Abstract
The entomopathogenic bacterium, Xenorhabdus nematophila was isolated from the hemolymph of Galleria mellonella infected with Steinernema carpocapsae. The bacterial cells and its metabolic secretions have been found lethal to the Galleria larvae. Toxic secretion in broth caused 95% mortality within 4 d of application whereas the bacterial cells caused 93% mortality after 6 d. When filter and sand substrates were compared, the later one was observed as appropriate. Similarly, bacterial cells and secretion in broth were more effective at 14% moisture and 25 °C temperature treatments. Maximum insect mortality (100%) was observed when bacterial concentration of 4×106 cells/ml was used. Similarly, maximum bacterial cells in broth (95%) were penetrated into the insect body within 2 h of their application. However, when stored bacterial toxic secretion was applied to the insects its efficacy declined. On the other hand, when the same toxic secretion was dried and then dissolved either in broth or water was proved to be effective. The present study showed that the bacterium, X. nematophila or its toxic secretion can be used as an important component of integrated pest management against Galleria.
Keywords: Biological control, Bacterial symbionts, Entomopathogenic nematodes, Xenorhabdus nematophila, Galleria mellonella
INTRODUCTION
Entomopathogenic nematodes of the genera Steinernema and Heterorhabditis have been used to control a wide range of agriculturally important insect pests (Kaya and Gaugler, 1993). The mechanism by which these nematodes are able to infect and reproduce in the insect host involves a mutual relationship between the nematode and the symbiotic bacteria, Xenorhabdus spp. and Photorhabdus spp. (Akhurst and Dunphy, 1993; Forst and Nealson, 1996). Poinar and Thomas (1966) established that a single species of bacterium in the family Enterobacteriaceae was present in the anterior region of the infective juvenile (IJ/IJs) of Steinernema carpocapsae. Since then it has been known that Steinernema spp. carry bacteria of the genus Xenorhabdus while Heterorhabditis nematodes harbour a species of the genus Photorhabdus (Akhurst and Boemare, 1988). Once an IJ penetrates into the haemocoele the bacterial symbiont is released from the nematode gut, septicemia becomes established and insect death occurs within 48 h. Although IJs play an important role in insect death by vectoring the bacteria, but, in most cases the bacteria alone are sufficient to cause insect mortality when injected into the haemocoele (Gotz et al., 1981).
Xenorhabdus nematophila is a gram-negative facultatively anaerobic, non-spore forming bacterium with numerous peritrichous flagella mutualistically associated with the nematode S. carpocapsae and is only found inside IJs or infected hosts (Akhurst and Boemare, 1990). The symbiotic association is essential for the survival of both nematode and its symbiotic bacteria (Poinar, 1979). Some recent evidence indicated that cells suspension of the bacterial symbiont X. nematophila may be toxic to the fire ant, Solenopsis invicta when sprayed on cotton leaves (Dudney, 1997) and larvae and pupae of Spodoptera exigua (Elawad et al., 1999). This bacterium has great potential as a biological control agent for noxious insects in cryptic environments. Bowen and Ensign (1998) suggested that it is feasible to use toxins produced from broth suspensions of the bacterial symbiont, to protect plants by the transformation of the toxin gene or genes into plants or by direct application of the toxin as a strategy for the control of insect pests.
In recent years the pathogenicity of toxic genes from entomopathogenic bacteria has been researched extensively (Bowen and Ensign, 1998; ffrench-Constant and Bowen, 1999). Recently a patent was filed in the USA (Ensign et al., 2002) for the use of toxins from Xenorhabdus spp. for insect control. Proper approaches will be needed to use these bacteria and their secretions. It is therefore anticipated that with the tools and research knowledge presently available a bio-pesticide can be developed. The major objective of present investigation was to determine the effects of bacterium alone and its secretions on mortality of Galleria mellonella larvae under different moistures, substrates, temperatures, cell concentrations, and methods of application.
