplastic astrocytoma; Cases 23–41: Glioblastoma
DISCUSSION
Previous reports demonstrated that eNOS-derived NO promotes vasodilation, inhibits smooth muscle cell growth and diminishes platelets aggregation. eNOS was excessively expressed in various tumor cells, promoting angiogenesis and providing essential blood supply for tumor growth (Ziche and Morbidelli, 2000). NO was mainly synthesized by eNOS and nNOS in glioblastoma. In present study, we demonstrated that eNOS expression in tumor cells and microvessels were correlated with histological grade in primary astrocytoma in situ, corresponding to Iwata et al.(1999)’s observations. We also found that there were no eNOS expressed in astrocytic hyperplasia. Our results suggested that over-expressed eNOS in astrocytoma play a crucial role in tumor angiogenesis.
Tumor angiogenesis is the process that the neovasculature sprouting from tumorous tissue and its surroundings which is important to invasiveness and metastasis, and related closely to malignancy and prognosis of tumor (Iwata et al., 1999). Angiogenesis consists of vasodilation and hyperpermeability, endothelial proliferation, remodeling of extracellular matrix, endothelial migration and formation of cellular cord, tubular structure formation. The processes are tightly regulated by actions of NO. NO is synthesized by eNOS in endothelial cell via GC/cGMP (guanylate cyclase/cyclic guanosine monophosphate) inhibit platelet aggregating, keep vessels in dilation and hyperpermeability.
NO can act on MAPK (mitogen activated protein kinase), promote mitosis and proliferation of endothelial cell (Bian et al., 2000). However, free radical-ONOO− derived from interstitial NO act on MMPs (matrix metalloproteases) or inhibit the effect of TIMPs (tissue inhibitor of metalloproteases), this make MMPs more active (Phillips et al., 2001), change the ingredients of extracellular matrix, help the movement of endothelial cell and formation of cellular cord.
In the present study, eNOS was found not only in endothelial cells but also in tumor cells, NO produced by endothelial cells and tumor cells could work in vascular structure and extracellular matrix simultaneously. But because of the semi-life of NO is very short and its effective distance is very close, the location of eNOS indicated its possible that NO produced by endothelial cell act mainly on the surrounding endothelial cells, blood ingredients and other vascular structures, keep vessels in dilation, promote the proliferation and movement of endothelial cells. However, NO produced by tumor cells works on the interstitium mainly, changes the construction of extracellular matrix, and helps endothelial cells move to form cellular cord. The correlation between positivity of tumor cell and positive degree of microvessels demonstrated that NO produced by endothelial cells and tumor cells have a cooperative effect in tumor angiogenesis.
Vascular growth factor family (esp. VEGF) have a definite effect of dilating vessels, promoting tumor blood supply, angiogenesis, proliferation, movement of endothelial cell and formation of tubular structure through several mechanisms (Ludwig et al., 2000). Nishikawa (1998) found VEGF expressed in all kinds of brain tumors, and its expression was related to tumor malignancy. Our study also found highly expressed VEGF had an obvious positive correlation with tumor malignancy and microvascular density in astrocytoma.
VEGF promote angiogenesis through a lot of pathway (Zachary and Gliki, 2001). Several studies show that lots of effect of VEGF was achieved through eNOS/NO (Morales-Ruiz et al., 2000). VEGF act on Flk-1/KDR (Koistinen et al., 2001), then through PI3K/Akt act on Ser1177 or Ser1179 in eNOS of endothelial cells to active eNOS (Fulton et al., 1999), produce NO to dilate vessels and promote angiogenesis. Murohara et al.(1998) and co-workers guessed that angiotrophic effect of VEGF would weaken, and even disappear when eNOS is lacking in eNOS knocked-out mouse. Fukumura et al.(2001) thought that VEGF only had effect on eNOS to produce NO, but no effect on iNOS. Besides, VEGF can promote eNOS expression by stimulating endothelial through Flk-1/KDR (Bouloumie et al., 1999) and NO can promote VEGF expression directly or through second messages such as protein enzymes, calcium in cell (Wang et al., 2001).
We found the distribution of eNOS was unified with VEGF finely, which suggest that eNOS and VEGF have a cooperative effect. This give a morphological support in situ to the studies mentioned above. The status is more obvious in the necrotic area in coordination with the research of Kimura et al.(2000), we suggested that NO can act on NRE (hypoxia response element) or elevate HIF-1 (hypoxia inducible factor-1), then promote the expression of VEGF.
