Abstract
Aquilaria agallocha can produce fragrant agarwood used for incense, traditional medicine and other products. An efficient plant regeneration system was established via organogenesis from shoots developed from seedlings of Aquilaria agallocha. Shoots generated many buds on MS medium supplemented with 1.3 μmol/L BA (6-benzylaminopurine) in the first 7 weeks, and the buds elongated on MS medium with 1.3 μmol/L BA+0.5 μmol/L NAA (naphthaleneacetic acid) in another 7 weeks, 2.3 shoots 2 cm in length per explant were obtained within 14 weeks. Plantlets were rooted on 1/2 MS medium after being immersed in 5 μmol/L NAA for 48 h, 96.7% of the roots grew up two weeks later. All plantlets that survived acclimatization grew well in the pots.
Keywords: Agarwood, Micropropagation, Aquilaria agallocha
INTRODUCTION
Many tree species have become the focus of increasing conservation concern in recent years, primarily because of the current high rates of forest clearance and over-exploitation (Newton et al., 1999). As an illustration, recent surveys have indicated that around 9000 tree species are threatened with extinction (Oldfield et al., 1998). Aquilaria agallocha (Thymelaeaceae) is one of very few species of tropical trees and is the principal source of agarwood, one of the most highly valuable forest products currently traded internationally. Agarwood (also known as aloeswood, eaglewood and gaharu, among many other common names) is a fragrant wood that has been traded since biblical times for use in religious functions and for medicinal and aromatic preparations. High consumer demand, particularly from Middle Eastern and Asian markets, combined with decreasing supply has pushed prices progressively higher to the extent that top grade agarwood can sell for over USD 10000/kg in end-use markets (Barden et al., 2000). A. sinensis was the traditional resource of agarwood in China dating from ancient times, but is now being replaced by agarwood from A. agallocha (named imported agarwood) in the market due to its better quality. Since the 15th century, agarwood has been collected and used as a drug in China. Studies revealed that agarwood has remarkable anticancer activity (Gunasekera et al., 1981). Benzene extractable compounds possess potent central nervous system antidepression activities (Okugawa et al., 1993; 1996), so agarofuran is considered as new promising nervous system drug (Chen, 1999). The normal propagation of A. agallocha by seed is difficult. Because the seeds’ moisture content is rapidly decreased during the first few hours/days, so the viability is lost rapidly. Furthermore, insect pests infestation often of the seed inhibits the growth of the tree (Su, 1994). The present study was undertaken to develop a reproducible protocol for in vitro micropropagation using shoot.
MATERIALS AND METHODS
The basal medium was MS medium (Murashige and Skoog, 1962) supplemented with 3% (w/V) sucrose and gelled with 0.8% (w/V) agar. The pH of the medium was adjusted to 5.8 with 1 mol/L HCl or NaOH before autoclaving at 120 °C for 20 min. Depending on the experiment, the basal medium was supplemented with 6-benzylaminopurine (BA) (0.13~5.2 μmol/L) alone or in combination with naphthaleneacetic acid (NAA) (0.05~0.5 μmol/L). The cultures were incubated at 26 °C and 55%±10% relative humidity under a 16 h/8 h-light/dark regime and provided with a photon flux of 30 μmol/(L·m2·s) by cool-white fluorescent lamps. Each treatment consisted of 20 explants and was replicated 3 times. The percentage of differentiation and the number of buds and shoots per explant were calculated and differences between means were tested for significance using Tukey’s Test at the level of P≤0.05.
Seeds of A. agallocha collected from the South China Botanical Garden in July were first stripped carefully to get the embryos, which were surface-sterilized by immersion in 0.2% (w/V) HgCl2 with a few drops of Tween-20 for 12 min and then washed thoroughly in sterile water and cultured on 1/2 MS medium. Three weeks later, these embryos developed into about 4 cm high seedlings with three leaves, which were used as explant source. The surface-sterilization of the seeds achieved 100% aseptication of the cultures.
For shoot bud induction, the shoots of seedlings without the buds at the apex were cut into about 0.5 cm long segments, and placed on bud induction medium composed of MS medium supplemented with different concentrations of BA alone or in combination with NAA. For root induction, 2 cm long or longer shoots were cut and immersed in sterilized 5 μmol/L NAA for about 48 h and then transferred into 1/2 MS.
RESULTS
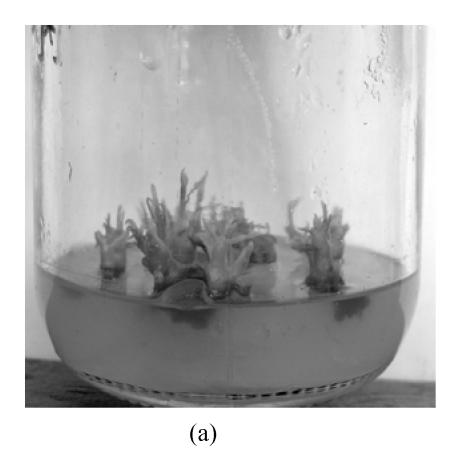
After being placed in MS, shoot bud induction medium supplemented with BA alone, the middle part of explants expanded without differentiation one week later, and adventitious buds developed after 3 weeks’ cultivation. In MS medium with two kinds of auxin, explants differentiated and developed calli one week later, and within 2 weeks developed buds, most of which originated from axillary buds of the shoots. After 4 weeks of culture, a number of adventitious buds generated (Fig.1a). About 7 weeks later, the buds developed into microshoots. The percentage of differentiation and the number of buds and shoots/explant was noted after 14 weeks (Table 1).