MATERIALS AND METHODS
Larvae of greater wax moth, Galleria mellonella infected with the IJs of S. carpocapsae (all isolates) were obtained from ‘Mealworm Co.’ (Sheffield), UK and cultured at 25 °C. IJs suspension of nematodes was supplied by ‘CAB Institute of Parasitology’ (St. Albans), UK. Nematodes were cultured in the G. mellonella and then stored at 7 °C.
Isolation of bacterial symbionts and their secretions
Xenorhabdus nematophila was obtained from the haemolymph of G. mellonella infected with IJs of S. carpocapsae. Dead G. mellonella larvae were surface-sterilized in 70% alcohol for 10 min, flamed and allowed to dry in a laminar airflow cabinet for 2 min. Larvae were opened with sterile needles and scissors, care being taken not to damage the gut, and a drop of the oozing haemolymph was streaked with a needle onto nutrient agar (NBTA) plates (37 g nutrient agar (BDH); 25 mg Bromothymol blue powder (Raymond); 4 ml of filtrates of 1% 2,3,5-Triphenyltetrazolium Chloride (BDH); 1000 ml distilled water). The agar plates, sealed with Parafilm, were incubated at 28 °C in the dark for 24 h, when single colony of bacterium was selected and streaked onto new plates of nutrient agar. Sub-culturing was continued until colonies of uniform size and morphology were obtained. The pathogenicity of the isolates was confirmed by inoculating (injecting) the bacterial cells into the body of G. mellonella larvae and streaking the haemolymph of the infected larvae on NBTA plates. A single colony of the bacterium was selected and inoculated into 500 ml of nutrient broth solution, containing 15 g nutrient broth (BDH) and 500 ml of distilled water in a flask stoppered by sterile cotton wool and placed in a shaking incubator at 150 rpm for one day at 28 °C. The bacterial concentration of the broth suspension was determined by measuring the optical density using a spectrophotometer adjusted to 600 nm wavelength. Based on results obtained by Elawad (1998) the concentration of the bacterial cells used in the present experiments was adjusted to 4×107 cells/ml and 3% Tween 80 was added as an emulsifier.
To obtain toxic secretions from the bacterial symbionts, the broth suspension was centrifuged at 4100 rpm for 20 min. As a result, bacterial pellet was formed at the bottom of the centrifuge tube; the supernatant broth solution was drawn off and replaced by distilled water. The concentration of bacterial cells was then estimated as stated previously and adjusted to 4×107 cells/ml. To obtain cells-free solution of the metabolites from the bacterial symbionts, the bacterial suspensions in broth or water were filtered using a Whatman 25 Mm GD/X filter with pore size of 0.2 μm. The purity of the cells-free toxin solutions was tested on agar plates before their application against G. mellonella larvae.
Experiment 1: Mortality of G. mellonella larvae following the inoculation of bacterial cells and their toxic secretion
This experiment was designed to test how fast larvae of G. mellonella die after the inoculation of X. nematophila suspensions and its toxic secretion in broth and water under sand arena. Fresh cells suspension and secretions of X. nematophila cells in broth and water were prepared at concentration of 4×107 cells/ml and 3% Tween-80 was mixed in all treatments in all experiments. In 100 g of sterilized sand 16.4 ml of cells suspension or their secretion was mixed in order to obtain 14% moisture content. Late instar of G. mellonella larvae of similar age and size were surface sterilized with 2% Hymine for five minutes and then dried under laminar airflow. Ten larvae were placed in the moist sand in sterilized plastic containers (110 mm×25 mm) with bacterial suspension or secretions. Water and broth alone were also used as control. All containers with content were incubated at 25 °C. The mortality was assessed daily for seven days. Replication was four fold in all experiments. The dead larvae were sterilized in 70% industrial methylated spirit for five minutes to kill the bacteria on the surface of the G. mellonella larvae. A sample from dead insects was then taken from the haemocoele of the abdomen and streaked onto nutrient agar to determine whether or not bacteria were present in the haemocoele.
