Abstract
The activities of enzymes responsible for lignification in pepper, pre-inoculation with arbuscular mycorrhizal (AM) fungus of Glomus intraradices and/or infection with pathogenic strain of Phytophthora capsici, and the biological control effect of G. intraradices on Phytophthora blight in pepper were investigated. The experiment was carried out with four treatments: (1) plants pre-inoculated with G. intraradices (Gi), (2) plants pre-inoculated with G. intraradices and then infected with P. capsici (Gi+Pc), (3) plants infected with P. capsici (Pc), and (4) plants without any of the two microorganisms (C). Mycorrhizal colonization rate was reduced by about 10% in pathogen challenged plants. Root mortality caused by infection of P. capsici was completely eliminated by pre-inoculation with antagonistic G. intraradices. On the ninth day after pathogen infection, Peroxidase (POD) activity increased by 116.9% in Pc-treated roots but by only 21.2% in Gi+Pc-treated roots, compared with the control, respectively. Polyphenol oxidase (PPO) and Phenylalanine ammonia-lyase (PAL) activities gradually increased during the first 3 d and dramatically decreased in Pc-treated roots but slightly decreased in Gi+Pc-treated roots, respectively. On the ninth day after pathogen infection, PPO and PAL decreased by 62.8% and 73.9% in Pc-treated roots but by only 19.8% and 19.5% in Gi+Pc-treated roots, compared with the control, respectively. Three major POD isozymes (45 000, 53 000 and 114 000) were present in Pc-treated roots, while two major bands (53 000 and 114 000) and one minor band (45 000) were present in spectra of Gi+Pc-treated roots, the 45 000 POD isozyme was significantly suppressed by G. intraradices, suggesting that the 45 000 POD isozyme was induced by the pathogen infection but not induced by the antagonistic G. intraradices. A 60 000 PPO isozyme was induced in Pc-treated roots but not induced in Gi+Pc-treated roots. All these results showed the inoculation of antagonistic G. intraradices alleviates root mortality, activates changes of lignification-related enzymes and induces some of the isozymes in pepper plants infected by P. capsici. The results suggested that G. intraradices is a potentially effective protection agent against P. capsici.
Keywords: Arbuscular mycorrhizal (AM) fungus, Glomus intraradices, Phytophthora capsici, Peroxidase (POD), Polyphenol oxidase (PPO), Phenylalanine ammonia-lyase (PAL)
INTRODUCTION
Plants have defense mechanisms against pathogen infection by inducing systemic resistance in response to localized pretreatment with biological control agents, thus making them resistant to subsequent pathogen infection (Caruso et al., 1999; Hammerschmidt, 1999; Mohammadi and Kazemi, 2002; Piyada et al., 1995; Pozo et al., 2002; Ray et al., 1998). Biological control of plant pathogens has received much more attention. It is well known that plants and microorganisms symbiosis is a defense mechanism against pathogen infection (Agrios, 1997; Azcon-Aguilar and Barea, 1996; Guenoune et al., 2001; Jung et al., 2004; Koike et al., 2001; Pozo et al., 2002). Kilic-Ekici and Yuen (2004) reported that certain plant growth promoting microorganisms (PGPM) could enhance defensive activity and stimulate plant resistance against soil borne pathogens.
Among the PGPM, arbuscular mycorrhizal (AM) fungus is involved in the most universal intimate and important symbiosis in terrestrial ecosystems (Blee and Anderson, 1996; Guenoune et al., 2001; Pozo et al., 2002). This symbiosis has been shown to assist the plants in overcoming biotic and abiotic stresses (Cordier et al., 1998; Gianinazzi et al., 1996; Guenoune et al., 2001; Trotta et al., 1996; Zheng et al., 2004).
In compatible or incompatible interactions between plants and microorganisms, salicylic-dependent pathway, jasmonic-acid-dependent pathway (Agrios, 1997) and shikimic acid pathway (Haslan, 1983) are involved in plant defense. Among them, shikimic acid pathway has received much more attention due to the secondary metabolites such as lignin and phenolic phytoalexins, which are responsible for adding mechanical rigidity and strength to cell walls and for providing barriers to infection of pathogens. In these protection processes, Peroxidase (POD), Polyphenol oxidase (PPO) and Phenylalanine ammonia-lyase (PAL) are directly involved in lignin biosynthesis metabolites (Haslan, 1983).
Peroxidase (POD) is an oxido-reductive enzyme that participate in the cell wall polysaccharides processes such as oxidation of phenols, suberization, and lignification of host plant cells during the defense reaction against pathogenic agents (Ray et al., 1998). The resistance is associated with the induction of peroxidase in host tissues (Breusegem et al., 2001; Lin and Kao, 2001). Accumulation of lignin and phenolic compounds has been correlated with disease resistance in a number of plant-pathogen interactions. These include wheat-Fusarium graminearum (Mohammadi and Kazemi, 2002) and cucumber-Pythium aphanidermatum (Chen et al., 2000).
