Abstract
The effect of tea saponins (TS) on rumen fermentation and methane emission was examined using an in vitro gas production technique named Reading Pressure Technique. Three levels of TS addition (0, 0.2, 0.4 mg/ml) were evaluated in the faunated and defaunated rumen fluid. Compared to the control, TS addition decreased the 24 h gas production in the faunated rumen fluid, but had a minor effect on gas yield in the defaunated rumen fluid. The TS significantly reduced methane production in vitro. In the faunated rumen fluid, 0.2 or 0.4 mg/ml TS decreased the 24 h methane emission by 12.7% or 14.0%, respectively. Rumen fluid pH value was affected neither by TS addition nor by defaunation. The TS addition had only minor effects on volatile fatty acids, but the yield and pattern of volatile fatty acids were greatly affected by defaunation. While the molar proportion of acetate was not affected by defaunation, the propionate was significantly increased and the butyrate significantly decreased. Ammonia-N concentration and microbial protein yield were influenced by TS inclusion and defaunation. Inclusion of 0.4 mg/ml TS increased the microbial protein mass by 18.4% and 13.8% and decreased the ammonia-N concentration by 8.3% and 19.6% in the faunated and defaunated rumen fluid, respectively. Protozoa counts were significantly reduced by TS inclusion. The current study demonstrated the beneficial effect of TS on methane production and rumen fermentation, and indicated that this may be due to the effect of the associated depression on protozoa counts.
Keywords: Tea saponin, Faunated, Defaunated, Ruminal fermentation, Methane, In vitro
INTRODUCTION
Livestock is one of the largest sources of methane emission with 80~115 million tons produced per year, equivalent to 15%~20% of total anthropogenic methane (IPCC, 2001). The global cattle population is responsible for 73% of methane emissions of all livestock, and methane produced during ruminal fermentation represents a loss of 2%~15% of gross energy intake and may contribute to global warming (Johnson and Johnson, 1995). Many researches had been carried out to find ways to lower the methane production in ruminant animals. The symbiosis of protozoa with methanogenic Archaea was described by Finlay et al.(1994), and selective suppression of the rumen protozoa had been suggested to be a promising approach to reduce methane release (Whitelaw et al., 1984; Dohme et al., 1999; Moss et al., 2000).
It was reported that saponins or saponin-like substances had the potential to suppress the methane emission, reduce rumen protozoa counts, and change fermentation patterns (Wang et al., 1997; Makkar and Becker, 1997; Hristov et al., 1999). In a preliminary study, Liu et al.(2003) observed that saponins from tea seed could modify the rumen fermentation and reduce rumen protozoa counts. However, results of Dohme et al.(1999) showed that the methane-suppressing effects of the active feed components from coconut oil were not necessarily mediated through the decline in protozoa counts, even when a simultaneous suppression of protozoa took place. The objective of this study was to investigate the effects of tea saponins on methane release, rumen fermentation and protozoa counts in the rumen. In order to be able to distinguish between direct and protozoa-mediated effects, faunated and defaunated rumen fluid were used.
MATERIALS AND METHODS
Materials
Saponins extracted from tea seed (TS) were purchased from Zhejiang Orient Tea Development Co. Ltd., affiliated to Tea Research Institute, Chinese Academy of Agricultural Sciences. It was in powder form, light-yellow, and contained above 60% triterpenoid saponin and foamility score of 160~190 mm and pH of 5.0~6.5.
