Abstract
Total RNA was isolated from the hybridoma cell line (LC-1), which secretes anti-lung adenocarcinoma monoclonal antibody, and was transferred into cDNA. Based on the FR1 (framework region 1) and FR4 conserved regions of LC-1 gene, the variable regions of heavy chain (Vh) and light chain (Vl) were amplified, and the Vh and modified Vl were connected to single chain Fv (ScFv) by SOE-PCR (splice overlap extension PCR). The modified ScFv was fused with green fluorescent protein (GFP) and introduced into E. coli JM109. The fusion protein induced by IPTG (Isopropylthiogalactoside) was about 57000 on a 10% SDS-PAGE gel (10% Sds Polyacrylamide Gel Electrophoresis), and primarily manifested as inclusion bodies. The renatured protein purified by Ni-NTA Superflow resins showed ability to bind to antigen on SPC-A-1 lung adenocarcinoma. In addition, the induced host cells fluoresced bright green under 395 nm wavelength, which indicated that the expected protein with dual activity was expressed in the prokaryotic system. The ScFv with GFP tag used in this research can be applied as a new reagent to detect immunological dye, and provide a feasible way to detect adenocarcinoma in a clinical setting.
Keywords: ScFv (single chain variable fragment), GFP (green fluorescent protein) tag, Protein fusion, Purification
INTRODUCTION
Because of its low molecular weight and ability to fluoresce independently (George, 1997), the new molecular tag, green fluorescent protein (GFP), has become more and more popular after Prasher et al.(1992) cloned its cDNA in 1992. There are many reports describing the co-expression of GFP and a specific antibody or cytokine gene, with the fusion protein expressing the fluorescent activity and biological activity of the complement protein (Haraguchi et al., 1999; Mclean et al., 1999; Walker et al., 1999; Otsuki et al., 1999; Zhu et al., 1999). This fusion protein can be used for tumor assessment, drug screening, and localization of cell receptors. Cheng et al.(2001) achieved the co-expression of GFP and anti-liver cancer ScFv. But until now, there is no report describing co-expression of GFP and anti-lung adenocarcinoma ScFv. In the study described here, LC-1 ScFv was constructed and linked with GFP for co-expression. Theoretically, the expected protein should possess dual activity so that it can be used as a new reagent to detect immunological dye and provide a feasible way to detect adenocarcinoma in clinical setting.
MATERIALS AND METHODS
Materials
Total RNA of the LC-1 hybridoma and the pProEx HTb vector were kindly provided by Dr. Chen Liang of Yale University. The pAVA319 vector was provided by Dr. Arnim. The pUCm-T vector, E. coli JM109, DH5α, and lung adenocarcinoma cell line are available in our institute.
The heavy chain primers were Vh-1 (AGGTCCAACTGCAGGAGTCAGG Pst I) and Vh-2 (TGAGGAGACGGTGACCGTGGTCCCTTGGCCCAG BstE II); the light chain primers were Vl-1 (GACATTGAGCTCACCCAGTCTCCA Sac I) and Vl-2 (GTTTGATCTCGAGCTTGGTCCC Xho I); the linker for Vh and Vl was link-1 (CTGGGGCCAA GGGACCACGGTCACCGTCTCCTCAGGTGGAGGCGGTTCAGGCGGAGGTGCTCTGGCGGTGGCGGATCGGACATTGAGCTCACCCAGTCTC); and the upstream primer for modifying ScFv was ScFv-1 (GAGCTAGATCTAAGGTCCAACTGCAG Bgl II).
Construction of ScFv
Vh and Vl were amplified from the cDNA of the LC-1 hybridoma using RT-PCR; we used primers for Vh-1, Vh-2, and Vl-1, Vl-2, respectively. They were cloned into a pUCm-T vector, respectively. The Vl was modified to be Vl′, primered with link-1 and Vl-2, and linked with Vh through SOE-PCR. Primered with ScFv-1 and Vl-2, the Bgl II and Xho I sites were added to the resultant ScFv′, which also was cloned into the pUCm-T vector. The recombinant plasmid, called T-ScFv,-was transformed into competent E. coli DH5α using the previously described CaCl2 method (Joseph and David, 2001) and sequenced.
