Abstract
MGTA1, a putative fungal Zn(II)2Cys6 transcriptional activator-encoding gene, was isolated from rice blast pathogen Magnaporthe grisea, which is homologous to CLTA1 from Colletotrichum lindemuthianum with 51% identity at protein level. MGTA1 cassette contains a 2370 bp open reading frame, consisting of 6 exons, coding a 790 amino acid peptide. MGTA1 gene exists as a single copy in genomes of 7 strains of M. grisea, and is expressed in tip hyphae, conidia, and mature appressoria of strain Guy11.
Keywords: Magnaporthe grisea, MGTA1 gene, Zn(II)2Cys6
INTRODUCTION
Fungi cause most plant diseases of all groups of microbes. During their infection cycles, fungal pathogens must undergo two key processes: first, penetration through cuticles into host plant cells; second, colonization in host cells utilizing nutrients from their hosts. To penetrate host cells, fungi develop a series of specialized infection structures such as appressorium, penetration peg, and infection hypha. The appressorium-mediated penetration is a process typical of some filamentous fungal pathogens, e.g., Magnaporthe grisea (Talbot, 1995), Colletotrichum lindemuthianum (Bailey and Jeger, 1992; O’Connell et al., 1985), and Erysiphe graminis (Kunoh et al., 1979). During colonization in plant hosts, fungal pathogens exhibit two main modes to obtain nutrients: biotrophy, where nutrients are obtained from living host cells, e.g., E. graminis (Aist and Bushnell, 1991); and necrotrophy, where nutrients are obtained from dead host cells killed by the fungi.
C. lindemuthianum has an intermediary nutrition strategy called hemibiotrophy. After penetrating its host cuticle and epidermal cell wall, it initially grows as a biotroph with primary intracellular hyphae for one or a few days. Subsequently, secondary narrow hyphae are formed, killing its host cells and proliferating by necrotrophic growth (Perfect et al., 1999). CLTA1 gene, a putative fungal Zn(II)2Cys6 family transcriptional activator is involved in the switch between these two phases. A null mutant of CLTA1 gene stops at biotrophic phase and loses its pathogenicity on common bean. In infected cells, mutants can form primary intracellular hyphae but cannot differentiate infectious secondary hyphae (Dufresne et al., 2000).
In M. grisea, different from C. lindemuthianum, no distinct switch between biotrophic and necrotrophic phases in the post-penetration processes was found, although M. grisea does not induce visible necrotic damages on the infected rice leaves until 4~5 d after infection. Firstly, the penetration peg of M. grisea differentiates into bulbous primary infection hyphae in the plant epidermal cells, and then primary hyphae differentiate narrow secondary hyphae and spread in the leaf (Heath et al., 1990a; 1990b). The mechanism involved in colonization of M. grisea in rice leaf leaves much to be understood.
In view of the similarity in penetration of C. lindemuthianum as M. grisea and the importance of CLTA1 in the colonization of C. lindemuthianum, CLTA1 homologous genes in M. grisea probably play an important role in the colonization of M. grisea and account for the differences of nutrition between these two fungi. A homologous gene (MGTA1) to CLTA1 with 51% identity to CLTA1 at the protein level in M. grisea was identified in this study.
MATERIALS AND METHODS
Strains, media, and growth of M. grisea
M. grisea strains Guy11, Y91-11, Y90-1, 84-7-3, 2000-034E3, 2001-068F1 and 2001-060G1 were used in this study. The fungi were grown routinely on complete medium (Talbot et al., 1993). Long-term storage of M. grisea was carried out by growing the fungus through sterile filter paper discs, desiccating for 48 h and storing them at −20 °C. Mycelia collected from 2-day-old complete medium cultures shaken at 150 r/min at 27 °C were used for the isolation of fungal genomic DNA.
Nucleic acid manipulation and analysis
Genomic DNA was extracted from fungal mycelium using a CTAB (hexadecyltrimethylammonium bromide) procedure described by Talbot et al.(1993). Routine PCR, gel electrophoresis, restriction enzyme digestion and cloning in Escherichia coli were carried out using standard procedures (Sambrook et al., 1989). Elongase Amplification System (Invitrogen, USA) was used to amplify relatively long DNA molecules (>5 kb) form genomic DNA, and the PCR products were cloned into PCR-XL-TOPO vector (Invitrogen, USA). DIG high prime DNA labelling and detection starter kit I (Roche, Germany) was used in the DNA gel blot hybridization.