Equipment and technique
A gas test was conducted using a semi-auto mated Reading Pressure Technical (RPT, Mauricio et al., 1999). Incubations were conducted in 180 ml flasks. About 750 mg of substrates (50% corn meal and 50% grass meal, m/m) were accurately weighed into the flasks, and then 90 ml reduced buffer medium (Theodorou et al., 1994) was added to each flask which were then sealed with a butyl rubber stopper and stored overnight at 4 °C. The rumen fluid inoculum was obtained from two donor sheep that were fed on a mixed diet (60% wild ryegrass hay plus 40% concentrate) at 1.3 times maintenance before morning feeding. The rumen fluid was strained through four layer of cheesecloth and held under CO2 in a water-bath at 39 °C until used. Each flask (pre-warmed to 39 °C) was then injected with 10 ml prepared inoculum through the stopper using a needle. The displaced gas was allowed to escape before the needle was removed. The flasks were then swirled to mix the contents and placed in an incubator at 39 °C. At the time for measuring the gas production, a pressure transducer, interfaced with a computer allowed the accumulated head-space gas pressure values to be directly entered into the computer. These pressure measurements were then used to estimate the generated gas volumes (Theodorou et al., 1994, Mauricio et al., 1999). The organic matter digestibility (OMD) was calculated from the gas production at 24 h incubation according to the formula by Menke and Steingass (1988).
Experimental design and diet preparation
Half of the flasks were injected with defaunated rumen fluids. Defaunation was achieved by nonyl phenol ethoxylate (Synperonic NP9® ICI, Middlesbrough, UK) administered at a concentration of 2.5 ml/L rumen fluid (Dohme et al., 1999). Then the TS were added at levels of 0, 0.2 and 0.4 mg/ml fluids on the faunated and defaunated rumen fluid, and four bottles (replicates) were used for each adding level of TS. At 3, 6, 9, 12 and 24 h incubation, gas pressure was recorded and methane concentration, pH, ammonia-N, volatile fatty acids (VFA), protozoa counts and microbial protein (MCP) yield were determined.
Measurement of in vitro fermentation parameters
The pH of in vitro rumen liquor was determined immediately after removal using a pH meter (Model PB-20, Sartorius). Ammonia-N concentration was determined by a spectrometer (Model 721) using colorimetry with the NH4Cl solution as a standard (Feng and Gao, 1993). For determination of VFA concentration, 1 ml of the fermentation medium sample was placed in centrifugal tubes, mixed uniformly with 0.25 ml of 25% ortho-phosphoric acid, and then centrifuged at 10000 r/min for 10 min. The supernatant was decanted into another test tube, capped and stored in a refrigerator at −20 °C until analyzed. The VFA was analyzed by gas chromatograph (GC-2100, Shimadzu) equipped with a Flame Ionization detector (FID). The column (HP-INNOWAX, 19091N-133) was 30 m×0.25 mm×0.25 μm in size. Two μl of fluid samples were injected with a syringe, and the temperature of the injector/detector and the column was 260 °C and 220 °C, respectively. Nitrogen was used as a carrier. Methane concentration was analyzed using the same gas chromatograph with the same column, and the temperatures of the injector/detector and the column were 130 °C and 80 °C, respectively.
Ciliate protozoa were counted by the method of Ogimoto and Imai (1981). In which, 0.5 ml of fermentation fluid was mixed with 0.5 ml methylgreen-formaldehyde-saline solution and shaken gently. The mixture was held for 5 min and then pipetted into a refitted counting haemocytometer of 0.3 mm depth. The protozoa were then counted under microscope. Concentrations of the MCP were determined based on purines using the method of Zinn and Owens (1986), modified by Makkar and Becker (1999), and estimated from the ratio of purines to N of isolated bacteria with yeast RNA as standard.
Statistical Analysis
Data were analysed using the general linear model (GLM) procedure of SAS (1997, SAS Inst., Inc., Cary, NC). All multiple comparisons among means were performed using Duncan’s new multiple range test.
RESULTS
Influence of TS and protozoa status on the in vitro gas production and methane release
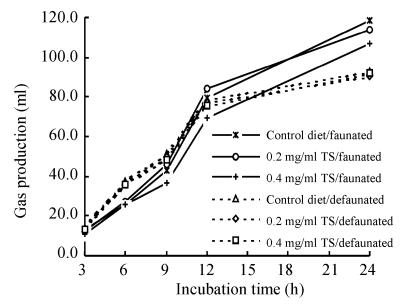
The gas production (GP) decreased with the adding levels of TS in the faunated rumen fluid, but the TS had no effect on the GP in the defaunated rumen fluid (Fig.1). Addition of 0.2 and 0.4 mg/ml TS decreased the 24 h GP by 4.0% and 10.1% respectively in the faunated rumen fluid (Table 1). The 24 h GP in the defaunated rumen fluid was significantly lower compared to the control. The OMD was indicative index of the GP value. While addition of TS had minor effect on the OMD, the defaunation significantly reduced the OMD.
