Abstract
The potential biodegradation of crude oil was assessed based on the development of a fermentative process with a strain of Pseudomonas aeruginosa which produced 15.4 g/L rhamnolipids when cultured in a basal mineral medium using glycerol as a sole carbon source. However, neither cell growth nor rhamnolipid production was observed in the comparative culture system using crude oil as the sole carbon source instead. As rhamnolipid, an effective biosurfactant, has been reported to stimulate the biodegradation of hydrocarbons, 1 g/L glycerol or 0.22 g/L rhamnolipid was initially added into the medium to facilitate the biodegradation of crude oil. In both situations, more than 58% of crude oil was degraded and further converted into accumulated cell biomass and rhamnolipids. These results suggest that Pseudomonas aeruginosa could degrade most of crude oil with direct or indirect addition of rhamnolipid. And this conclusion was further supported by another adsorption experiment, where the adsorption capacity of crude oil by killed cell biomass was negligible in comparison with the biologic activities of live cell biomass.
Keywords: Rhamnolipid, Crude oil, Biodegradation, Pseudomonas aeruginosa
INTRODUCTION
Oily wastewater, especially from oil field, has posed a great hazard for terrestrial and marine ecosystems. The traditional treatment of oily wastewater, such as containment and collection using floating booms, adsorption by natural or synthetic materials, etc., can not degrade the crude oil thoroughly (Ollis, 1992). So far, biodegradation suggests an effective method. During biodegradation, crude oil is used as an organic carbon source by a microbial process, resulting in the breakdown of crude oil components to low molecular weight compounds.
However, the bioavailability of weekly soluble hydrophobic compounds for microbial conversion is usually low and thus limits their degradation rate in aqueous medium. The use of surfactants has been found to enhance degradation of crude oil (Urum et al., 2003; Balba et al., 2002) or other hydrocarbons (Nakahara et al., 1981). Among various surfactants, rhamnolipids are considered to be the most in degrading hydrocarbons (Itoh and Suzuki, 1972). For example, the biodegradation of long-chain alkanes was stimulated by addition of rhamnolipid (Wouter and Dick, 2002). This facilitated biodegradation is probably due to the increase of cell surface hydrophobicity after extraction of lipopolysaccharides from the cellular envelope by rhamnolipids, which subsequently stimulates uptake via direct contact between cells and hydrocarbon droplets. Thus, the interaction between addition of rhamnolipid and biodegradation of hydrocarbons seems to be highly specific.
However, it is not known whether biodegradation of more recalcitrant crude oil can be facilitated by addition of rhamnolipid. Although rhamnolipid was reportedly produced by a bacterium (NCC-DSS6) isolated from an acclimated semicontinuous reactor fed with crude oil (Chhatre et al., 1996), its function in biodegradation of crude oil is not clear. The authors of this paper are not aware of researchers on the interaction between addition of rhamnolipid and biodegradation of crude oil.
The objective of this paper was to evaluate the biodegradation of crude oil by Pseudomonas aeruginosa in laboratory. Pseudomonas aeruginosa is a typical strain for rhamnolipid production and can utilize vegetable oil or glycerol as the sole carbon source. We investigated the feasibility of crude oil degradation by microbial process, focusing mainly on the effect of rhamnolipid on biodegradation. In spite of this, the adsorption by killed cell mass was presented to provide additional support to reports of biological activities of Pseudomonas aeruginosa during biodegradation of crude oil. In this work, the biodegradation of crude oil was conducted with the same initial basal mineral salt medium to allow comparison of the different supplements.
MATERIALS AND METHODS
Microorganism and culture conditions
Pseudomonas aeruginosa was purchased from the Shanghai Microbiology Institute. This strain produces rhamnolipids.
The composition of the basal mineral salt medium used in this study was as follow (g/L): NaNO3 4.0, NaCl 1.0, KCl 1.0, CaCl2·2H2O 0.1, KH2PO4 3.0, Na2HPO4·12H2O 3.0, MgSO4 0.2, FeSO4·7H2O 0.001; 2 ml trace element stock solution composed of (g/L): FeCl3·6H2O 0.08, ZnSO4·7H2O 0.75, CoCl2·6H2O 0.08, CuSO4·5H2O 0.075, MnSO4·H2O 0.75, H3BO3 0.15, Na2MoO4·2H2O 0.05. The initial pH was adjusted to 6.8.