Polyphenol oxidase (PPO) is involved in the oxidation of polyphenols into quinines (antimicrobial compounds) and lignification of plant cells during microbial invasion. A number of studies indicated that phenol-oxidizing enzymes may participate in the responding defense reaction and hypersensitively by inducing plant resistance against fungi. These include common potato-Fusarium sambucinum (Ray et al., 1998) and wheat-F. graminearum (Mohammadi and Kazemi, 2002).
Phenylalanine ammonia-lyase (PAL) is the entry-point enzyme in the phenylpropanoid biosynthesis pathway and its presence has been demonstrated in pathogen-infected plants (Chen et al., 2000). Studies with several different species of plants showed that PAL activity increases with the biotic and abiotic stresses, such as bacterial infection (Hammerschmidt, 1999).
Study of a possible role for AM fungus symbiosis in protection against plant pathogens started in the 1970s (Azcon-Aguilar and Barea, 1996). Trotta et al. (1996), Cordier et al.(1998), and Pozo et al.(2002) reported that phenol-oxidizing enzymes such as POD, PPO and PAL are induced in tomato plant pre-inoculated with AM fungus and post-infected with Phytophthora spp. However, little is known about the changes of POD, PPO and PAL activity in host plants as related to inoculation with AM fungus and plant pathogens in pepper plants. The present study aimed at investigating the variations of the enzymes responsible for lignification in pepper pre-inoculated with arbuscular mycorrhizal (AM) fungus of Glomus intraradices and/or infected with Phytophthora capsici which could cause Phytophthora blight in pepper, and the potential of G. intraradices as a biological control agent against the disease.
MATERIALS AND METHODS
AM fungus and pathogen strains
The AM fungal species, Glomus intraradices, was purchased from the International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungus (West Virginia University, USA). The AM fungal inoculum was obtained from pot cultures of sudan grass as a host plant. Isolate KACC 40483 Phytophthora capsici was obtained from Korea Agriculture Culture Collection (KACC).
Plant materials and growth condition
Soil was mixed with quartz sand and vermiculate in a ratio of 1:2:1 (V/V/V), and rock phosphate was diluted to 0.2%. Chemical characteristics of the mixture utilized in this study consisted of 0.35% organic matter (OM), 0.15% nitrogen, 56×10−6 P2O5, 3.4 mmol K, 34.2 mmol Ca, 11.5 mmol Mg, 48 mmol cation exchangeable capacity (CEC), and 0.065 s/m electrical conductivity (EC).
Four treatments of pepper (Capsicum annuum L. Chungok) were used: G. intraradices added at planting (Gi); P. capsici added 5 weeks after inoculation with G. intraradices (Gi+Pc); pepper infected with P. capsici 5 weeks after planting (Pc); and control without G. intraradices and P. capsici (C). Pepper seeds were surface-sterilized with 3% (m/V) NaOCl for 2 min, and thoroughly washed with sterile distilled water. Three seeds were planted in each pot. The seeds were put in the site at which the AM fungus was pre-introduced by adding 150~200 spores in the planting hole. Pepper plants in each pot were thinned to one in 2 weeks after planting.
Phytophthora capsici was grown on a V8 juice agar medium for 4 d and then induced to sporulate under fluorescent light at 28 °C for 2 d. The mycelia and sporangia were incubated in sterile water for 1 h at 4 °C and then for 30 min at room temperature. Zoospores released from the sporangia were collected by filtering through two layers of cheese-cloth. Ten ml of collected zoospores (5×104 zoospores/ml) were used to inoculate plants 5 weeks after planting. Such a delayed infection time with P. capsici was chosen, because biological protection by AM fungus occurred mainly when symbiosis was well established before the pathogen attack (Azcon-Aguilar and Barea, 1996).
Plants were watered with nutrient solution as described in Zheng et al.(2004) throughout the experiment. Plants were grown in greenhouse conditions at 25~30 °C and 60%~70% relative humidity. Samples were harvested at 0, 1, 3, 5, 7, and 9 d after P. capsici infection. The samples were washed with tap water, rinsed in deionized water, and weighed. Plant tissues were immediately frozen in liquid nitrogen, and lyophilized.
Mycorrhizal colonization
Mycorrhizal colonization was assayed by using modified method of Brundrett et al.(1984). Roots of pepper plant were gently collected, rinsed free of soil with distilled water, and then immerged in FAA solution (mixture of formalin/acetic acid/ethanol, 13/5/200, V/V/V) for 24 h. After washing and rinsing several times with distilled water, the mycorrhizal roots were placed into 20 ml vials containing 10% (m/V) KOH, and then incubated at 90 °C for 45 min. After incubating, mycorrhizal roots were washed with distilled water and stained overnight with stain solution (400 ml 85% (V/V) lactic acid, 1.2 g chlorazole black E, 400 ml glycerine, 400 ml distilled water) at 50 °C. The root samples were rinsed with water until it was clear and put into destaining solution (50% (V/V) glycerol) overnight. Mycorrhizal colonization was visualized with a microscope (Olympus Model HB2-RFC). Total mycorrhizal colonization rate (%) was expressed as the percentage of the number of colonized roots versus total number of roots examined as described by Read et al.(1976).