Experiment 2: Optimization of substrates (filter paper and sand box) containing bacterial cells and their toxic secretion
Cells suspension and toxic secretion of X. nematophila on the filter paper and sand substrate were tested against the larvae of G. mellonella. Two Whatman filter papers were placed in 9 cm sterilised Petri-dish and 2 ml from each cells suspension and their secretion was sprayed on the filter paper with a hand sprayer. Like the previous experiment, 100 g sterilised fine sand containing bacterial suspension and their toxic secretion in plastic containers was also tested (experiment 1). Water and broth alone were sprayed as controls. Ten G. mellonella larvae were placed on both substrates to observe the mortality.
Experiment 3: Response of G. mellonella larvae to moisture content, temperature, concentration and bacterial penetration
Xenorhabdus cells suspension and their toxic secretion in broth and water were prepared as described already in experiment 1. To investigate the importance of moisture, the experiment was carried out in sand at 10%, 14% and 18% moisture content whereas to determine temperature, wax moths were incubated at 20 °C, 25 °C and 30 °C post inoculation. Similarly, to observe an appropriate bacterial cells concentration, six concentrations of 4×102 to 4×107 cells/ml in broth and water were applied. Finally, to determine the time for the bacterial cells to enter into the larvae, haemolymph was taken from each treated larvae and streaked on agar plates to confirm the bacterial growth. If bacteria grow on agar plates it will confirm their penetration into the larval body. Data of bacterial penetration were taken after 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 16 h and 32 h on agar plates.
Experiment 4: Longevity of stored bacterial toxic secretion against G. mellonella larvae
Bacterial toxin secretion in broth and water was produced as described in experiment 1. These solutions were either stored at 25 °C for four weeks or dried for two days under laminar airflow at the same temperature. The former content was then mixed with 100 g sterilized sand while the dried toxin was rewetted with either broth or water and then mixed with the sterilized sand. Ten Galleria larvae were placed in each container in the sand arena and mortality of larvae was assessed after one week of application. Data taken from all experiments were transformed (arcsin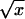 ) prior to statistical analysis using regression technique of SAS (version 8) statistical package (SAS Institute Inc., Cary, North Carolina, USA). The same statistical package was used to determine any significant difference between means using t-test.
) prior to statistical analysis using regression technique of SAS (version 8) statistical package (SAS Institute Inc., Cary, North Carolina, USA). The same statistical package was used to determine any significant difference between means using t-test.
RESULTS
The regression analysis of the data showed that mortality of the G. mellonella larvae significantly (P<0.05) increased with the time (Fig.1). Maximum mortality of insects (100%) was achieved when they were treated with toxic secretion in broth after 5 d (R 2=0.94 at 6 d.f.). The same mortality percentage was obtained when the insects were treated with bacterial cells suspension in broth after 7 d of application, a linear increase was observed (R 2=0.98 at 6 d.f.). However, toxic secretion in water caused 95% mortality (R 2=0.96 at 6 d.f.) whereas bacterial cells suspension in water caused 77.5% mortality (R 2=0.98 at 6 d.f.) after 7 d of observation. In control treatments, only 15% (in broth) and 12.5% (in water) mortality was found after 7 d.
Fig. 1.