DNA Sequencing and Sequence Analysis
The DNA clones and cDNA clones were sequenced using AB3730 autosequencer (ABI, USA). BLAST program (Altschul et al., 1997) was used to search for homologues against GenBank database (http://www.ncbi.nlm.nih.gov/blast/) and fungal genome database (Broad Institute, http://www.broad.mit.edu/annotation/). Multiple sequence alignments were determined using CLUSTAL V software (Higgins et al., 1992).
Construction of MGTA1(p)::eGFP::HPH fusion vector
pEGFP (Clontech, USA) was digested with PvuII and HpaI to remove lacZ promoter, and self-ligated to generate pEGFP11. A 1.5 kb promoter fragment of MGTA1 was amplified using the primer MGTA150 (5′-GCATTCCTTGGGCCCCGCATAAC-3′) and MGTA130n (5′-GTGCCATGGTGGTCGAAGTGCTGAAGCCAC-3′), and cloned into easy vector pGEM-T (Promega, USA) to generate pMP4. Then this promoter fragment was cut with NcoI and cloned into pEGFP11. A plasmid with MGTA1 promoter and eGFP gene in the same orientation was selected and named pLM7. A 2.3 kb ApaI MGTA1(p)::eGFP fragment from pLM7 was cloned into the ApaI site of pCB1004 (Caroll et al., 1994) to generate two plasmids with different insertion orientation: pELM3 and pELM6. A 1.4 kb KpnI-BamHI fragment covering HPH gene under control of trpC was cut from pCSN43 (Staben et al., 1989) and cloned into pLM7 to generate pLMH. The MGTA1(p)::eGFP::HPH vectors pELM3, pELM6 and pLMH were verified by restriction digestion and used to transform protoplast after linearization.
Fungal transformations
Protoplast preparation and transformation were done as described previously (Talbot et al., 1993). DNA was transformed into the M. grisea strain Guy11 and transformants selected for hygromycin resistance in complete media with 200 μg/ml hygromycin.
Examination of eGFP fluorescence
Fluorescence of MGTA1(p)::eGFP::HPH fusion transformed strains were detected using an excitation wavelength of 488 nm and an emission between 500 and 530 nm. Transformants were viewed using an Olympus-BX51 microscope with UV epifluorescence and appropriate filters.
RESULTS
Isolation of MGTA1 gene
The 746 amino acid sequence of CLTA1 was used to search for homologous genes against M. grisea genome database (Broad Institute, http://www.broad.mit.edu/annotation/fungi/magnaporthe/) with tblastN and blastP program (Altschul et al., 1997). Seven genome contigs and 14 hypothetical genes with E-values less than 8e-04 were found in the M. grisea database (Table 1 and Table 2).
Table 1.
Result of tblastN with Clta1p against M. grisea genome database
| Contig noes | Score (bits) | E-value |
| M. grisea contig 2.2025 | 223 | 4e-65 |
| M. grisea contig 2.275 | 71 | 4e-12 |
| M. grisea contig 2.1096 | 68 | 4e-11 |
| M. grisea contig 2.274 | 51 | 3e-06 |
| M. grisea contig 2.1386 | 48 | 4e-05 |
| M. grisea contig 2.474 | 46 | 1e-04 |
| M. grisea contig 2.488 | 44 | 7e-04 |
Table 2.