Root mortality
Root mortality was measured by using the modified method of Liu and Huang (2000). About 500 mg of fresh roots were incubated with 10 ml 0.6% 2,3,5-triphenyltetrazolium chloride in 0.05 mol/L phosphate buffer (pH 7.4) for 24 h in the dark at 30 °C. Roots were then rinsed twice with sterilized water. Formazan was twice extracted from the roots with 95% (V/V) ethanol at 70 °C for 4 h. Combined extracts from the two extractions were adjusted to a final volume of 50 ml with 95% ethanol, and absorbance was measured at 490 nm. A standard curve was prepared by using different proportions of living roots and roots killed in an autoclave to calculate root mortality expressed as percentage of dead root dry weight versus total root dry weight.
Lignification-related enzyme activities
For extraction of enzymes, 2 g frozen fresh sample was homogenized with 2.5 ml of 100 mmol/L K3PO4 buffer solution, pH 7.0, containing 2 mmol/L of EDTA, 1% (m/V) PVP, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF). The homogenates were centrifuged at 12 000 g for 15 min at 4 °C and the supernatant fractions were kept frozen at −20 °C until enzyme analysis. Protein concentration was determined by using the method of Bradford (1976).
Peroxidase (POD) activity was determined according to the method of Lin and Kao (2001). The reaction mixture contained 50 μl of 20 mmol/L guaiacol, 2.8 ml of 10 mmol/L phosphate buffer (pH 7.0), and 0.1 ml enzyme extract. The reaction was initiated by the addition of 20 μl of 40 mmol/L H2O2. Increase in absorbance at 470 nm was recorded for 1 min using UV-visible spectrophotometer (Shimadzu, UV-1601). The POD activity was calculated by using an absorption coefficient (26.6 mmol/(L·cm) at 470 nm) for the tetraguaiacol. One unit of POD activity was defined as the amount of enzyme required for formation of 1 μmol of tetraguaiacol per minute at room temperature.
Polyphenol oxidase (PPO) activity was determined according to the method of Siriphanich and Kader (1985). One ml reaction mixture contained 20 μl enzyme extract and 10 mmol/L phosphate buffer, pH 7.0. Each sample was aerated for 2 min in a small test tube followed by the addition of catechol as the substrate at a final concentration of 20 mmol/L. PPO activity was presented as the change in one unit of absorbance at 420 nm per minute per gram fresh weight of sample.
Phenylalanine ammonia-lyase (PAL) activity was assayed by using an assay modified from Godwin et al.(1996). The reaction mixture contained 100 mmol/l Tris-HCl buffer, pH 8.5, 1 mmol/L 2-mercaptoethanol, 15 mmol/L L-phenylalanine, and 100 μl enzyme extract. The reaction mixture was incubated at 30 °C for 15 min, and reaction was terminated by the addition of 6 mol/L HCl and then measured at 290 nm. One unit represents the amount of enzyme that produces 1 μmol of cinnamic acid per hour.
Active staining of peroxidase and polyphenol oxidase
For active staining of POD after 12.5% (m/m) polyacrylamide gel electrophoresis (PAGE), the gels (30 μg proteins) were soaked for 10 min in 50 mmol/L Tris buffer, pH 6.8, then incubated with 0.46% (V/V) guaiacol, and 13 mmol/L H2O2 in the same buffer until red bands appeared and the gels were subsequently fixed in water/methanol/acetic acid (6.5:2.5:1, V/V/V) (Chen et al., 2000).
For active staining of PPO, the gels were rinsed in deionized water several times and then placed in 100 mmol/L pH 7.0 sodium phosphate buffer, containing 10 mmol/L DL-1,3-dihydroxy phenylalanine. After 3 h of incubation on a rotary shaker, dark bands indicative of PPO isozymes appeared in the gel spectrum (Mohammadi and Kazemi, 2002).
Statistical analysis
Treatment effects were determined by analysis of variance according to the general linear model procedure of the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA). Differences among various treatment means were separated by least significant difference (LSD). Significance of between-treatment means was tested at the 0.05 level of probability.