Fig. 1.
Effect of tea saponin (TS) additon and protozoa status on gas production
Table 1.
Effect of tea saponin and defaunation on in vitro gas production, estimated organic matter digestibility (OMD), methane emission and rumen fluid characteristic in the faunated and defaunated rumen fluid at 24 h
| Items | Faunated |
Defaunated |
SEM |
P value |
||||||
| 0 mg/ml | 0.2 mg/ml | 0.4 mg/ml | 0 mg/ml | 0.2 mg/ml | 0.4 mg/ml | Protozoa | TS | TS×Protozoa | ||
| Gas production (ml) | 118.5A | 113.8AB | 106.5B | 93.0C | 90.5C | 92.0C | 2.20 | 0.0001 | 0.0370 | 0.0651 |
| OMD (%) | 63.9A | 62.4AB | 60.1B | 55.6C | 54.7C | 55.2C | 0.73 | 0.0001 | 0.0425 | 0.0754 |
| Methane (mmol) | 1.05A | 0.91B | 0.90B | 0.42C | 0.39C | 0.41C | 0.023 | 0.0001 | 0.0049 | 0.0347 |
| Methane (mmol/g OMD) | 2.17A | 1.94B | 1.98B | 1.01C | 0.95C | 0.97C | 0.039 | 0.0001 | 0.0078 | 0.0764 |
| pH | 6.80 | 6.78 | 6.82 | 6.81 | 6.80 | 6.82 | 0.025 | 0.5630 | 0.5056 | 0.9140 |
| Ammonia-N (mg/L) | 14.0A | 13.6AB | 12.9AB | 12.4BC | 11.3C | 10.0D | 0.26 | 0.0001 | 0.0001 | 0.0912 |
| VFA (mmol/L) | 41.97A | 43.36A | 44.25A | 35.59B | 34.36B | 35.46B | 1.112 | 0.0001 | 0.5799 | 0.4505 |
| Molar proportions (%) | ||||||||||
| Acetate | 70.92 | 70.78 | 70.88 | 71.97 | 71.78 | 71.22 | 1.486 | 0.5256 | 0.9648 | 0.9646 |
| Propionate | 15.71B | 16.52B | 16.35B | 25.01A | 24.93A | 25.92A | 1.472 | 0.0001 | 0.8729 | 0.9184 |
| Butyrate | 13.37A | 12.70A | 12.77A | 3.02B | 3.29B | 2.86B | 0.385 | 0.0001 | 0.6207 | 0.4833 |
| A/P | 4.54A | 4.28A | 4.37A | 2.91B | 2.88B | 2.85B | 0.269 | 0.0001 | 0.8501 | 0.9133 |
| Protozoa (105 ml−1) | 0.61A | 0.53B | 0.51B | 0.03C | 0.01C | 0.01C | 0.008 | 0.0001 | 0.0001 | 0.0045 |
| Microbial protein (mg/ml) | 0.71CD | 0.76BCD | 0.84BC | 0.87BC | 0.93AB | 0.99A | 0.024 | 0.0001 | 0.0007 | 0.9145 |
Means with different superscripts within the same row differ at P<0.01
Means with different superscripts within the same row differ at P<0.01
Means with different superscripts within the same row differ at P<0.01
Means with different superscripts within the same row differ at P<0.01
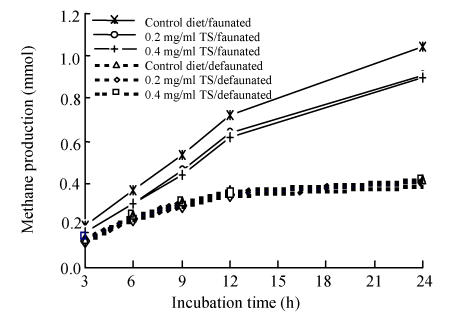
Methane production was reduced by the TS inclusion in the faunated rumen fluid and by defaunation (Fig.2). Inclusion of 0.2 and 0.4 mg/ml TS decreased the methane emission by 12.7% and 14.0% respectively in the faunated rumen fluid. Defaunation was a much more efficient way to inhibit the methane production. After 24 h incubation, methane production was 57.4% reduced on average by defaunation (Table 1).