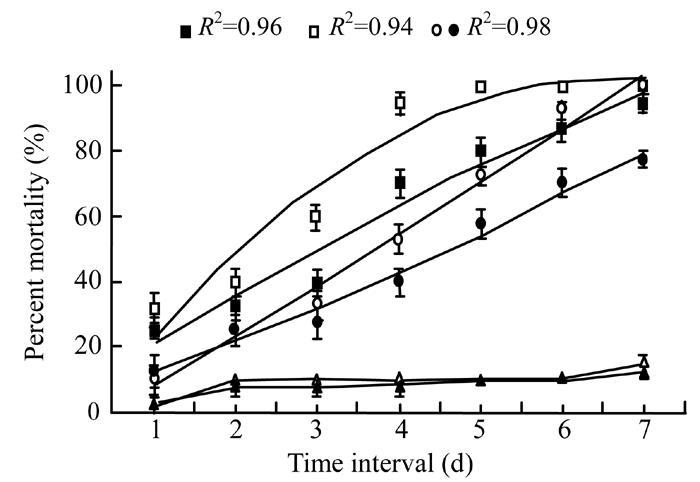
Mortality response of Galleria larvae to Xenorhabdus cells in broth (◦) and water (•), Xenorhabdus secretion in broth (▫) and water (▪), broth alone (∆) and water alone (▲) after different time intervals. The data were fitted on polynomial model (at 6 d.f.) excluding control treatments whereas vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
Analysis of variance of the data showed a statistically significant (P<0.05) difference between filter and sand substrates however there was no significant difference between cells or cells-free secretion (Fig.2). In sand culture, maximum mortality of G. mellonella larvae (100% and 97.5%) occurred when treated with secretion and cells suspension of X. nematophila in broth however in filter paper arena, only 45% and 40% mortality was observed for the same treatments. Bacterial secretion in water caused 92.5% and cell suspension in water caused 85% mortality in sand media although minimum mortality of 35% was observed when X. nematophila suspension and its secretion were applied using filter paper.
Fig. 2.
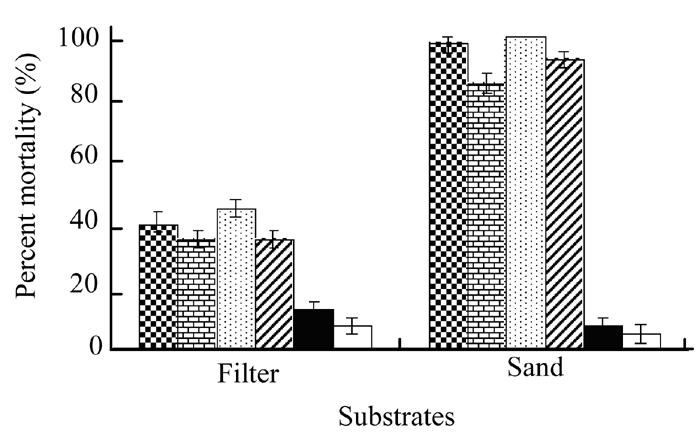
Effect of Xenorhabdus cells in broth (checker bars) and water (brick bars), Xenorhabdus secretion in broth (dotted bars) and water (zebra lines bars), broth alone (black bars) and water alone (white bars) on mortality of Galleria larvae using filter and sand substrates. Vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
The effect of three moisture contents on the pathogenicity of X. nematophila bacterium and its secretion showed a significant (P<0.05) difference between the three moisture levels (Fig.3). Analysis of variance indicated higher mortality of G. mellonella larvae (80% to 97.5%) at 14% moisture in all cells or their secretion treatments as compared to the other two levels. However, 65% to 75% insect mortality was observed at 18% moisture level followed by 45% to 67.5% at 10% moisture treatment. Both control treatments showed very poor mortality percentage.
Fig. 3.
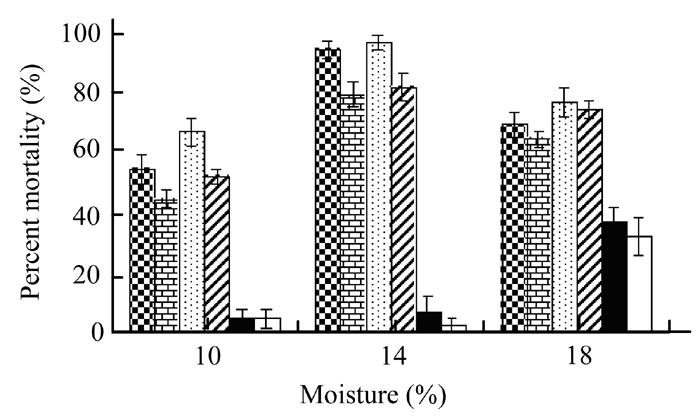
Effect of Xenorhabdus cells in broth (checker bars) and water (brick bars), Xenorhabdus secretion in broth (dotted bars) and water (zebra lines bars), broth alone (black bars) and water alone (white bars) on mortality of Galleria larvae at three moisture contents. Vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
Fig.4 shows the significant (P<0.05) effect of different temperature on the mortality of G. mellonella. Analysis of variance showed no significant difference in mortality caused by either cell of X. nematophila suspension or cells-free secretion. The results indicated that higher mortality, 70% to 97.5% of the G. mellonella larvae was recorded when the larvae were reared at 25 °C or 30 °C and received either of the bacterial treatments (cells and their secretion). However, minimum insect mortality (55%–72%) was observed at 20 °C. Control treatments of broth and water showed poorest effect on insect mortality when compared to the bacterial treatments.