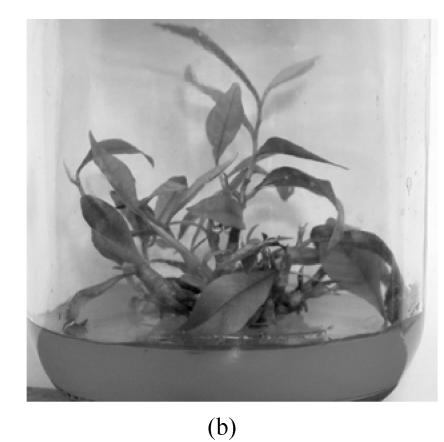
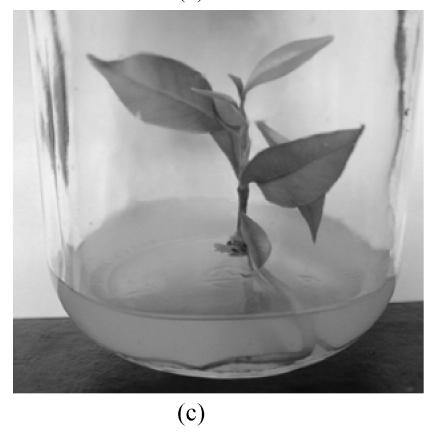
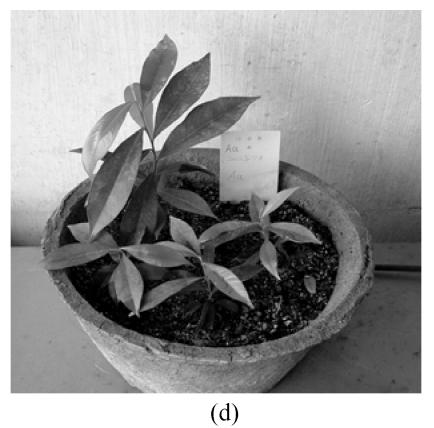
Fig. 1.
In vitro propagation of Aquilaria agallocha (a) Buds induction on MS+1.3 μmol/L BA medium in the first 7 weeks; (b) Buds elongation on MS+1.3 μmol/L BA+0.5 μmol/L NAA medium in subsequent 7 weeks; (c) Shoot with roots on 1/2 MS medium; (d) Well grown plant (after 2 months)
Table 1.
Effect of growth regulators on shoot induction after 14 weeks of cultivation
| BA (μmol/L) | NAA (μmol/L) | Differentiation percentage (%) | No. of buds/explant | No. of shoots/explant |
| 0.13 | 0.00 | 100.00a | 2.58a | 0.72ab |
| 0.26 | 0.00 | 83.30ab | 1.74ab | 0.46ab |
| 1.30 | 0.00 | 78.70b | 2.48a | 0.17ab |
| 2.60 | 0.00 | 78.70b | 1.89ab | 0.22ab |
| 5.20 | 0.00 | 67.33bc | 2.05ab | 0.23ab |
| 0.13 | 0.05 | 80.67b | 1.30ab | 0.17ab |
| 0.13 | 0.25 | 35.33c | 1.02b | 0.08ab |
| 0.13 | 0.50 | 55.33bc | 0.96b | 0.04b |
| 1.30 | 0.05 | 62.33bc | 1.27ab | 0.24ab |
| 1.30 | 0.25 | 55.33bc | 1.09b | 0.66ab |
| 1.30 | 0.50 | 80.67b | 1.11b | 0.89a |
| 2.60 | 0.05 | 66.67bc | 1.46ab | 0.37ab |
| 2.60 | 0.25 | 55.67bc | 1.59ab | 0.11ab |
| 2.60 | 0.50 | 86.67ab | 1.26ab | 0.74ab |
Means in each column followed by different letters are different according to Turkey’s Multiple Range Test (P≤0.05)
The buds in MS medium supplemented with higher BA (≥2.6 μmol/L) were dumpy and twisty (Fig.2). Medium supplemented with NAA and BA, low concentration of BA (1.3 μmol/L) in combination with NAA, led to explants differentiation whereas high concentration of BA in combination with NAA led to explants development into microshoots.
Fig. 2.

Dumpy and twisty buds induced by MS medium supplemented with higher concentrations of BA (≥2.6 μmol/L)
For root induction, before being transferred to 1/2 MS medium without growth regulators, 2 cm long or longer shoots were cut and immersed in sterilized different concentration auxin for different time. The frequency of rooting and the number of roots were observed about 2 weeks later (Table 2).