Construction of Pro319
The GFP was cloned into pProEx HTb by digesting the pAVA319 with Nco I and Xba I. The resultant Pro319 was transformed into JM109.
Construction of ProGFP-ScFv
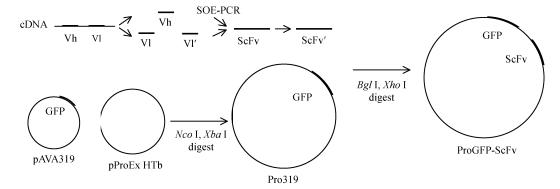
The ScFv was cloned into Pro319 by digesting T-ScFv with Bgl II and Xho I. The recombinant plasmid, called ProGFP-ScFv, was transformed into JM109 (Fig.1).
Fig. 1.
Construction of ProGFP-ScFv plasmid
Expression of GFP-ScFv fusion protein
A positive cell clone was inoculated onto 500 ml LB (50 µg/ml Amp) including HEPES buffer (pH 6.0~6.5) and incubated until OD 60=1.0 (Shi and Su, 2000). LB without HEPES buffer served as the negative control. The cells that were induced in the presence of IPTG gradient concentrations (0.1, 0.5, and 1 mmol/L) at 24 °C for 5 h were collected. Expressed fusion protein was analyzed on a 10% SDS-PAGE gel.
Purification of the fusion protein
Sedimentation was achieved by disrupting the induced cells using sonication (500 W, 2 min×5), and washing with wash buffer I (1 mmol/L EDTA, 5% glycerol, 0.5% Triton X-100) and buffer II (wash buffer I with 2 mol/L carbamide). Then the sedimented material was dissolved in lysis buffer (50 mmol/L Tris-HCl, 0.3% Sodium lauryl sulfate, 0.1 mmol/L Dithiothreitol) overnight. The fusion protein was renatured in lysis buffer without SLS. Finally, the renatured protein was purified by Ni-NTA Superflow resins; elution was carried out in the presence of an imidazole gradient concentration (10~150 mmol/L) and was collected per millitre, and A 280 was measured. The eluted protein was analyzed on a 10% SDS-PAGE gel.
Competitive ELISA assay of fusion protein
The SPC-A-1 cells were coated onto each well of a 96-well plate at a density of 5×105 cells/well. The 0.01 µg/µl LC-1 antibody and the purified fusion protein of the gradient concentrations (original liquid was about 0.1 µg/µl, 0.01 µg/µl, or 0.001 µg/µl) were used to bind cells; goat anti-mouse labelled anti-IgM secondary antibody was used to bind to the LC-1 antibody. We read signal at A 490 using an automatic reader. At the same time, the LC-1 antibody (0.01 µg/µl, 0.001 µg/µl, or 0.000 1 µg/µl) and PBS served as the positive control and negative control, respectively.
Assay of fluorescent activity
The fluorescent activity of induced cells was assayed at 395 nm by fluorescent microscope (640X); cells that had not been induced served as the negative control.
Prediction of tertiary structure
The ScFv sequence was submitted to SWISS-MODEL (http://swissmodel.expasy.org/) (Schwede et al., 2003; Guex and Peitsch, 1997; Peitsch, 1995) by ExPASy; templates were obtained by searching the ExNRL-3D database. Using the reported structure of GFP, the tertiary structure of GFP-ScFV was constructed.
RESULTS
Construction of ScFv′
Following Orlandi et al.(1989) regarding FR1 and FR4 conserved regions, the primers Vh-1/Vh-2, and Vl-1/Vl-2 were designed. The final ScFv′ was about 700 bp, as expected.