Fig. 4.
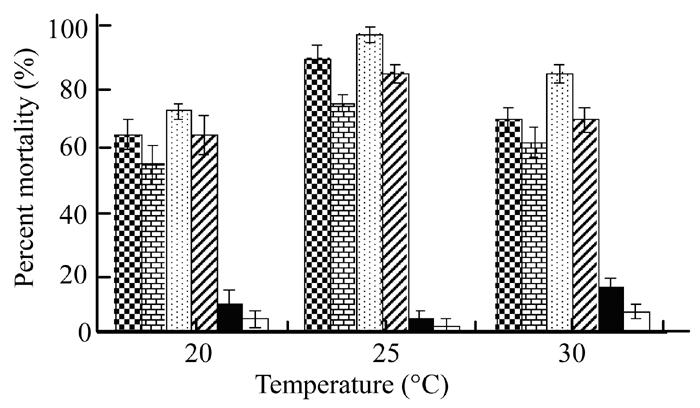
Effect of Xenorhabdus cells in broth (checker bars) and water (brick bars), Xenorhabdus secretion in broth (dotted bars) and water (zebra lines bars), broth alone (black bars) and water alone (white bars) on mortality of Galleria larvae at three temperature regimes. Vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
The regression analysis of the data regarding different concentrations of bacterial cells (Fig.5) in broth (R 2=0.99 at 5 d.f.) was found to be more significantly (P<0.05) effective than cells in water (R 2=0.98 at 5 d.f.). Lower concentration of X. nematophila cells in broth (4×102 cells/ml) was even more harmful (77.5% mortality) than the same bacterial concentration in water (50% mortality). However, mortality was increased with the increase in bacterial concentration. Maximum mortality (100%) was recorded when 4×106 or 4×107 cells/ml dose in broth was used whereas minimum mortality (50%) of larvae was observed at lowest cells concentration (4×102 cells/ml) in water.
Fig. 5.
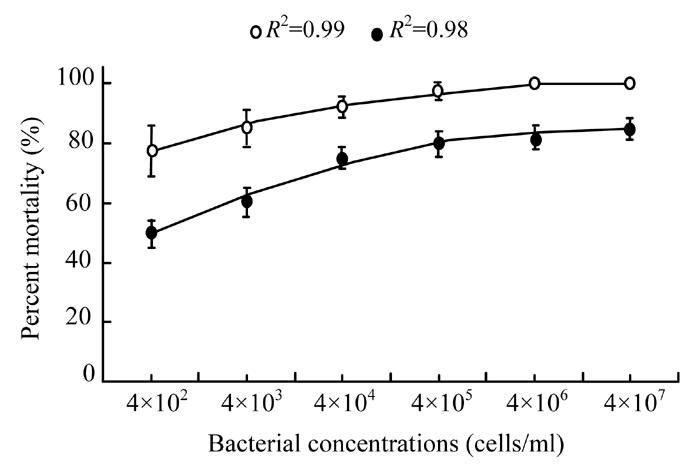
Effect of different bacterial cell concentrations (4×102, 4×103, 4×104, 4×105, 4×106 and 4×107 cells/ml) in broth (◦) and water (•) on mortality percentage of Galleria larvae. The data were fitted on polynomial model (at 5 d.f.) whereas vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
Eighty percent of bacterial cells in broth entered into the insect larvae within 30 min whereas 50% bacterial cells in water entered into the larvae (Fig.6). The regression analysis showed a significant (P<0.05) difference between broth (R 2=0.89 at 7 d.f.) and water (R 2=0.98 at 7 d.f.). In one hour’s time, 90% cells in broth entered into the larvae as compared to 60% cells in water. All G. mellonella larvae were infected with bacterial cells in broth within 4 h time however bacterial cells in water took 16 h (R 2=0.98 at 7 d.f.). Similarly, only 35% larvae were found dead when treated with cells suspension in broth but cells suspension in water caused 30% mortality of G. mellonella larvae within 32 h.