For subculture of Pseudomonas aeruginosa, bacterial cultures were inoculated into the basal mineral salt medium with 3% glycerol as carbon source. To investigate the effect of different carbon sources on rhamnolipid production, Pseudomonas aeruginosa was cultivated in the basal salt medium supplemented with 3 g/L of a specific carbon source. The cultivations were conducted in 1 L shaking (150 r/min) flasks containing 250 ml medium for a period of 48 h. The temperature was controlled at 35 °C.
Rhamnolipid production in glycerol
Cultures were carried out in 1 L Erlenmeyer flasks filled with 250 ml of medium (shaking at 150 r/min) for 5 d at temperature of 35 °C. The growth curve was measured at specific time period during which a 5 ml sample was taken to assay glycerol, biomass and rhamnolipid concentration.
Biodegradation of crude oil
The effect of biosurfactants on degradation of crude oil by the biosurfactant-producing strains was determined by measuring the residual amount of crude oil present in cultures that had been incubated with or without biosurfactants.-Crude oil was obtained from the Shengli Oilfield (Shandong, China). Experiments were carried out in 1 L shaking flasks containing 250 ml basal mineral salt medium with or without glycerol and rhamnolipid. The shaking flasks were rotated at 150 r/min constant temperature of 35 °C. Some amounts of crude oil were added so that the initial amount of crude oil in the flask was set around 0.7 g/L.
Adsorption of crude oil by cell mass
The biomass of Pseudomonas aeruginosa was first isolated from 3-day-culture broth by centrifugation at 350 g for 20 min. The pellets were first immersed in 1% sodium hypochloride for 30 min before Pseudomonas aeruginosa was confirmed to be dead according to viability staining. Then the killed Pseudomonas aeruginosa were precipitated by centrifugation and washed three times with a 20-fold basal mineral medium. The subsequent adsorption test adopted the same operation condition as the microbial culture above, such as temperature, pH etc., to allow comparison between biodegradation and biosorption. The basal mineral salt medium was used to dilute the cell pellets to make a specific concentration biomass suspension. And the adsorption of crude oil by cell mass was implemented in a 1 L shaking flask at temperature of 35 °C and rotary speed of 150 r/min. Forty-eight hours later, the whole suspension was centrifuged and the supernatant was extracted by petroleum ether for assay of the final oil concentration. The initial crude oil and the final crude oil were measured for determination of the adsorption capacity.
Extraction and identification of rhamnolipids
After fermentation, the culture medium was centrifuged at 350 g for 20 min and then the isolated supernatant was adjusted to pH of 2.0 by adding 5 mol/L H2SO4 for the rhamnolipid precipitation. The precipitates were extracted with two volumes of diethyl ether/methanol (1:1, V/V) mixture. Evaporation of the solvent yielded crude rhamnolipids. Three hundred mg pellets of which were applied onto thin-layer chromatography (TLC) plates and developed with a solvent mixture containing chloroform, methanol, and water (65:25:4, V/V/V). Rhamnolipid spots were detected by using orcinol reagent.
Analytical methods
Biomass content was determined by measuring culture densities, the spectrophotometric absorption at 620 nm, and the actual biomass in dry weight (g/L) were obtained from the constructed calibration curve (y=31.2x). Glycerol concentration was measured by titrimetric method (Yong et al., 2001). Rhamnolipid concentration was determined in triplicate by the orcinol assay. Its value was calculated from a standard curve prepared with L-rhamnose and expressed as rhamnolipid values by multiplying rhamnose values by a coefficient of 3.4, obtained from the correlation of pure rhamnolipids/rhamnose (Chandrasekaran and Bemiller, 1980).
Crude oil concentrations were determined as follows: crude oil samples were mixed with equal volume of petroleum ether to extract crude oil. Then the extracted crude oil was detected spectrophotometrically at 228 nm (Rahman et al., 2002).
All data presented are mean values of three determinations and statistically compared using Student’s test with significance assumed if P<0.05.
RESULTS AND DISCUSSION
Typical fermentation of rhamnolipid
The fermentations of Pseudomonas aeruginosa were first investigated by using a couple of carbon sources (Table 1). Table 1 shows that rhamnolipid production by glycerol is much higher than that of other substrates including glucose, vegetable oil and liquid paraffin. So, for this cell line (Pseudomonas aeruginosa), glycerol is the most effective substrate for producing rhamnolipids among other substrates.