Construction of ProGFP-ScFv
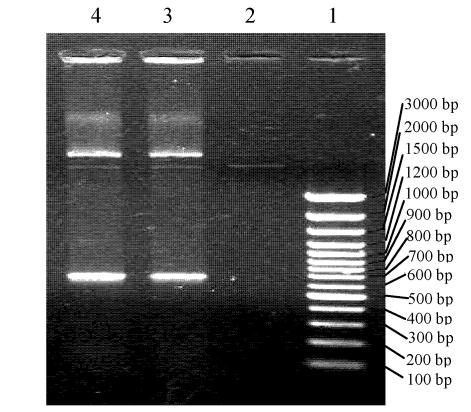
We obtained the expected bands (Fig.2) after digesting ProGFP-ScFv with Bgl II/Xho I and Nco I/Bgl II. The sequence of ProGFP-ScFv showed that the target plasmid had been constructed successfully, and that the ScFv and GFP were within the correct reading frame (Fig.3).
Fig. 2.
Digesting result of ProGFP-ScFv in 1% agrose gel. 1: Marker; 2: Nothing; 3:Digesting result of ProGFP-ScFv by Bgl II and Xho I, 4: Digesting result of ProGFP-ScFv by Nco I and Bgl II
Fig. 3.
Nucleotide and deduced amino acid sequence of GFP-ScFv
The bold words represents the hypervariable regions of ScFv
Expression and purification of GFP-ScFv
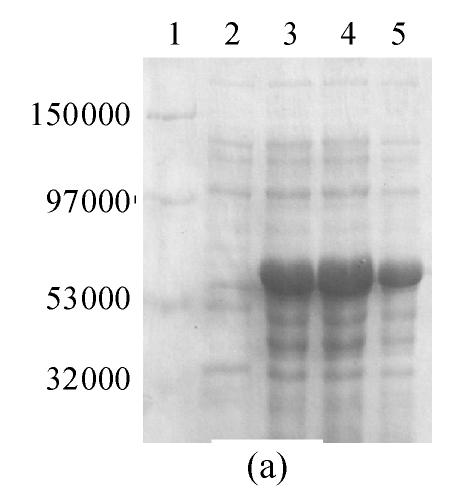
It was reported (Ogawa et al., 1995) that GFP is very likely to form chromophore at a low temperature. So, the expression was induced by IPTG at 24 °C. A 10% SDS-PAGE gel showed a band of about 57000, which was in accordance with the theoretical molecular weight of GFP-ScFv. In addition, the protein was primarily identified in inclusion bodies. When the expression was induced by 0.5 mmol/L IPTG, the fusion protein was composed of approximately 40% of total bacterial protein (Fig.4a).
Fig. 4.
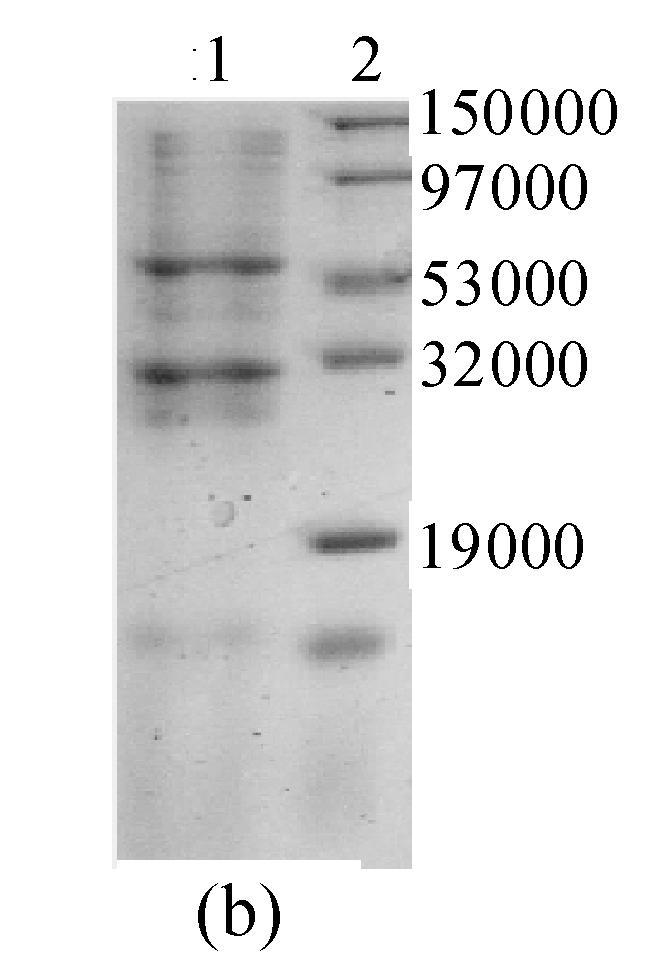
10% SDS-PAGE gel of GFP-ScFv fusion protein (a) Expression result of ProGFP-ScFv vector. 1: Marker; 2: Negative control; 3, 4, 5: Cell induced in different [IPTG] (0.1, 0.5, 1 mmol/L) (b) Purified GFP-ScFv in 10% SDS-PAGE gel. 1: Purified GFP-ScFv; 2: Marker