Result of blastP with Clta1p against M. grisea genome database
| Hypothetical gene | Score (bits) | E-value |
| MG10529.4 | 540 | e-154 |
| MG10528.4 | 80 | 3e-15 |
| MG01486.4 | 71 | 1e-12 |
| MG05829.4 | 68 | 1e-11 |
| MG10212.4 | 55 | 9e-08 |
| MG03848.4 | 54 | 2e-07 |
| MG07450.4 | 51 | 1e-06 |
| MG09443.4 | 47 | 1e-05 |
| MG08418.4 | 47 | 1e-05 |
| MG02377.4 | 46 | 3e-05 |
| MG09118.4 | 45 | 6e-05 |
| MG02408.4 | 44 | 2e-04 |
| MG06086.4 | 42 | 6e-04 |
| MG00318.4 | 42 | 8e-04 |
Among those hypothetical genes, the gene most identical (Score=540, E-value=e-154) to CLTA1 was MG10529.4 (GenBank accession No. EAA46835.1) located in M. grisea contig 2.2025 (Broad Institute). So, the hypothetical gene MG10529.4, namely MGTA1 in this paper, was the potential homologous gene to CLTA1.
A 5.2 kb DNA fragment covering MGTA1 gene full length was amplified from genomic DNA of M. grisea strain Guy11 using forward primer MGTA150 and reverse primer MGTA130 (5′-GCTGCGTGGGATGGGGGTGTG-3′) (Fig.1a). The fragment was cloned into PCR-XL-TOPO vector (Invitrogen, USA). Three independent clones (pTL12, pTL20, pTL22) were selected and verified exactly as recombination clones by restriction digestion (Fig.1b).
Fig. 1.
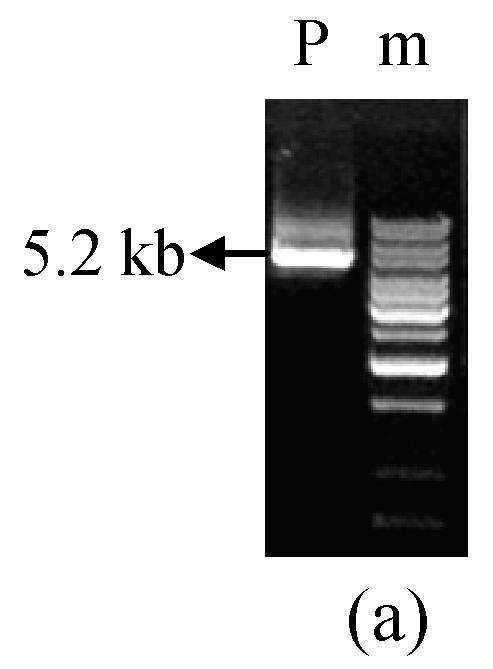
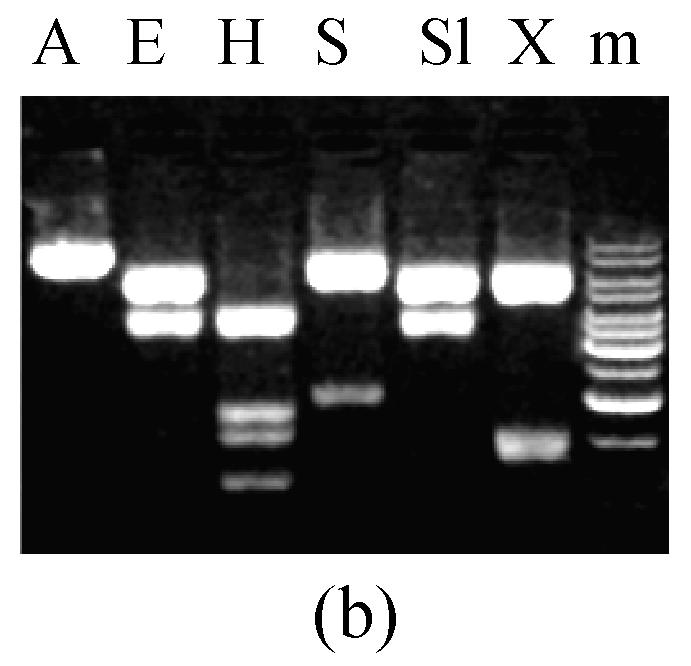
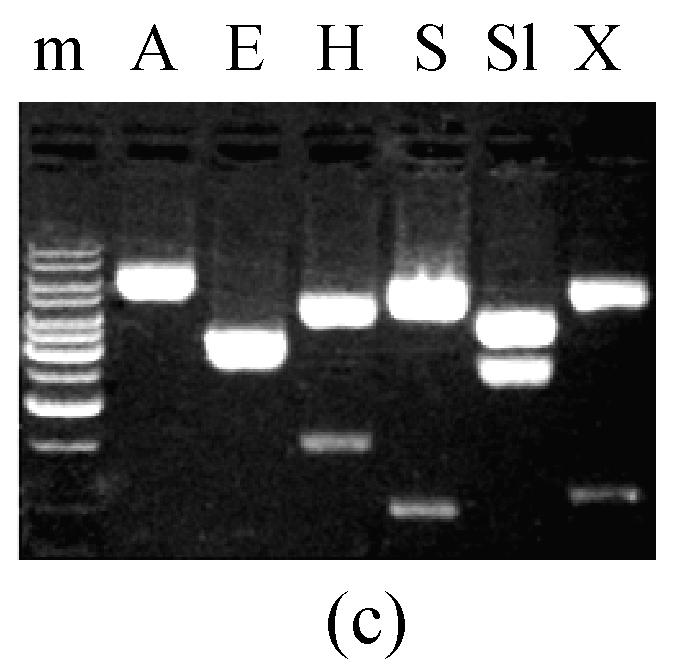
5.2 kb PCR product and digestion of pTL20 and pW2FW2R4 (a) 5.2 kb fragment containing MGTA1 gene amplified from M. grisea strain Guy11 genomic DNA; (b) pTL20 digestion with restriction enzymes; (c) pW2FW2R4 digestion with restriction enzymes. P, 5.2 kb PCR product; m, 1 kb DNA ladder; A, ApaI; E, EcoRI; H, HindIII; S, SacI; Sl, SalI; X, XhoI