Fig. 2.
Effect of tea saponin (TS) additon and protozoa status on methane production
Influence of TS and protozoa status on rumen fermentation characteristics
Rumen fermentation characteristics influenced by TS addition and defaunation at 24 h are summarized in Table 1. The mean rumen fluid pH in all treatments was 6.81, and the effects of TS and defaunation were minor. Total VFAs were significantly reduced by defaunation (P<0.05), but were not affected by the TS inclusion in faunated or defaunated rumen fluid (P>0.05). Molar proportion of acetate was similar among all treatments. Compared to the control, inclusion of TS showed no effect on the molar proportion of proprinate, but propionate was significantly higher in defaunated rumen fluid than in the faunated fluid. Thus, the A/P (acetate/propionate) was significantly decreased in defaunated rumen fluid. Molar proportion of butyrate was not influenced by TS, but was siginificantly reduced by the defaunation.
Ammonia-N concentration was siginificantly reduced by TS addition in both faunated and defaunated rumen fluid, and defaunation significantly reduced the ammonia-N concentration compared to the control faunated rumen fluid. Inclusion of 0.2 or 0.4 mg/ml TS reduced protozoa number by 13.1% or 16.4% in the faunated rumen fluid. Defaunation reduced the protozoa number by 97.0% on average. The MCP synthesis was significantly increased by TS addition in faunated and defaunated rumen fluid. Defaunation significantly increased MCP synthesis compared to the control faunated rumen fluid.
DISCUSSION
TS treatment
Saponins extracted from many plants have been reported to have effects on rumen protozoa counts, ammonia-N, MCP yield and fermentation patterns (Wang et al., 1997; Hess et al., 2003; Hristov et al., 1999). In this study, inclusion of TS significantly reduced protozoa counts (Table 1). One possible mechanism explaining the effect of saponins on protozoa is the changes in cell membrane permeability (Klita et al., 1996). Of all rumen microbes, protozoa are particularly susceptible to saponin-induced changes in cell membrane properties (Moss et al., 2000).
Methane produced by enteric fermentation in ruminants leads to a severe loss of feed energy for the animals, and ecological problems through greenhouse gas emissions. Therefore, reducing methane production has significant economical and environmental benefits. Inclusion of 0.2~0.4 mg/ml TS reduced the 24 h methane emission by 12.7%~14.0% (Table 1), which was similar to the results reported by Wang et al.(2000) who observed that the methane production was 15% lower in Yucca saponin-added group compared with the control. The methane suppressing-effects of saponins were presumably a direct action against the rumen microbes involved in methane formation (including methanogens and protozoa). Apart from methanogens, rumen protozoa were believed to be of importance in methane formation because of the association of protozoa with ecto- and endosymbiotic methanogen (Finlay et al., 1994). Inclusion of TS significantly reduced methane production in faunated rumen fluid, but not in the defaunated rumen fluid, suggesting that inhibition of methanogenesis by tea saponins was primarily due to their anti-protozoal activity.
Ammonia-N concentration was decreased and MCP yield was increased when TS were added (Table 1). Addition of 0.4 mg/ml TS led to the lowest ammonia-N concentration and highest MCP yield. The VFA are considered as one of the rumen fermentation parameters, and they represent the rumen fermentation pattern. The proportions of individual VFA were not significantly changed with addition of TS, indicating that TS had little effect on rumen fermentation pattern, just as Hess et al.(2003) and Wang et al.(1998) reported. The 24 h GP declined when TS was included, and this reduction might have been due to the TS-mediated inhibition of cellulolytic bacterial and fungal growth (Wang et al., 2000).