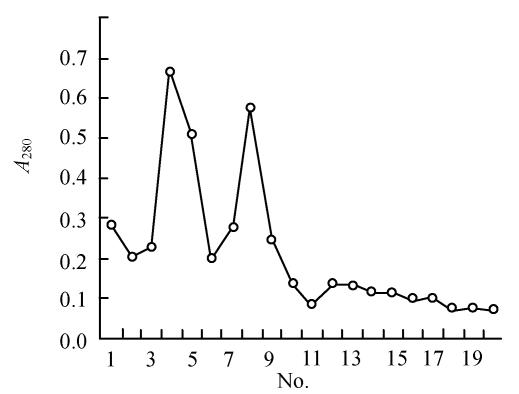
The Ni-NTA elution (Fig.5) showed two clear peaks. The 10% SDS-PAGE gel of these peaks showed that the fusion protein was comparatively pure (Fig.4b). In addition, the molecular weight of peak 2 was in accordance with what was anticipated for the protein (about 57000), while the molecular weight of peak 1 was close to that anticipated for GFP (about 28000). It is possible that the fusion protein had degraded automatically and released GFP.
Fig. 5.
The elution curve for protein purified by Ni-NTA
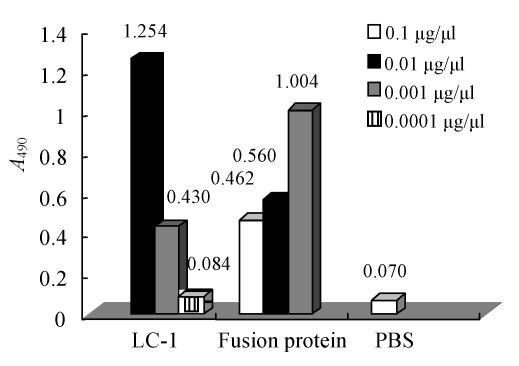
Competitive ELISA assay of the fusion protein
Fig.6 shows that the 0.01 µg/µl LC-1 antibody had relatively strong ability to bind to lung adenocarcinoma (A 490=1.254±0.02); so, this concentration of LC-1 was used in our competitive ELISA. According to the formula:
| IR(%)=(1−Asample/Apositive control)×100% |
, the IR of the fusion protein with the high concentration (0.1 µg/µl, A 490=0.462±0.01) was 63%. It indicated that numerous epitope sites on lung adenocarcinoma were bound to the fusion protein.
Fig. 6.
The ELISA curve of GFP-ScFv in A 490
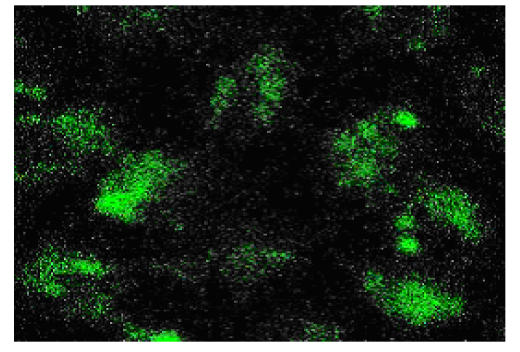
Assay of fluorescent activity
Though the secreted fusion protein was small, the cells induced by IPTG fluoresced bright green under 640X fluorescent microscope (Fig.7), while the negative control did not.