RESULTS
Mycorrhizal colonization and root mortality
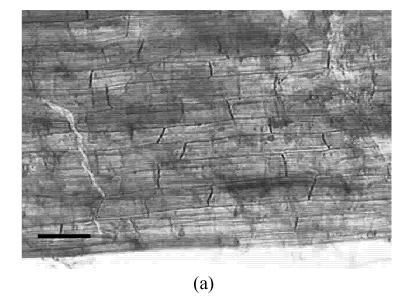
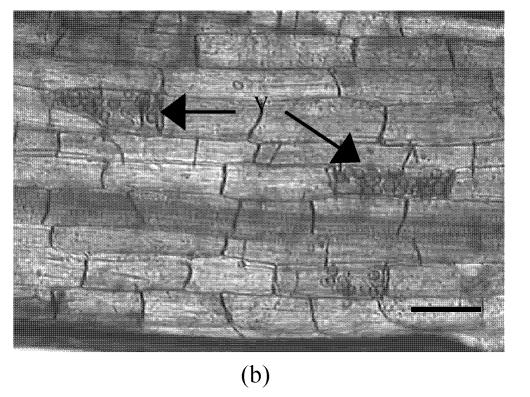
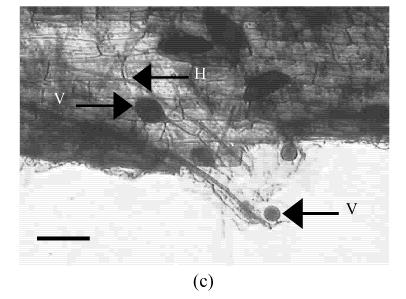
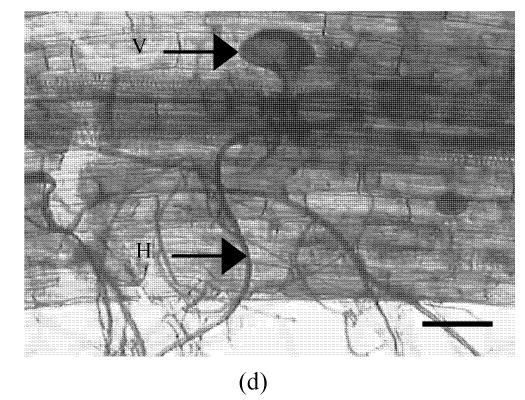
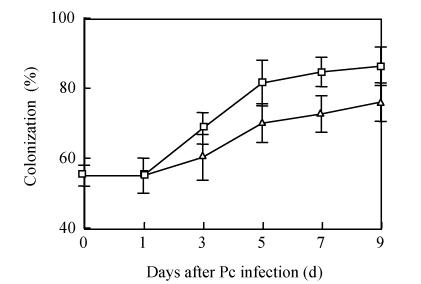
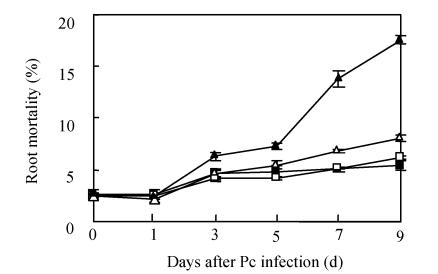
The AM fungus colonized in root cortex containing numerous hyphae and vesicles are shown in Fig.1. Hyphae quickly branched and penetrated the walls of cortical cells to form vesicles (Figs.1b, 1c, 1d). So that, multiple hyphae and vesicles were observed within cortical cells. Colonization with AM fungus was established in pepper roots at a level of 55% in five weeks after planting, with an increase to about 88% after nine days more of growth (Fig.2). Infection of the mycorrhizal roots by P. capsici decreased the rate of increase in G. intraradices colonization to a level of about 70% after nine days of challenge by the pathogen. Root mortality of pepper plants dramatically increased after 5 d infection with the P. capsici pathogen but was almost completely not induced by pre-inoculation with antagonistic G. intraradices (Fig.3), thus resulting in little difference of root mortality between C and Gi+Pc. Pepper plants inoculated with the pathogen had root decay, and the leaves were chlorotic and wilted after nine days infection with the pathogen (data not shown). Pathogen infection on mycorrhizae colonized roots showed little disease, as demonstrated by root mortality (Fig.3).
Fig. 1.
Colonization of pepper roots inoculated with G. intraradices. Colonized roots, after staining, photographed using bright-field microscope (a) Control; (b) Yong arbuscules; (c) Aging arbuscules and vesicle formation; (d) Intracelluar location of vesicles becomes apparent as they enlarge. V: Vesicle; H: Hyphal. Bar=50 μm
Fig. 2.
Effect of P. capsici infection on G. intraradices colonization in pepper roots. P. capsici infection occurred after 5 weeks inoculation with G. intraradices. G. intraradices (□), G. intraradices+P. capsici (∆). Each value is the mean±SE for n=6. Bars represent standard error
Fig. 3.