A 3.0 kb MGTA1 cDNA fragment was amplified from a cDNA library constructed with RNA extracted from 24 h-old appressoria of M. grisea strain Guy11 (Lu et al., 2005) using forward primer Tl20.w2f (5′-AAACCATAATAGCCGCAAGG-3′) and reverse primer Tl20.w2r (5′-GTCATTCCCAAGATCTAGTC-3′). The fragment was cloned into easy vector pGEM-T (Promega, USA). Three independent clones (pW2FW2R4, pW2FW2R7, pW2FW2R8) were selected and verified as recombination clones by restriction digestion (Fig.1c).
Sequencing analysis of MGTA1 gene
Three DNA clones (pTL12, pTL20 and pTL22) and 3 cDNA clones (pW2FW2R4, pW2FW2R7 and pW2FW2R8) were sequenced on both strands. The genomic DNA sequence, cDNA sequence and deduced protein sequence of MGTA1 were submitted to GenBank (accession No. AY786158). Six nt differing was identified among 5.2 kb DNA sequence between our result (strain Guy11) and M. grisea database (strain 70-15, Broad Institute).
Analysis of these 3 cDNA sequences revealed an open reading frame of 2370 bp, coding a 790 amino acid peptide (GenBank accession No. AY786158). Compared with MGTA1 cDNA sequence, MGTA1 genomic DNA cassette contains 6 introns and 6 exons. Of these 6 introns, a 100 bp long 5′-intron locates at the 405 bp upstream of the MGTA1 initiation codon. MG10529.4 not having exactly the same location as that of the second and the third intron of MGTA1 leads to a difference in a stretch of 28-amino acids between the two proteins (Fig.2). Since intron locations of MGTA1 were determined experimentally using a cDNA sequence, the automatic annotation of these two introns in MG10529.4 was erroneously estimated.
Fig. 2.