Fig. 7.
Host cells excited by 395 nm under fluorescent microscope (640X)
There was an interesting observation in our findings: when cells were induced in LB without HEPES for 5 h, the final pH of LB decreased to 5.1. In addition, the cells expressed much less protein (accounting for about 8% of total bacterial protein, primarily as inclusion bodies) and hardly fluoresced at 395 nm. This finding indicated the necessity for invariable pH in this expression system.
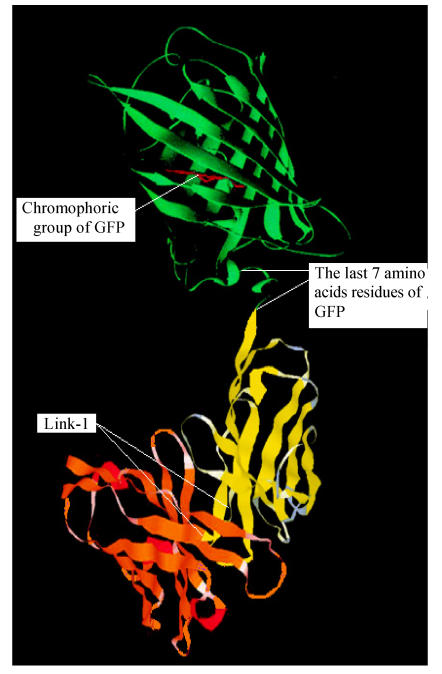
Prediction of tertiary structure
The tertiary structure of GFP-ScFv was determined as depicted in Fig.8.
Fig. 8.
Tertiary structure of GFP-ScFv
The GFP, the chromophoric group of GFP, the variable heavy domain and light domain is in green, red, yellow and orange. The figure was made by RasMol 2.6
CONCLUSION
Usually, the antibody gene and another gene with some biological activity are co-expressed, and the resultant molecule can be used for clinical evaluation and gene therapy (Myers et al., 2002; David et al., 2000; Mccall et al., 1999). In theory, the main benefits of our GFP-ScFv as opposed to conventional immunostaining methods are that this approach is rapid, purification is not essential, and it is relatively inexpensive. Also, the GFP tag is capable of eliminating background resulting from nonspecific binding of primary and secondary antibodies to targets other than the antigen, which is often a problem in immunostaining (Casey et al., 2000).
To preserve the antibody activity of ScFv, we cloned the integrated variable regions, including all six hypervariable regions (Fig.3). The ScFv eliminates the invariable regions so that ScFv has much lower immune binding than does McAb (monoclonal antibody) (Tina et al., 2004; Gao et al., 2003). The linker was designed as (GGGGS)3 to ensure accurate folding of Vh and Vl; the Gly made the chain more flexible; and the Ser made the linker more hydrophilic. Finally, the molecular weight of the ScFv we constructed was as low as 26000, which was one-sixth of an integrated natural antibody. Accordingly, this ScFv is so small that it can penetrate and enter the blood circulation around a tumor.
The GFP structure had been reported to be a barrel, but the final seven C-terminus amino acid residues are not part of the barrel; so, their absence should not weaken its fluorescent activity (Youvan and Michel, 1996). Therefore, we constructed the GFP-ScFv, but not ScFv-GFP, so that these seven amino acid residues could be used as a linker to weaken the spatial interference between GFP and ScFv. The dual activity of the fusion protein indicated that these seven amino acid residues indeed weakened their spatial interference. On the basis of these observations, we created Fig.8 from the model, these seven amino acid residues separate both proteins well; the antigen-binding site of ScFv is fully exposed so that it can easily be recognized by its antigen on the surface of lung adenocarcinoma; and the integrity of the barrel leads to the high fluorescent activity of GFP.