Root mortality of pepper plants as affected by pre-inoculation of G. intraradices and/or P. capsici. Control (■), G. intraradices (□), G. intraradices+P. capsici (∆) and P. capsici (▲). Each value is the mean±SE for n=6. Bars represent standard error
Lignification-related enzyme activities in roots
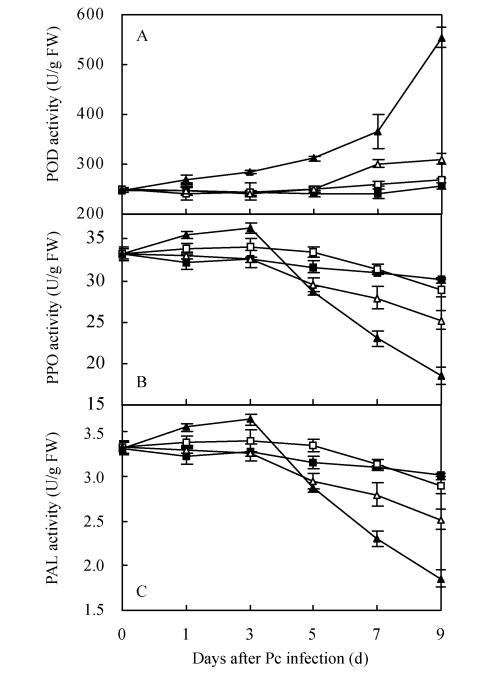
POD activity in Pc-treated roots gradually increased during the first 7 d and sharply increased thereafter, while the POD activity in Gi+Pc-treated roots was unchanged in the first 5 d and slightly increased thereafter (Fig.4a). POD activity increased by 116.9% in Pc-treated roots but by only 21.2% in Gi+Pc-treated roots on the ninth day, compared with the control. Meanwhile, POD activity in Pc-treated roots showed about 1.8-fold increase compared with Gi+Pc-treated roots. PPO activity in Pc-treated roots gradually increased in the first 3 d after pathogen infection and then markedly decreased, while the PPO activity in Gi+Pc-treated roots was unchanged in the first 3 d and slightly decreased thereafter (Fig.4b). PPO activity decreased by 62.8% in Pc-treated roots but by only 19.8% in Gi+Pc-treated roots on the ninth day, compared with the control. Meanwhile, PPO activity in Pc-treated roots decreased by about 1.4-fold compared with Gi+Pc-treated roots. PAL activity in Pc-treated roots gradually increased in the first 3 d after pathogen infection and then markedly decreased, while the PAL activity in Gi+Pc-treated roots gradually decreased in the first 7 d and remained at that level thereafter (Fig.4c). PAL activity decreased by 73.9% in Pc-treated roots but by 19.5% in Gi+Pc-treated roots, compared with the control on the ninth day. Meanwhile, PPO activity in Pc-treated roots decreased by about 1.5-fold compared with Gi+Pc-treated roots. However, there was no significant difference in POD, PPO and PAL activities between control and Gi-treated roots during the period of the experiment on pepper roots.
Fig. 4.
Changes of peroxidase (A), polyphenol oxidase (B) and phenylalanine ammonia-lyase (C) in pepper roots upon G. intraradices and/or P. capsici infection. Control (■), G. intraradices (□), G. intraradices+P. capsic (∆) and P. capsic (▲). Each value is the mean±SE for n=6. Bars represent standard error
Polyacrylamide gel electrophoresis (PAGE)
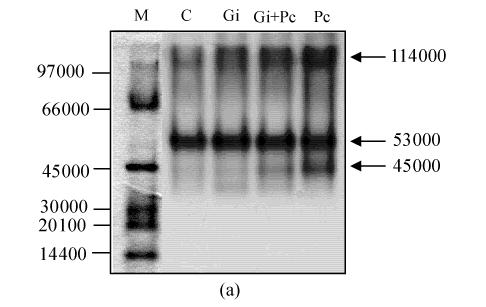
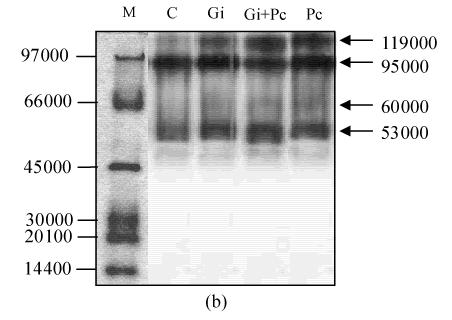
After PAGE, active staining of POD was performed to observe the change in the isozymes in pepper roots. On day 9, three major bands (45 000, 53 000 and 114 000) were appeared in spectra of Pc-treated roots, while two major (53 000 and 114 000) and minor bands (45 000) appeared in spectra of Gi+Pc-treated roots, suggesting that the 45 000 POD isozyme was actively induced by pathogen infection but not induced by the antagonistic G. intraradices. Two major bands (53 000 and 114 000) were present in spectra of C and Gi-treated roots (Fig.5a). Active staining of PPO in pepper roots revealed the presence of three isozymes (53 000, 95 000, and 119 000) in C, Gi and Gi+Pc-treated roots (Fig.5b). The isozyme of 60 000 appeared in Pc-treated roots only, suggesting that the 60 000 PPO was induced by pathogen infection but not induced by the antagonistic G. intraradices.