28-amino acid divergence between deduced protein sequences of MG10529.4 and MGTA1
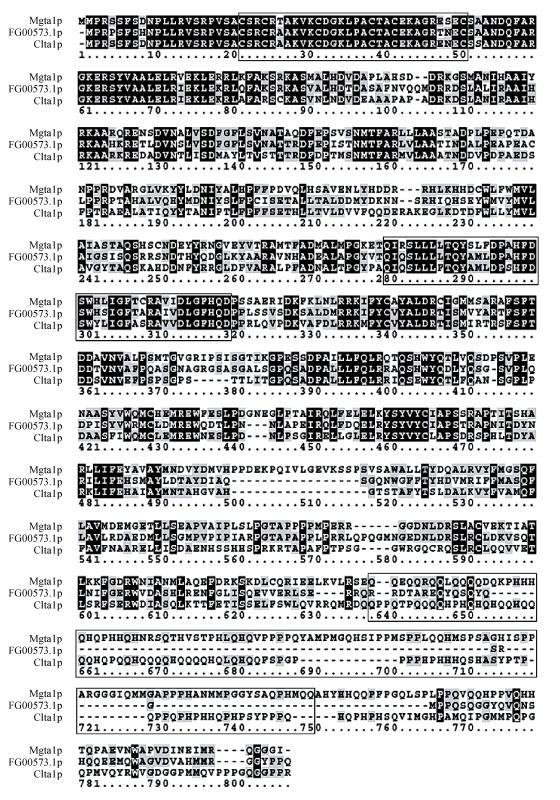
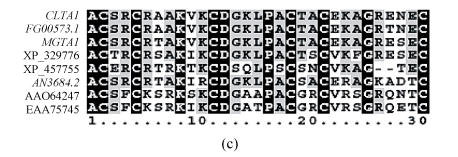
The 790 amino acid polypeptide of MGTA1 gene has 51% identity to CLTA1 gene (E-value=e-173). Three conserved functional domains, characteristic of transcription factors from the zinc binuclear cluster family (Zn(II)2Cys6 DNA binding domain, middle homology region and activation domain), appear at the regions from aa 22 to 51, 276 to 315 and 628 to 738 respectively in Mgta1p (Fig.3). These 3 domains are identical to the homologous domains of Clta1p with 93%, 75%, 22% identity respectively. The features of Mgta1p sequence indicate that this protein is a potential transcriptional activator from the fungal zinc-binuclear cluster family.
Fig. 3.
Sequence alignment of Mgta1p*, FG00573.1p and Clta1p. The 3 conserved functional domains were indicated in boxes
*The sequence of Mgta1p deduced from cDNA sequence, not the MG10529.4p automatic estimated from M. grisea genome database (Broad Institute)
Copies of MGTA1 gene in 7 M. grisea strains
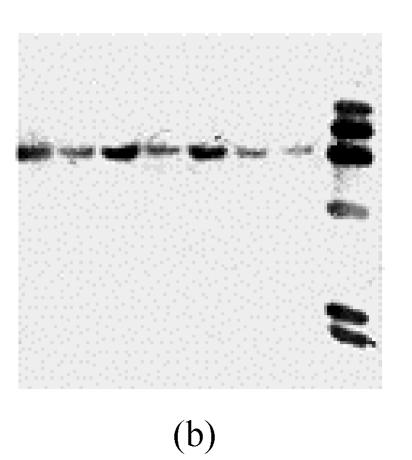
In M. grisea genome database (strain 70-15, Broad Institute), only one copy of MGTA1 was found. The number of copies of MGTA1 in 7 M. grisea strains isolated from 5 host plants in different geographic locations (Y91-11, Y90-1, 84-7-3, 2000-034E3, 2001-068F1, 2001-060G1 and Guy11) was assessed by Southern hybridization. Firstly, genomic DNA from strain Guy11 was digested with six restriction enzymes (ApaI, EcoRI, HindIII, SacI, SalI, and XhoI), separated by agarose gel electrophoresis, blotted on Nylon membrane and hybridized with a 1 kb probe of MGTA1 internal fragment. Only one band emerged in each lane of digested DNA (Fig.4a). Then, genomic DNA of other 6 M. grisea strains and strain Guy11 were digested by Sac I and blotted by the same probe as above. Only one band emerged in each lane of digested DNA for each strain (Fig.4b). These facts implied that only one copy of MGTA1 gene exists in each genome of these 7 strains.
Fig. 4.