In the purification of fusion protein, much fusion protein was degraded automatically (Fig.5) and a 28000 molecule was released (Fig.4b); this occurrence was reported (Hu et al., 1999). Fig.8 shows that the structures of GFP and ScFv were both relatively tight; so that, the seven flexible amino acid residues of GFP between these two rigid fragments broke easily (Mark et al., 2000). In addition, the 28 000 molecule fluoresced faintly at 395 nm. Accordingly, we concluded that the fusion protein degraded at the end of the GFP.
Although ProGFP-ScFv expressed very effectively (Fig.4a), the major expression pattern of the fusion protein was as inclusion bodies, which will weaken the activity of ScFv. The reasons for formation of inclusion bodies vary; for one thing, the pProEx HTb has a strong promoter so that the ScFv can be transcribed effectively, but it always leads to the accumulation of precursor molecules in the cell (Gerard and Andreas, 1999), which appear as inclusion bodies. Additionally, the sequence of ScFv is possibly a factor in the formation of inclusion bodies. Jung et al.(1999) found that with stable ScFv, after two rounds of mutagenesis and selection, the quantity of the secretive protein from the new ScFv was three times that of the original one. These findings implied that the ScFv itself greatly affected the form of expression.
Our future work will focus on application: in the clinical setting, the ScFv with a GFP tag will be used as a new reagent to detect SPC-A-1 adenocarcinoma; in an applied assay, it will be used to mark directly the specific antigen-antibody reaction.
Footnotes
Project (No. 396007) supported by the National Natural Science Foundation of China
References
- 1.Casey JL, Coley AM, Tilley LM, Foley M. Green fluorescent antibodies: Novel in vitro tools. Protein Engineering. 2000;13:445–452. doi: 10.1093/protein/13.6.445. [DOI] [PubMed] [Google Scholar]
- 2.Cheng H, Liu YF, Zhang HZ, Shen WA, Zhang SZ. Constrution and expression of eukaryotic vector with recombinant secratory anti-liver cancer double fuction antibody and GFP fusion gene. World Chinese Journal of Digestion. 2001;9(6):640–644. (in Chinese) [Google Scholar]
- 3.David GM, Kaija A, Kirsi O. Baculoviral display of functional ScFv and synthetic IgG-binding domains. Biochemical and Biophysical Research Communications. 2000;275(1):84–90. doi: 10.1006/bbrc.2000.3264. [DOI] [PubMed] [Google Scholar]
- 4.Gao YD, Xiong DS, Peng H. Expression and characterization of recombinant ScFv antibody detecting p-glycoprotein. Chinese Journal of Immunolog. 2003;20(4):267–271. (in Chinese) [Google Scholar]
- 5.George NP. Structrue and dynamics of green fluorescent protein. Current Opinion in Structure Biology. 1997;7:821–827. doi: 10.1016/s0959-440x(97)80153-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerard W, Andreas P. The hierarchy of mutations influencing the folding of antibody domains in Escherichia coli . Protein Engineering. 1999;12:605–611. doi: 10.1093/protein/12.7.605. [DOI] [PubMed] [Google Scholar]
- 7.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 8.Haraguchi T, Ding DQ, Yamamoto A, Kaneda T, Koujin T, Hiraoka Y. Multiple-color fluorescene imaging of chromosomes and microtubules in living cells. Cell Struct Funct. 1999;24:291–298. doi: 10.1247/csf.24.291. [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Lu P, Wang LF. Expression and analysis of recombinant human IL-18 and human IL-18-GFP fusion protein. Chinese Journal of Microbiology and Immunology. 1999;19(4):306–310. (in Chinese) [Google Scholar]
- 10.Joseph S, David WR. Molecular Cloning. New York, America: Cold Spring Harbor Press; 2001. pp. 96–99. [Google Scholar]