Fig. 5.
Active staining of peroxidase (a) and polyphenol oxidase (b) in pepper roots. Molecular size standards (M), control (C), G. intraradices (Gi), G. intraradices+P. capsici (Gi+Pc) and P. capsici (Pc)
DISCUSSION
The AM fungus was well colonized in pepper roots after 5 weeks planting (Fig.1). Mycorrhizal colonization slightly reduced 9 d after P. capsici infection (Fig.2). This result indicated localized competition for infection/colonization sites between G. intraradices and P. capsici (Cordier et al., 1998; Pozo et al., 2002). Increasing root mortality was observed after P. capsici infection of non-mycorrhizal plants, and root mortality was markedly reduced in Gi+Pc-treated roots compared with Pc-treated roots (Fig.3). It indicated that plant resistance to P. capsici infection was increased by AM fungus symbiosis in pepper plants. Dassi et al.(1998) suggested that the presence of mycorrhizal hyphae drastically reduced the percentage of zoospore germination, resulting in reduction of penetration to root via epidermal grooves. This result was also supported by Pozo et al.(2002) who reported that Phytophthora infected non-mycorrhizal roots, but failed to infect pre-inoculated mycorrhizal roots.
Furthermore, Trotta et al.(1996) showed that Phytophthora development was reduced in mycorrhizal colonized and adjacent uncolonized regions of mycorrhizal root system, suggesting a systemic effect. According to this study, hyphae-vesicles-containing cortical cells of mycorrhizal plants exhibited resistance against P. capsici infection. These Gi+Pc-treated roots would help sustainable growth by absorbing nutrients while the damaged Pc-treated roots could not.
Many plant enzymes are involved in defense reactions against plant pathogens. These include oxidative enzymes such as POD and PPO, which catalyse the formation of lignin and other oxidative phenols that contribute to the formation of defense barriers for reinforcing the cell structure (Avdiushko et al., 1993). Other enzymes such as PAL are involved in phytoalexin or phenolic compound biosynthesis. These enzymes have been correlated with defense against pathogens in several plants, including tomato (Borden and Higgins, 2002), pepper (Jung et al., 2004) and wheat (Mohammadi and Kazemi, 2002).
POD, a member of the PR-9 family, is of the lignin-forming type of plant defense response (Ray et al., 1998). POD activity is associated with disease resistance in plants (Lin and Kao, 2001), and increases in host plants following pathogen infection (Borden and Higgins, 2002; Koike et al., 2001; Zheng et al., 2004). Plant POD has been reported to catalyse the last step in the biosynthesis of lignin and hydrogen peroxide (Breusegem et al., 2001). Mohammadi and Kazemi (2002) reported that POD was induced in wheat head by infection with pathogenic strain of F. graminearum. In our study, a continuous increase in POD activity was observed with an increase in root mortality in Pc-treated roots during a period of 9 d following infection with P. capsici. However, POD activity was slightly induced in Gi+Pc-treated root (Fig.3 and Fig.4a), possibly due to the antagonistic effect of G. intraradices, which suppressed hydrogen peroxide generation in Gi+Pc-treated roots much more than that in Pc-treated roots described in Zheng et al.(2004). Moreover, the reduction of hydrogen peroxide in Gi+Pc-treated plants might act as secondary messenger to generate even less POD than that in Pc-treated plants (data not shown).
PPO usually accumulates during pathogenic attack, in wounds, or tissue senescence in plants. Plant cells membrane disruption occurrence might initiate formation of quinines following an increase in accessibility of PPO to its substrate. The increased activity of PPO could be detected in the cucumber leaf in the vicinity of lesions caused by some foliar pathogens or phosphate application (Avdiushko et al., 1993). In our study, PPO activity in Pc-treated roots gradually increased in the first 3 d after the pathogen infection and then rapidly decreased, while the activity in Gi+Pc-treated roots was unchanged till the third day, and slightly decreased thereafter. There was no significant difference in POD activities between control and Gi-treated plants during the experimental period in roots (Fig.4b). This might suggest that the induced PPO activity in Pc-treated roots could be a defensive response against P. capsici infection at the early stage. Similar results obtained in plant-pathogenic fungal interactions such as wheat-F. graminearum (Mohammadi and Kazemi, 2002). In Gi+Pc-treated roots, the PPO activity remained similar to that of the control, suggesting the antagonistic effect of G. intraradices. The capability of adaptation to phytopathogenic stress was related to the increase or sustained ability to form defense barriers for reinforcing the cell structure by lignification-related enzymes. However, decrease in the activities of lignification-related enzymes, particularly PPO activity, might be the result from prolonged pathogenic stress.