Copies of MGTA1 gene in M. grisea strains (a) Southern blot of genomic DNA from M. grisea strain Guy11 strain with 1 kb fragment of MGTA1 gene. Genomic DNA was digested with different enzymes (left to right: ApaI, EcoRI, HindIII, SacI, SalI, XhoI); (b) Southern blot of genomic DNAs of 7 M. grisea strains by 1 kb fragment of MGTA1 gene (The strains and hosts (from left to right): Y91-11, summer grass; Y90-1, goose grass; 84-7-3, millet; 2000-034E3, rice; 2001-068F1, rice; 2001-060G1, rice; Guy11). Genomic DNA was digested with SacI
MGTA1 expression in conidia, mycelia and appressoria
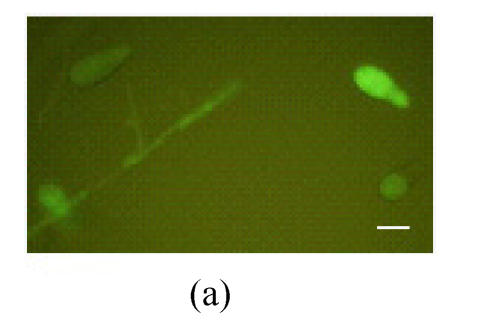
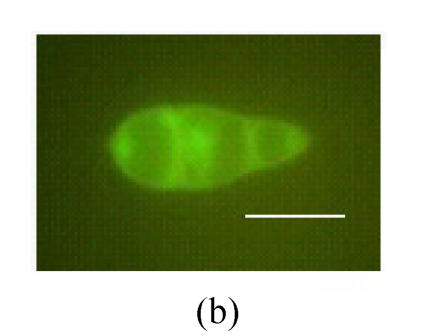
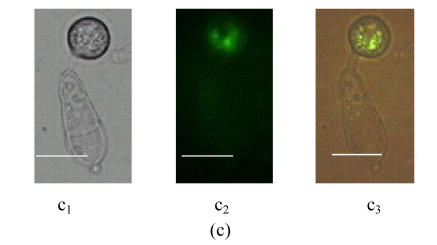
To investigate the expression pattern of MGTA1 gene, an MGTA1(p)::eGFP::HPH fusion vector was constructed and introduced into M. grisea strain Guy11. Eight transformants containing single insertion were selected from 30 hygromycin-resistant transformants by Southern analysis (data not showed). Mycelia and conidia at different stages of development of these 8 transformants were examined for eGFP fluorescence under control of MGTA1 promoter. Green eGFP fluorescence appeared faintly in hyphal tips from mycelia cultivated on complete media and increased lightly in conidia (Fig.5a and Fig.5b). The conidia of 8 transformants were induced to germinate and form appressoria on terylene membranes and sterile onion epidermis. During the germinating process, the eGFP fluorescence of conidia decreased rapidly. In germ tubes and young appressoria, no eGFP fluorescence was detected. While in mature appressoria, eGFP fluorescence was observed again (Fig.5c). On onion epidermis, infection hyphae were observed clearly in the infected cells after incubation of 28 h, but no eGFP fluorescence was detected.
Fig. 5.
Fluorescence in M. grisea MGTA1(p)::eGFP transformants (Bars=10 µm) (a) Fluorescence of conidia and tip hyphae; (b) Zoom of a conidium’s fluorescence; (c) Fluorescence of appressorium. c1, c2 and c3 show the image under bright-field illumination, UV light and bright-UV compound light, respectively
DISCUSSION
M. grisea is a well-known ascomycete that causes rice blast (Ou, 1985). As a model fungal pathogen, its pre-penetration processes, including conidium dissemination, adhesion (Hamer et al., 1988) and germination, appressorium differentiation (Howard et al., 1991) and maturation (Howard and Ferrari, 1989), penetration peg differentiation (Bourett and Howard, 1990; 1992), had been reported. Several chemical and physical factors activating these processes have been found and many genes related to these processes have been cloned and characterized (Balhadere and Talbot, 2001; Talbot et al., 1993; Xu and Hamer, 1996). The post-penetration processes of M. grisea, including infection hyphae differentiation and colonization, are far less known (Balhadere and Talbot, 2001). Our work focuses on genes involved in such processes. In this study, we isolated MGTA1, a novel gene homologous to CLTA1 involved in the post-penetration processes of C. lindemuthianum (Dufresne et al., 2000). Clarification of MGTA1 functions, especially in pathogenicity, is helpful for us to understand the infection process of M. grisea and the difference of nutrition in post-penetration processes between M. grisea and C. lindemuthianum. Work on MGTA1 gene replacement, mutant analysis, functional recovery, and cross-complement of MGTA1 and CLTA1 is being carried out now in our lab. The MGTA1 gene replacement vector was constructed successfully (data not shown).