Table 2.
The effects of different auxin on rooting
| Auxin (μmol/L) | Dealing time (days) | No. of shoots inoculated | No. of shoots rooted | No. of roots | Frequency of rooting (%) | Average roots/shoot |
| NAA 0.0 | 0 | 20 | 20 | 0 | 0.0 | 0.00 |
| NAA 2.5 | 2 | 30 | 27 | 75 | 90.0 | 2.50 |
| NAA 5.0 | 2 | 30 | 29 | 80 | 96.7 | 2.67 |
| BA 2.5 | 2 | 30 | 24 | 70 | 80.0 | 2.33 |
| BA 4.9 | 2 | 30 | 21 | 46 | 70.0 | 1.53 |
Above 90% of the shoots that had been immersed in 5 μmol/L NAA for 48 h developed roots (Fig.1c).
Before being transplanted into pots, regenerated plantlets were cultivated in open-tubes with their roots immersed in water for one month. The pots contained vermiculite, soil and pond mud in 1:1:1 ratio. Sixty-five plantlets were planted in pots and 94.3% of them survived after 55 d (Fig.1d).
DISCUSSION
It was observed that BA was the best growth regulator for the induction of adventitious buds (Table 1). The number of the buds increased with the concentration of BA, but high concentrations of BA (2.6~5.2 μmol/L) resulted in the expansion and translucency of explants and inhibited the buds’ elongation, whereas low concentrations of BA led to normal development. On the other hand, MS medium supplemented with NAA alone (0.5~10 μmol/L) could induce calli only and there were no buds but roots sprouted from the calli in these cases (data not shown). As shown in Table 1, it appeared that 1.3 μmol/L BA+0.5 μmol/L NAA was appropriate for shoot formation and 0.89 shoots/explant were obtained at last. Considering the needs of micropropagation, the explants were grown on MS+1.3 μmol/L BA for bud induction in the first 7 weeks and then the buds were transferred onto MS+1.3 μmol/L BA+0.5 μmol/L NAA for the elongation and development (Fig.1b). In that case, 2.3 shoots 2 cm in length per explant were obtained within 14 weeks.
If the microshoots were transferred onto 1/2 MS medium or supplemented with low concentrations of NAA directly, they developed roots slowly. The method of root induction here was relatively more effective and had been used in some Temperate Zone fruit trees (Dodds, 1983).
CONCLUSION
In conclusion, using shoot segments as explants, direct plantlet regeneration of A. agallocha can be achieved via organogenesis in 20 weeks, and the regenerated plantlets can be used for further propagation. This study provided a very useful method for propagation of this medicinal plant.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30070066) and the Science and Technology Project of Guangzhou City (No. 2003J1-C0241), China
References
- 1.Barden A, Anak NA, Mulliken T, et al. Heart of the Matter: Agarwood Use and Trade, and CITES Implementation for Aquilaria Malaccensis . 2000 (Available from: http://www.traffic.org)
- 2.Chen KX. Modern theory and method of innovative medicinal research–progress in 1998. Basal Research. 1999;7(6):7–10. [Google Scholar]
- 3.Dodds JH. Tissue Culture of Trees. American Edition. Westport, Connecticut: the Avipublishing Company, Inc; 1983. pp. 71–74. [Google Scholar]
- 4.Gunasekera SP, Kinghorn AD, Cordell GA, Farnsworth NR. Plant anticancer agents. XIX. Constituents of Aquilaria Malaccensis . J Nat Prod. 1981;44:569–572. doi: 10.1021/np50017a010. [DOI] [PubMed] [Google Scholar]
- 5.Murashige T, Skoog F. A revised medium for rapid growth bioassays with tobacco tissue cultures. Physilo Plant. 1962;15:473–497. [Google Scholar]
- 6.Newton AC, Allnutt T, Gillies ACM, Lowe AJ, Ennos RA. Molecular phylogeography, intraspecific variation and the conservation of tree species. Trends in Ecology and Evolution. 1999;14(4):140–145. doi: 10.1016/s0169-5347(98)01555-9. [DOI] [PubMed] [Google Scholar]
- 7.Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A. Effects of agarwood extracts on the central nervous systems in mice. Planta Med. 1993;59:32–36. doi: 10.1055/s-2006-959599. [DOI] [PubMed] [Google Scholar]
- 8.Okugawa H, Ueda R, Matsumoto K, Kawanishi K, Kato A. Effects of jinkoh-eremol and agarospirol from agarwood on the central nervous systems in mice. Planta Med. 1996;62:2–6. doi: 10.1055/s-2006-957784. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield S, Lusty C, Mackinven A. The World List of Threatened Trees. Cambridge, UK: World Conservation Press, WCMC; 1998. [Google Scholar]
- 10.Su YP. Biological characters of Heortia vitessoides . Zhong Yao Cai. 1994;17(12):412–414. (in Chinese) [Google Scholar]