- 11.Jung S, Honegger A, Plückthun A. Selection for improved protein stability by phage display. J Mol Biol. 1999;294(1):163–180. doi: 10.1006/jmbi.1999.3196. [DOI] [PubMed] [Google Scholar]
- 12.Mark A, Remko AG, Jan WB. Structural dynamics of green fluorescent protein alone and fused with a single chain Fv protein. J Biol Chem. 2000;275:17556–17560. doi: 10.1074/jbc.M001348200. [DOI] [PubMed] [Google Scholar]
- 13.Mccall AM, Adams GP, Amoroso AR. Isolation and characterization of an anti-CD16 single-chain Fv fragment and construction of an anti-HER2/neu/anti-CD16 bispecific ScFv that triggers CD16-dependent tumor cytolysis. Molecular Immunology. 1999;36(7):433–446. doi: 10.1016/s0161-5890(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 14.Mclean AJ, Bevan N, Rees S, Milligan G. Visualizing differences in ligand regulation of wild-type and constitutively active mutant β2-adrenoceptor-green fluorescent protein fusion proteins. Mol Pharmacol. 1999;56:1182–1191. doi: 10.1124/mol.56.6.1182. [DOI] [PubMed] [Google Scholar]
- 15.Myers K, Ryan MG, Stern PL, Shaw DM, Embleton MJ, Kingsman SM, Carroll MW. Targeting immune effector molecules to human tumor cells through genetic delivery of 5T4-specific ScFv fusion proteins. Cancer Gene. 2002;9(11):884–896. doi: 10.1038/sj.cgt.7700513. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa H, Inouye S, Tsuji FI, Yasuda K, Umesono K. Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc Natl Acad Sci USA. 1995;92(25):11899–11903. doi: 10.1073/pnas.92.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlandi R, Gussow DH, Johns PT. Cloning immunoglobulin variable domain for expression by the polymerase chain reaction. Proceedings of the National Academy of Sciences. 1989;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otsuki M, Fukami K, Kohno T, Yokota J, Takenawa T. Identification and characterization of a new phospholipase C-like protein, PLC-L2 . Biochem Biophys Res Commun. 1999;266:97–103. doi: 10.1006/bbrc.1999.1784. [DOI] [PubMed] [Google Scholar]
- 19.Peitsch MC. Protein modelling. Bio/Technology. 1995;13:658–660. [Google Scholar]
- 20.Prasher DC, Echenrode VK, Ward WW. Primary structure of the Aequorea victorial green fluorescent protein. Gene. 1992;111:229–233. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- 21.Schwede T, KOPP J, Guex N, Peitsch MC. MC SWISS-MODEL: An automated protein homology-modelling server. Nucleic Acids Research. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi HD, Su WW. Display of fluorescent protein on Escherichia coli cell surface. Enzyme and Microbial Technology. 2000;28(2001):25–34. doi: 10.1016/s0141-0229(00)00281-7. [DOI] [PubMed] [Google Scholar]
- 23.Tina K, Dirk MN, Tina V. Recombinant bispecific antibodies for the targeting of adenoviruses to CEA-expressing tumour cells: A comparative analysis of bacterially expressed single-chain diabody and tandem ScFv. Journal of Gene Medicine. 2004;6:642–651. doi: 10.1002/jgm.555. [DOI] [PubMed] [Google Scholar]
- 24.Walker D, Htun H, Hager GL. Using inducible vectors to study intracellular trafficking of GFP-tagged steroid/nuclear receptors in living cells. Methods Companion Methods Enzymol. 1999;19:386–393. doi: 10.1006/meth.1999.0874. [DOI] [PubMed] [Google Scholar]
- 25.Youvan DC, Michel BM. Structwe and flurescence mechanism of GFP. Nat Biotechnol. 1996;14(10):1219–1220. doi: 10.1038/nbt1096-1219. [DOI] [PubMed] [Google Scholar]
- 26.Zhu HY, Yamada H, Jiang YM, Yamada M, Nishiyama Y. Intracellular localization of the UL31 protein of herpes simplex virus type 2. Arch Virol. 1999;144:1923–1935. doi: 10.1007/s007050050715. [DOI] [PubMed] [Google Scholar]