MGTA1 gene expression in M. grisea strain Guy11 was demonstrated by MGTA1(p)::eGFP::HPH fusion expression experiment. Unlike the CLTA1 gene expressed in later phase of the C. lindemuthianum infection cycle (Dufresne et al., 2000), MGTA1 gene is expressed in several phases of the M. grisea infection cycle: tip hypha, conidium and mature appressorium. While in infectious hypha, eGFP fluorescence cannot be detected. It is possible that the fluorescence is too weak to be detected under the shades of host cell cuticles. The different expression patterns indicate that some potential differences probably exist between MGTA1 and CLTA1 gene functions.
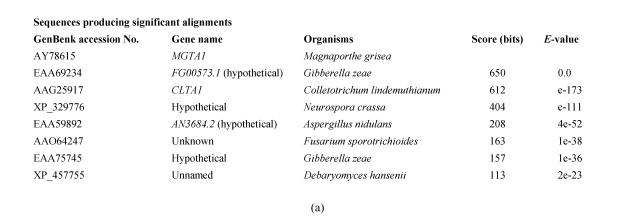
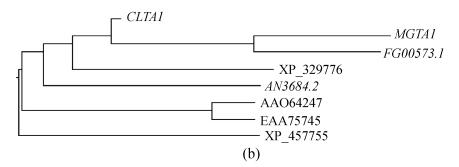
Several homologous genes of MGTA1 were found in some fungal genomes by searching against GenBank database with Mgta1p (Fig.6). In these homologues, a predicted protein FG00573.1 from Gibberella zeae had 54% identity to the protein Mgta1p and 56% identity to Clta1p (Fig.3). G. zeae does not form appressorium for plant penetration, and the CLTA1 null mutants of C. lindemuthianum can form appressoria normally (Dufresne et al., 2000), so MGTA1 is possibly involved in other processes. It will be interesting to determine the function of MGTA1, FG00573.1 and their homologous genes fungal pathogens.
Fig. 6.
Scores of 8 top hits of blast to GenBank using Mgta1p (a) GenBank accession No., gene name, organisms, and scores of 8 top hits; (b) Phylogeny of the 8 top hit protein sequences (marked with gene name or GenBank accession No.); (c) Sequence alignment of Zn(II)2Cys6 domains of the 8 top hits
MGTA1 encodes a putative transcription factor of the Zn(II)2Cys6 family. Zn(II)2Cys6 DNA binding proteins are unique fungal transcriptional activators involved in a wide range of processes, including primary and secondary metabolism, drug resistance, and meiotic development (Todd and Andrianopoulos, 1997). Fifty-four hypothetical proteins with Zn(II)2Cys6 domain were found in the M. grisea genome database (Broad Institute). Up to now, these genes have not been researched well yet.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30270869 and 30470064) and the National Hi-Tech Research and Development Program (863) of China (No. 2002AA245041)
References
- 1.Aist JR, Bushnell WR. Invasion of Plants by Powdery Mildew Fungi, and Cellular Mechanisms of Resistance. In: Cole GT, Hoch HC, editors. The Fungal Spore and Disease Initiation in Plants and Animals. New York: Plenum Press; 1991. pp. 321–345. [Google Scholar]
- 2.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey JA, Jeger MJ. Colletotrichum: Biology, Pathology and Control. Wallingford: CAB International; 1992. [Google Scholar]
- 4.Balhadere PV, Talbot NJ. PDE1 encodes a P-type ATPase involved in appressorium-mediated plant infection by the rice blast fungus Magnaporthe grisea . Plant Cell. 2001;13:1987–2004. doi: 10.1105/TPC.010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourett TM, Howard RJ. In vitro development of penetration structures in the rice blast fungus Magnaporthe grisea . Can J Bot. 1990;69:329–342. [Google Scholar]
- 6.Bourett TM, Howard RJ. Actin in penetration pegs of the fungal rice blast pathogen, Magnaporthe grisea . Protoplasma. 1992;168:20–26. [Google Scholar]