Table 1.
Rhamnolipid production after 3 d incubation of Pseudomonas aeruginosa with different carbon sources at an initial concentration of 30 g/L
| Carbon source | Biomass (g/L) | Rhamnolipids (g/L) |
| Glucose | 1.45±0.12 | 0.45±0.09 |
| Glycerol | 1.23±0.18 | 12.92±0.19 |
| Vegetable oil | 1.01±0.10 | 10.77±0.18 |
| Paraffin | 1.03±0.11 | 8.05±0.11 |
The fermentation of rhamnolipid was carried out using 30 g/L glycerol as the sole carbon source. Fig.1 shows the profiles of cell growth, glycerol concentration and rhamnolipid production obtained when the strain was cultivated with 30 g/L glycerol. As shown in Fig.1, the cell growth corresponded well with glycerol consumption and approached to the stationary stage after two days’ culture. It seems that the main carbon source is the limiting nutrient. Most rhamnolipids were found to accumulate at the stationary stage of cell growth. Rhamnolipid production of 15.4 g/L was attained almost two days after cell mass reached the maximum (2.3 g/L). The solvent extract of the culture medium contained two clear orcinol-positive spots (Rf 0.32 and 0.52) on TLC. These two spots were considered as rhamnolipids (Matsufuji et al., 1997).
Fig. 1.
Profiles of cell growth, rhamnolipid production, and glycerol consumption of a typical Pseudomonas aeruginosa fermentation with 30 g/L glycerol (Values shown here are from one typical experiment of three independent experiments)
After Pseudomonas aeruginosa had been identified as rhamnolipids producer using glycerol as the sole carbon source, the possibility of biodegradation of the crude oil was subsequently explored.
Direct biodegradation of crude oil
Crude oil degradation was first conducted by culturing Pseudomonas aeruginosa on basal mineral salt medium supplemented with 0.7 g/L crude oil. After 10 d incubation, no cell growth and emulsification were observed. Crude oil still either floated above the aqueous layer or attached to the surface of glassware without shaking. Also, no rhamnolipids were detected by chemical analysis.
The inability of Pseudomonas aeruginosa to uptake crude oil was most possibly due to the inaccessibility of weekly soluble petroleum to microbials. So, two approaches, supplementation of either glycerol or rhamnolipid to the initial basal medium, were used to improve the emulsification of the crude oil.
Effect of glycerol or rhamnolipid addition on crude oil utilization
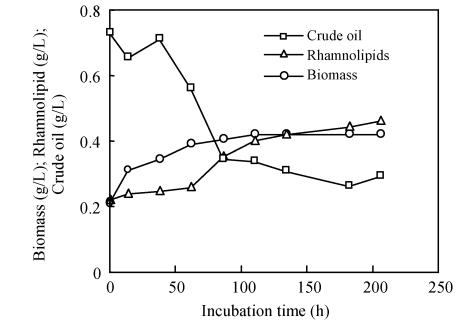
The effect of glycerol on crude oil degradation is shown in Fig.2. One g/L Glycerol and 0.7 g/L crude oil were first added into the basal medium before inoculation with bacterial. After 8 d incubation, 58% of the initial 0.7 g/L crude oil was consumed while 0.7 g/L rhamnolipid was simultaneously accumulated. Two stages of rhamnolipid production were observed: the first rapid production stage (0~4 d) and the second slow production stage (4~8 d). In contrast to the slow decrease of crude oil concentration, the glycerol concentration decreased much more rapidly in the first two days. Fig.2 shows that crude oil concentration data were noticeably scattered during the initial two days, which was due to the difficulties in handling the samples covered with a layer of crude oil before rhamnolipids was accumulated. The TLC assay confirmed the accumulation of rhamnolipids.
Fig. 2.
Profiles of cell growth, rhamnolipid production, and crude oil degradation of Pseudomonas aeruginosa incubated at basal mineral medium with 0.7 g/L crude oil and 1 g/L glycerol (Values shown here are from one typical experiment of three independent experiments)
During the incubation, the crude oil morphology went through three obvious changes. In the first two days, crude oil appeared as either a large stretch covering the upper part of the aqueous medium or attached on glass wall. Later, the large stretch or attachment of petroleum turned gradually into dispersed oil drops at a medium of 0.5~1.5 mm. Immediately after that, the oil drops disappeared even without shaking. This phenomenon is closely related to the emulsification of rhamnolipids.