PAL, one of the key enzymes in the phenylpropanoid pathway and the flavonoid pathway, was increased in both incompatible and compatible interactions between plants and pathogens (Harllen et al., 2004). Recent work also demonstrated the existence in cucumbers of phenolic phytoalexins, which might be produced through a PAL pathway (Chen et al., 2000). In our study, the PAL was induced in Pc-treated roots at the early stage by infection with P. capsici. (Fig.4c), however, with prolonged pathogenic stress, the PAL levels were lower compared with other treatments or control. All these results suggested that early induction of PAL by P. capsici might result in the activation of defenses, but subsequent pathogen challenge might cause damage and necrosis that blocks the activation of PAL in pepper roots. In addition, antagonistic G. intraradices was able to induce resistance to P. capsici without detectable accumulation of PPO and PAL in pepper root. This result indicated that inhibited growth of the pathogen in the mycorrhizal roots was not correlated with PPO and PAL induction, and that the absence of PPO and PAL did not lower the level of protection.
The induction of isozymes in both POD and PPO was observed in pepper roots after their infection with P. capsici zoospores. Especially, 45000 POD and 60000 PPO isozymes in Pc-treated roots were strongly induced (Fig.5). But, in case of Gi+Pc-treated roots, those of POD and PPO were slightly induced or not induced, respectively, suggesting that the POD and PPO isozymes were induced by P. capsici attack and distinctly suppressed by G. intraradices. Many studies indicated that POD and PPO isozymes were induced in cucumber and tobacco with various abiotic and biotic inducers (Piyada et al., 1995; Ray et al., 1998) and in cucumber root following pathogen challenge with P. aphanidermatum (Chen et al., 2000). Also, a remarkable increase in POD activity and occurrence of new isozymes were observed in wheat infected with F. graminearum (Mohammadi and Kazemi, 2002).
All these results indicate that co-inoculation of G. intraradices with P. capsici alleviates the physiological symptoms affected by the pathogen challenge including root mortality, activation of lignification-related enzymes, and induction of the isozymes. Furthermore, it also proves G. intraradices potentially can act as one of the protection agents against plant pathogen P. capsici.
Acknowledgments
We greatly appreciate Dr. Y.W. Kim (Chonnam National University) for critical reading of our manuscript.
Footnotes
Project supported by Korea Science and Engineering Foundation (KOSEF) through the Agricultural Plants Stress Research Center (APSRC) at Chonnam National University, Korea
References
- 1.Agrios GN. Plant Pathology. 4th Edition. San Diego, USA: Academic Press; 1997. pp. 93–114. [Google Scholar]
- 2.Avdiushko SA, Ye XS, Kuc J. Detection of several enzymatic activities in leaf prints of cucumber plant. Physiological and Molecular Plant Pathology. 1993;42:441–454. [Google Scholar]
- 3.Azcon-Aguilar C, Barea JM. Arbuscular mycorrhizas and biological control of soil–borne plant pathogens–an overview of the mechanisms involved. Mycorrhiza. 1996;6:457–464. [Google Scholar]
- 4.Blee KA, Anderson AJ. Defense-related transcript accumulation in Phaseolus vulgaris L. colonized by the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith. Plant Physiology. 1996;110:675–688. doi: 10.1104/pp.110.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borden S, Higgins VJ. Hydrogen peroxide plays a critical role in the defense response of tomato to Cladosporicem fulvum . Physiological and Molecular Plant Pathology. 2002;61:227–236. [Google Scholar]
- 6.Bradford MM. A rapid sensitive method for quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Analytical Biochemistry. 1976;72:48–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 7.Breusegem FV, Vranova E, Dat JF, Inze D. The role of active oxygen species in plant signal transduction. Plant Science. 2001;161:405–414. [Google Scholar]
- 8.Brundrett MC, Piche Y, Peterson RL. A new method for observing the morphology of vesicular-arbuscular mycorrhizae. Canadian Journal of Botany. 1984;62:2128–2134. [Google Scholar]
- 9.Caruso C, Chilosi G, Caporale C, Leonardi L, Bertini L, Magro P, Buonocore V. Induction of pathogenesis-related proteins in germinating wheat seeds infected with Fusarium culmorum . Plant Science. 1999;140:107–120. [Google Scholar]
- 10.Chen CQ, Belanger RR, Benhamou N, Paulitz TC. Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum . Physiological and Molecular Plant Pathology. 2000;56:13–23. [Google Scholar]