- 7.Caroll AM, Sweigard JA, Valent B. Improved vectors for selecting resistance to hygromycin. Fungal Genet Newslett. 1994;41:22. [Google Scholar]
- 8.Dufresne M, Perfect S, Pellier AL, Bailey JA, Langin T. A GAL4-like protein is involved in the switch between biotrophic and necrotrophic phases of the infection process of Colletotrichum lindemuthianum on common bean. Plant Cell. 2000;12:1579–1590. doi: 10.1105/tpc.12.9.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamer JE, Howard RJ, Chumley FG, Valent B. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science. 1988;239:288–290. doi: 10.1126/science.239.4837.288. [DOI] [PubMed] [Google Scholar]
- 10.Heath MC, Valent B, Howard RJ, Chumley FG. Correlations between cytologically detected plant-funga1 interactions and pathogenicity of Magnaporthe grisea toward weeping lovegrass. Phytopathology. 1990;80:1382–1386. [Google Scholar]
- 11.Heath MC, Valent B, Howard RJ, Chumley FG. Interactions of two strains of Magnaporthe grisea with rice, goosegrass and weeping lovegrass. Can J Bot. 1990;68:1627–1637. [Google Scholar]
- 12.Higgins DG, Bleasby AJ, Fuchs R. CLUSTAL V: Improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 13.Howard RJ, Ferrari MA. Role of melanin in appressorium function. Exp Mycol. 1989;13:403–418. [Google Scholar]
- 14.Howard RJ, Bourett TM, Ferrari MA. Infection by Magnaporthe Grisea: An in vitro Analysis. In: Mendgen K, Lesemann DE, editors. Electron Microscopy of Plant Pathogens. Berlin: Springer-Verlag; 1991. pp. 251–264. [Google Scholar]
- 15.Kunoh H, Aist JR, Israel HW. Primary germ tubes and host cells penetrations from appressoria of Erysiphe graminis hordei . Ann Phytopathol Soc Japan. 1979;45:326–332. [Google Scholar]
- 16.Lu JP, Liu TB, Yu XY, Lin FC. Representative appressorium stage cDNA library of Magnaporthe grisea . J Zhejiang Univ Sci. 2005;6(2):132–136. doi: 10.1631/jzus.2005.B0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connell RJ, Bailey JA, Richmond DV. Cytology and physiology of infection of Phaseolus vulgaris by Colletotrichum lindemuthianum . Physiol Plant Pathol. 1985;27:75–98. [Google Scholar]
- 18.Ou SH. Rice Diseases. Kew, Surrey: Commonwealth Mycological Institute, CABI; 1985. pp. 109–201. [Google Scholar]
- 19.Perfect SE, Hughes HB, O’Connell RJ, Green JR. Colletotrichum: A model genus for studies on pathology and fungal-plant interactions. Fungal Genetics and Biology. 1999;27:186–198. doi: 10.1006/fgbi.1999.1143. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. Use of a bacterial Hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newsl. 1989;36:79–81. [Google Scholar]
- 22.Talbot NJ. Having a blast: Exploring the pathogenicity of Magnaporthe grisea . Trends Microbiol. 1995;3:9–16. doi: 10.1016/s0966-842x(00)88862-9. [DOI] [PubMed] [Google Scholar]
- 23.Talbot NJ, Ebbole DJ, Hamer JE. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea . Plant Cell. 1993;5:1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todd RB, Andrianopoulos A. Evolution of a fungal regulatory gene family: The Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genetics and Biology. 1997;21:388–405. doi: 10.1006/fgbi.1997.0993. [DOI] [PubMed] [Google Scholar]
- 25.Xu JR, Hamer JE. MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea . Genes Dev. 1996;10:2696–2706. doi: 10.1101/gad.10.21.2696. [DOI] [PubMed] [Google Scholar]