Similar to the experiment in Fig.2, the effect of rhamnolipid was conducted in a basal mineral medium with low addition of rhamnolipids and crude oil. The initial rhamnolipid concentration was measured as 0.22 g/L. The profiles of cell growth, rhamnolipid accumulation and crude oil degradation are shown in Fig.3. During 8 d culture, 0.5 g/L crude oil was consumed by microbials while 0.25 g/L rhamnolipids were produced at the same time. More than 60% of crude oil was degraded. It seems Pseudomonas aeruginosa could uptake crude oil to produce biomass and rhamnolipids. Although the initial addition of rhamnolipids improved the emulsification of crude oil, most crude oil was still isolated from the aqueous medium during the first two days’ incubation and gradually emulsified later. By 72 h culture, isolated oil droplets disappeared and bubbles could be seen if the flasks were shaken vibrantly by hand, an indicator the accumulation of biosurfactants. The TLC assay also confirmed the accumulation of rhamnolipids. However, in comparison with Fig.2, both accumulated biomass and rhamnolipids were about 50% lower in Fig.3.
Fig. 3.
Profiles of cell growth, rhamnolipid production, and crude oil degradation of Pseudomonas aeruginosa incubated at basal mineral medium with 0.7 g/L crude oil and 0.22 g/L rhamnolipids (Values shown here are from one typical experiment of three independent experiments)
Biodegradation of crude oil by microbials appears to be the natural process by which the bulk of the polluting oil is used as an organic carbon source, causing the breakdown of petroleum components to lower molecular compounds or transformed into the other organic compounds such as biosurfactants (Chhatre et al., 1996). In another words, biodegradation of crude oil contaminants can be described as the conversion of chemical compounds by microorganisms into energy, cell mass and biological products. As the compositions of organic compounds in crude oil are rather complicated, the rates of uptake of specific compounds by a microbial depend on their distributions in crude oil. High concentrations of hydrocarbons associated with heavy oil can even inhibit biodegradation. It has been expected that only one microbial could not biodegrade crude oil thoroughly, corresponding well with the results shown in both Fig.2 and Fig.3. Thus, a population of microbial was necessary to improve the biodegradation of crude oil.
On the whole, both cell growth and biodegradation of crude oil were observed even with small addition of either glycerol or rhamnolipid, which were closely associated with the facilitated biodegradations of crude oil caused by the supplemented or accumulated rhamnolipids. Because only 0.02% of crude oil is water soluble, emulsification of crude oil is thus necessary for improving the bioavailability of insoluble hydrocarbons to microorganisms by increasing the interfacial area between the aqueous-insoluble hydrocarbons (Gerson, 1993). Also, rhamnolipids are well-known biosurfactants, predominantly from Pseudomonas aeruginosa (Beal and Betts, 2000). It could be concluded that in the presence of rhamnolipids, Pseudomonas aeruginosa can convert crude oil into both cell mass and biosurfactant. As the biosorption of crude oil by accumulated biomass might have effect on the actual biodegradation of crude oil, the biosorption was subsequently explored.
Adsorption of crude oil by killed Pseudomonas aeruginosa
The adsorption of crude oil by killed Pseudomonas aeruginosa was investigated at three biomass concentrations (shown in Table 2). While being shaken, the crude oil appeared as a dark aqueous solution resulting from the dispersion of the crude oil. But, without shaking, the crude oil was completely isolated and floated above the aqueous surface. At the end of adsorption (48 h) periods, the crude oil was conveniently separated from the aqueous solution without shaking. The adsorption capacity was determined by dividing the difference between the initial crude oil concentration and the final crude oil concentration by the biomass concentration.
Table 2.
Adsorption of crude oil by killed Pseudomonas aeruginosa
| Cell mass conc. (g/L) | Initial oil conc. (g/L) | Final oil conc. (g/L) | Adsorption capacity (g oil/g cell) |
| 0.5±0.05 | 0.75±0.06 | 0.72±0.09 | 0.06±0.03 |
| 1.0±0.09 | 0.73±0.06 | 0.71±0.08 | 0.02±0.02 |
| 2.0±0.08 | 0.76±0.08 | 0.70±0.06 | 0.03±0.01 |
Table 2 shows that the adsorption of crude oil by cell mass was slight so that it can be concluded that the adsorption of crude oil by dead Pseudomonas aeruginosa can be negligible and that the biological activity of Pseudomonas aeruginosa contributed to the biodegradation of crude oil on condition that either glycerol or rhamnolipid was supplemented.