Defaunation treatment
Effects of defaunation on rumen fermentation had been intensively investigated and reviewed, including its effect on methanogenesis (Jouany, 1996; Moss et al., 2000). Jouany (1996) reported that ciliate protozoa contributed significantly to intraruminal cycling of microbial N and efficiency of MCP synthesis, so reducing protozoa counts can improve dietary N utilization and increase MCP flow to the intestine. We found that ammonia-N concentration decreased and MCP yield increased in defaunated rumen fluid (Table 1). Most of defaunation-induced changes found in the present study accorded with those of other studies (Jouany 1996; Moss et al., 2000). Total concentrations of VFA were reduced by elimination of protozoa. An increased molar proportion of propionate and a lowered proportion of butyrate accompanied the VFA reduction. In the defaunated rumen fluid, molar proportion of propionate was increased at the apparent expense of butyrate.
Methane emission was greatly reduced by defaunation (0.4217 mmol vs 1.0454 mmol at 24 h in defaunated and faunated rumen fluid, respectively), as was found in some other studies (Whitelaw et al., 1984; Dohme et al., 1999). Formation of acetate and butyrate are usually accompanied by production of hydrogen and carbon dioxide, whereas propionate formation involves a net uptake of hydrogen, thus, defaunation decreases the hydrogen supply for methanogens in the rumen, leading to lower methane emission.
Interaction between defaunation and TS treatment
Interactions between TS and protozoa status were mostly weak except for the methane emission and protozoa number. The lack of significant interactions for most variables investigated suggests that the effects of TS were not predominantly mediated by the simultaneously observed inhibition of the rumen protozoa. However, since the depression of methane release due to tea saponins addition was found only in faunated rumen fluid, it could be assumed that this effect was mediated through associated effects on protozoa. Further research is necessary to confirm the effects of TS on methanogens and other microbials.
CONCLUSION
Saponins from tea seeds have anti-protozoa effect, and have the potential to modulate the rumen fermentation pattern and reduce methane release from ruminal fermentation. Since the depression of methane release due to tea saponins addition was found only in faunated rumen fluid, it could be assumed that this effect was mediated through associated effects on protozoa. Combinations of TS addition and defaunation can be promising strategies for suppressing methane emissions.
Footnotes
Project (No. 12665/R0) supported partly by Co-ordinated Research Projects from Joint FAO/IAEA Division, IAEA
References
- 1.Dohme F, Machmuller A, Estermann BL, Pfister P, Wasserfallen A, Kreuzer M. The role of the rumen ciliate protozoa for methane suppression caused by coconut oil. Letters of Applied Microbialogy. 1999;29:187–192. [Google Scholar]
- 2.Feng ZC, Gao M. The determination of ammonia nitrogen concentration in the rumen fluid by a colorimetric method. Inner Mongolian J of Anim Sci and Produc. 1993;4:40–41. (in Chinese) [Google Scholar]
- 3.Finlay BJ, Esteban G, Clarke KJ, Williams AG, Embley TM, Hirt RP. Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiology Letters. 1994;117:157–162. doi: 10.1111/j.1574-6968.1994.tb06758.x. [DOI] [PubMed] [Google Scholar]
- 4.Hess HD, Kreuzer M, Diaz TE, Lascano CE, Carulla JE, Soliva CR, Machmuller A. Saponin rich tropical fruits affect fermentation and methanogensesis in faunated and defaunated rumen fluid. Animal Feed Science and Technology. 2003;109:79–94. [Google Scholar]