In this study, eNOS and VEGF are correlated to microvascular density, suggesting that anti-angiogenic therapy could be a new therapeutic strategy of astrocytoma. In fact, a few animal experiments focusing on this strategy had been done. For example, Swaroop et al.(2000) found that NOS inhibitor could decrease blood supply of glioma and add tumor necrosis, then give a therapeutic efficiency. Our research provide the morphological proof of tumor tissue in situ, suggesting that inhibit eNOS combined with VEGF receptor could probably produce the more effective anti-angiogenic effect.
Footnotes
Project (No. G50241) supported by the Start-up Fund for Study-abroad Returnee, Ministry of Education, China
References
- 1.Bian XW, Du LL, Shi JQ, Cheng YS, Liu FX. Correlation of bFGF, FGFR and VEGF expression with vascularity and malignancy of human astrocytomas. Anal Quant Cytol Histol. 2000;22(3):267–274. [PubMed] [Google Scholar]
- 2.Bouloumie A, Schini-Kerth VB, Busse R. Vascular endothelial growth factor up-regulates nitric oxide synthase expression in endothelial cells. Cardiovasc Res. 1999;41(3):773–780. doi: 10.1016/s0008-6363(98)00228-4. [DOI] [PubMed] [Google Scholar]
- 3.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98(5):2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata S, Nakagawa K, Harada H, Oka Y, Kumon Y, Sakaki S. Endothelial nitric oxide synthase expression in tumor vasculature is correlated with malignancy in human supratentorial astrocytic tumors. Neurosurgery. 1999;45(1):24–28. doi: 10.1097/00006123-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D’Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: Control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95(1):189–197. [PubMed] [Google Scholar]
- 7.Koistinen P, Siitonen T, Mantymaa P, Koistinen P, Siitonen T, Mantymaa P, Saily M, Kinnula V, Savolainen ER, Soini Y. Regulation of the acute myeloid leukemia cell line OCI/AML-2 by endothelial nitric oxide synthase under the control of a vascular endothelial growth factor signaling system. Leukemia. 2001;15(9):1433–1441. doi: 10.1038/sj.leu.2402217. [DOI] [PubMed] [Google Scholar]
- 8.Ludwig HC, Feiz-Erfan I, Bockermann V, Behnke-Mursch J, Schallock K, Markakis E. Expression of nitric oxide synthase isoenzymes (NOS I–III) by immunohistochemistry and DNA in situ hybridization: Correlation with macrophage presence, vascular endothellial growth factor (VEGF) and edema volumetric data in 220 glioblastomas. Anticancer Res. 2000;20(1A):299–304. [PubMed] [Google Scholar]
- 9.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86(8):892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 10.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101(11):2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishikawa R, Cheng SY, Nagashima R. Expression of vascular endothelial growth factor in human brain tumors. Acta Neuropathol(Berl) 1998;96(5):453–462. doi: 10.1007/s004010050919. [DOI] [PubMed] [Google Scholar]
- 12.Percy C, Van-Holten V, Muir C. International Classification of Diseases for Oncology. Second Edition. Geneva: World Health Organization; 1990. pp. M938–M948. [Google Scholar]
- 13.Phillips PG, Birnby LM, Narendran A, Milonovich WL. Nitric oxide modulates capillary formation at the endothelial cell-tumor cell interface. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L278–L290. doi: 10.1152/ajplung.2001.281.1.L278. [DOI] [PubMed] [Google Scholar]
- 14.Swaroop GR, Kelly PA, Bell HS, Shinoda J, Yamaguchi S, Whittle IR. The effects of chronic nitric oxide synthase suppression on glioma pathophysiology. Br J Neurosurg. 2000;14(6):543–548. doi: 10.1080/02688690020005554. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Xiong Q, Shi Q, Tan K, Le X, Xie K. Genetic disruption of host nitric oxide synthase II gene impairs melanoma-induced angiogenesis and suppresses pleural effusion. Int J Cancer. 2001;91(5):607–611. [PubMed] [Google Scholar]
- 16.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological action of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49(3):568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Pan JW, Ren TR, Zhu YF, Han YJ, Kühnel W. Correlated expression of inducible nitric oxide synthase and P53, Bax in benign and malignant diseased gallbladder. Ann Anat. 2003;185(6):549–554. doi: 10.1016/S0940-9602(03)80125-5. [DOI] [PubMed] [Google Scholar]
- 18.Ziche M, Morbidelli L. Nitric oxide and angiogenesis. J Neurooncol. 2000;50(1-2):139–148. doi: 10.1023/a:1006431309841. [DOI] [PubMed] [Google Scholar]