In summary, more than 58% of crude oil was degraded after addition of small amount of rhamnolipids or glycerol. When incubated in crude oil together with 1 g/L glycerol, Pseudomonas aeruginosa produced rhamnolipids in two stages, possibly reflecting that microbials began to use petroleum when glycerol was used up in medium. When rhamnolipids were added in place of 1 g/L glycerol, cells grew much slower and rhamnolipid production decreased, which contributed to the non-availability of glycerol. And the biosorption of crude oil by killed Pseudomonas aeruginosa can be negligible in comparison with its biological activity. Our results suggest that the additions of either rhamnolipid or glycerol could facilitate the biodegradation of crude oil by Pseudomonas aeruginosa.
Footnotes
Project supported by Science Foundation from China Petroleum and Chemical Corporation, China
References
- 1.Balba MT, Al-Shayji Y, Al-Awadhi N, Yateem A. Isolation and characterization of biosurfactant-producing bacteria from oil-contaminated soil. Soil and Sediment Contamination. 2002;11:41–55. [Google Scholar]
- 2.Beal R, Betts WB. Role of rhamnolipids biosurfactants in the uptake and mineralization of hexadecane by Pseudomonas aeruginosa . J Appl Microbiol. 2000;89:158–168. doi: 10.1046/j.1365-2672.2000.01104.x. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran EV, Bemiller JN. Constituent Analysis of Glycosaminoglycans. In: Whistler RL, Wolfrom ML, editors. Methods in Carbohydrate Chemistry. New York: Academic Press; 1980. pp. 89–96. [Google Scholar]
- 4.Chhatre SA, Purohit HJ, Shanker R, Chakrabarti T, Khanna P. Bacterial consortia for crude oil spill remediation. Wat Sci Tech. 1996;34:187–193. [Google Scholar]
- 5.Gerson DF. The Biophysics of Microbial Surfactants: Growth on Insoluble Substrates. In: Kozaric N, editor. Surfactant Science Series, Biosurfactants: Production, Properties, Applications. New York, USA: Marcel Dekker; 1993. pp. 269–286. [Google Scholar]
- 6.Itoh S, Suzuki T. Effect of rhamnolipids on growth of Pseudomonas aeruginosa mutant deficient in n-paraffin-utilizing ability. Agric Biol Chem. 1972;36:2233–2235. [Google Scholar]
- 7.Matsufuji M, Nakata K, Yoshimoto A. High production of rhamnolipids by Pseudomonas aeruginosa growing on ethanol. Biotechnology Letters. 1997;19:1213–1215. [Google Scholar]
- 8.Nakahara T, Hisatsuka K, Minoda Y. Effect of hydrocarbon emulsification on growth and respiration of microorganisms in hydrocarbon media. J Ferment Technol. 1981;59:415–418. [Google Scholar]
- 9.Ollis D. Slick solutions for oil spills. Nature. 1992;358:453–454. [Google Scholar]
- 10.Rahman KSM, Banat IM, Thahira J, Thayumanavan T, Lakshmanaperumalsamy P. Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant. Biores Technol. 2002;81:25–32. doi: 10.1016/s0960-8524(01)00105-5. [DOI] [PubMed] [Google Scholar]
- 11.Urum K, Pekdemir T, Gopur M. Optimum conditions for washing of crude oil-contaminated soil with biosurfactant solutions. Process Safety and Environm Protect: Transact of the Institut of Chem Engin. 2003;81:203–209. [Google Scholar]
- 12.Wouter HN, Dick BJ. Rhamnolipid stimulates uptake of hydrophobic compounds by Pseudomonas aeruginosa . Appl and Environm Microb. 2002;68:4502–4508. doi: 10.1128/AEM.68.9.4502-4508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong KC, Ooi TL, Dzulkefly K, Wanyunus WMZ, Hazimah AH. Characterization of glycerol residue from a palm kernel oil methyl ester plant. J of Oil Palm Research. 2001;13(2):1–6. [Google Scholar]