- 5.Hristov NA, McAllister TA, van Herk FH, Cheng KJ, Newbold CJ, Cheeke PR. Effect of Yucca schidigera on ruminal fermentation and nutrient digestion in heifers. Journal of Animal Science. 1999;77:2554–2563. doi: 10.2527/1999.7792554x. [DOI] [PubMed] [Google Scholar]
- 6.IPCC (Intergoverment Panel on Climate Change) Climate Change 2001. The Scientific Basis. Cambridge, UK: Cambridge Univeisity Press; 2001. [Google Scholar]
- 7.Johnson KA, Johnson DE. Methane emissions from cattle. J Anim Sci. 1995;73:2483–2492. doi: 10.2527/1995.7382483x. [DOI] [PubMed] [Google Scholar]
- 8.Jouany JP. Effect of rumen protozoa on nitrogen utilization by ruminants. Journal of Nutrition. 1996;126:1335–1346. doi: 10.1093/jn/126.suppl_4.1335S. [DOI] [PubMed] [Google Scholar]
- 9.Klita PT, Mathison GW, Fenton TW, Hardin RT. Effects of alfalfa root saponins on digestive function in sheep. Journal of Animal Science. 1996;74:1144–1156. doi: 10.2527/1996.7451144x. [DOI] [PubMed] [Google Scholar]
- 10.Liu JX, Yuan WZ, Ye JA, Wu YM. Effect of tea (Camellia sineis) saponin addition on rumen fermentation in vitro. Tropical Subtropical Agroecosystem. 2003;3:561–564. [Google Scholar]
- 11.Makkar HPS, Becker K. Degradation of Quillaja saponins by mixed culture of rumen microbes. Letters of Applied Microbialogy. 1997;25:243–245. doi: 10.1046/j.1472-765x.1997.00207.x. [DOI] [PubMed] [Google Scholar]
- 12.Makkar HPS, Becker K. Purine quantification in digesta from ruminants by spectrophotometric and HPLC methods. British Journal of Nutrition. 1999;81:107–113. [PubMed] [Google Scholar]
- 13.Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Animal Feed Science and Technology. 1999;79:321–330. [Google Scholar]
- 14.Menke KH, Steingass H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Research Development. 1988;28:7–55. [Google Scholar]
- 15.Moss AR, Jouany JP, Newbold J. Methane production by ruminants: Its contribution to global warming. Annal Zootechnology. 2000;49:231–253. [Google Scholar]
- 16.Ogimoto K, Imai S. Atlas of Rumen Microbiology. Tokyo, Japan: Japan Scientific Society Press; 1981. p. 311. [Google Scholar]
- 17.SAS (Statistical Analysis System) Base SAS Software Reference Card. Version 6.12. USA: Cary, N.C., SAS Institute Inc; 1997. pp. 211–253. [Google Scholar]
- 18.Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Animal Feed Science and Technology. 1994;48:185–197. [Google Scholar]
- 19.Wang YX, McAllister TA, Newbold CJ, et al. Effects of Yucca Extract on Fermentation and Degradation of Saponins in the Rusitec; Proceedings of Western Section, American Society of Animal Science; Nashville, Tennessee, USA. 1997. pp. 149–152. [Google Scholar]
- 20.Wang YX, McAllister TA, Newbold CJ, Rode LM, Cheeke PR, Cheng KJ. Effect of Yucca schidigera extract on fermentation and degradation of steroidal saponins in the rumen simulation technique (RUSITEC) Animal Feed Science and Technology. 1998;74:143–153. [Google Scholar]
- 21.Wang YX, McAllister TA, Yanke LJ, Xu ZJ, Cheeke PR, Cheng KJ. In vitro effects of steroidal saponins from Yucca Schidigere extract on rumen microbial protein synthesis and ruminal fermentation. Journal of Science and Food Agriculture. 2000;80:2114–2122. [Google Scholar]
- 22.Whitelaw FG, Eadie JM, Bruce LA, Shand WJ. Methane formation in faunated and ciliate-free cattle and its relationship with rumen volatile fatty acid proportions. British Journal of Nutrition. 1984;52:261–275. doi: 10.1079/bjn19840094. [DOI] [PubMed] [Google Scholar]
- 23.Zinn RA, Owens FN. A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Canadian Journal of Animal Science. 1986;66:157–166. [Google Scholar]