- 11.Cordier C, Pozo MJ, Barea JM, Gianinazzi S, Gianinazzi PV. Cell defense response associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Molecular Plant-Microbe Interactions. 1998;11(10):1017–1028. [Google Scholar]
- 12.Dassi B, Dumas GE, Gianinazzi S. Do pathogenesis-related (PR) proteins play a role in bioprotection of mycorrhizal tomato roots towards Phytophthora parasitica? Physiological and Molecular Plant Pathology. 1998;52:167–183. [Google Scholar]
- 13.Gianinazzi PV, Dumas GE, Gollotte A, Tahiri AA, Gianinazzi S. Cellular and molecular defence-related root responses to invasion by arbuscular mycorrhizal fungus. New Phytologist. 1996;133:45–57. [Google Scholar]
- 14.Godwin BD, Vaduvatha S, Nair PM. Stabilization of phenylalanine ammonia-lyase containing Rhodotorula glutinis cells for the continuous synthesis of phenylalanine methyl ester. Enzyme and Microbial Technology. 1996;19:421–427. [Google Scholar]
- 15.Guenoune D, Galili S, Phillips DA, Volpin H, Chet I, Okon Y, Kapulnik Y. The defense response elicited by the pathogen Rhizoctonia solani is suppressed by colonization of VM-fungus Glomus intraradices . Plant Science. 2001;160:925–932. doi: 10.1016/s0168-9452(01)00329-6. [DOI] [PubMed] [Google Scholar]
- 16.Hammerschmidt R. Induced disease resistance: How do induced plants stop pathogens. Physiological and Molecular Plant Pathology. 1999;55:77–84. [Google Scholar]
- 17.Harllen SAS, Reginaldo SR, Dirceu M, Bernardo AH, Maria CBP, Ann M. Rhizobacterial induction of systemic resistance in tomato plants: Non-specific protection and increase in enzyme activities. Biological Control. 2004;29:288–295. [Google Scholar]
- 18.Haslan E. Shikimic Acid: Metabolism and Metabolites. Chichester, UK: Wiley; 1983. pp. 50–80. [Google Scholar]
- 19.Jung WJ, Jin YL, Kim YC, Kim KY, Park RD, Kim TH. Inoculation of Paenibacillus illinoisensis alleviates root mortality, activates of lignification-related enzymes, and induction of the isozymes in pepper plants infected by Phytophthora capsici . Biological Control. 2004;30:645–652. [Google Scholar]
- 20.Kilic-Ekici O, Yuen GY. Comparison of strains of Lysobacter enzymogenes and PGPR for induction of resistance against Bipolaris sorokiniana in tall fescue. Biological Control. 2004;30:446–455. [Google Scholar]
- 21.Koike N, Hyakumachi M, Kageyama K, Tsuyumu S, Doke N. Induction of systemic resistance in cucumber against several diseases by plant growth-promoting fungus: Lignification and superoxide generation. European Journal of Plant Pathology. 2001;107:523–533. [Google Scholar]
- 22.Lin CC, Kao CH. Cell wall peroxidase activity, hydrogen peroxide level and NaCl-inhibited root growth of rice seedlings. Plant and Soil. 2001;230:135–143. [Google Scholar]
- 23.Liu XZ, Huang BR. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bent grass cultivars. Journal of American Society for Horticultural Science. 2000;125(4):442–447. [Google Scholar]
- 24.Mohammadi M, Kazemi H. Changes in peroxidase and polyphenol activity in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Science. 2002;162:491–498. [Google Scholar]
- 25.Piyada T, Michelle DH, John CS. Systemic wound induction of potato (Solanum tuberosum) polyphenol oxidase. Phytochemistry. 1995;40(3):673–676. [Google Scholar]
- 26.Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, Azcon-Aguilar C. Localized versus systemic effect of arbuscular mycorrhizal fungus on defense responses to Phytophthora infection in tomato plants. Journal of Experimental Botany. 2002;53(368):525–534. doi: 10.1093/jexbot/53.368.525. [DOI] [PubMed] [Google Scholar]
- 27.Ray H, Douches DS, Hammerschmidt R. Transformation of potato with cucumber peroxidase: Expression and disease response. Physiological and Molecular Plant Pathology. 1998;53:93–103. [Google Scholar]
- 28.Read DJ, Koucheki HK, Hodgson J. Vesicular-arbuscular mycorrhiza in native vegetation system. New Phytologist. 1976;77:641–653. [Google Scholar]
- 29.Siriphanich J, Kader AA. Effects of CO2 on cinnamic acid 4-hydroxylase in relation to phenolic metabolism in lettuce tissue. Journal of the American Society for Horticultural Science. 1985;110:333–335. [Google Scholar]
- 30.Trotta A, Varese GC, Gnavi E, Fusconi A, Sampo S, Berta G. Interaction between the soilborne root pathogen Phytophthora nicotianae var. parasitica and the arbuscular mycorrhizal fungus Glomus mosseae in tomato plants. Plant and Soil. 1996;185:199–209. [Google Scholar]
- 31.Zheng HZ, Kim YW, Lee HJ, Park RD, Jung WJ, Kim YC, Lee SH, Kim TH, Kim KY. Quantitative changes of PR proteins and antioxidant enzymes in response to Glomus intraradices and Phytophthora capsici in pepper (Capsicum annuum L.) plants. Journal of Microbiology and Biotechnology. 2004;14(3):553–562. [Google Scholar]