Fig. 6.
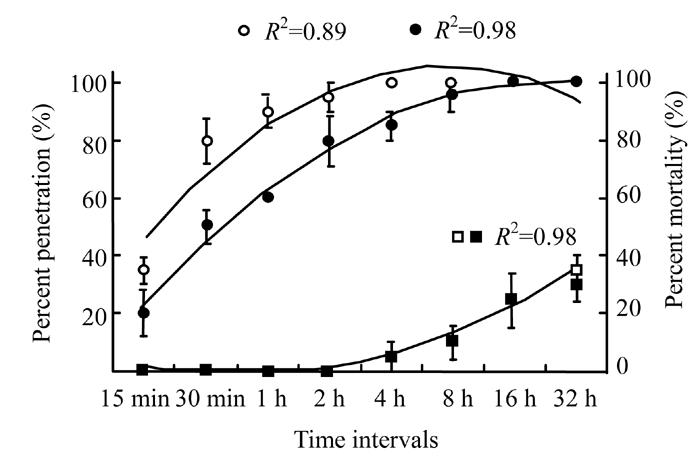
Percent penetration of Xenorhabdus cells in broth (◦) and water (•), on primary axis and mortality of Galleria larvae caused by Xenorhabdus cells in broth (▫) and water (▪), on secondary axis after different time intervals. The data were fitted on polynomial model (at 7 d.f.) whereas vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
Fresh toxin secretion taken from bacterium in broth and water, were found significantly (P<0.05) more effective against G. mellonella larvae than the stored one (Fig.7). The regression analysis indicated that mortality decreased linearly (R 2=0.98 at 4 d.f.) from 97.5% to 40% within four weeks when stored secretion in broth was used. A similar linear trend (R 2=0.99 at 4 d.f.) was observed when the same secretion was used in water and mortality rate decreased from 77.5% to 27.5% in four weeks time. Control treatments (broth and water only) showed lowest effects.
Fig. 7.
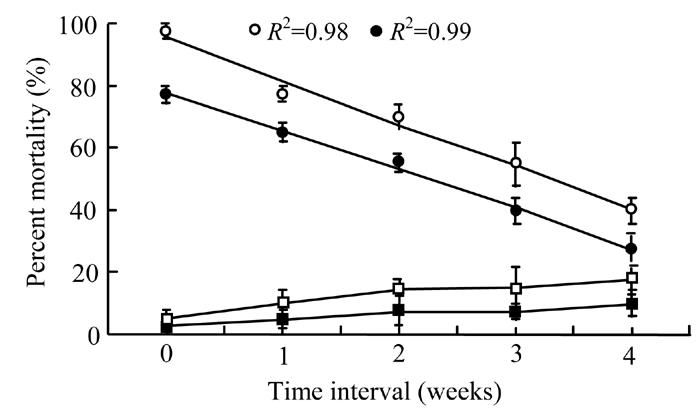
Effect of stored bacterial secretion in broth (◦) and water (•), broth alone (▫) and water alone (▪) on mortality percentage of Galleria larvae after different time intervals. The data were fitted on linear model (at 4 d.f.) excluding control treatments whereas vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
Bacterial secretion in dried form, when dissolved in broth and water significantly (P<0.05) increased the mortality percentage of Galleria when compared to the control treatment (Fig.8). However, analysis of variance showed a no significant difference between the bacterial secretions in broth or in water. Secretion of X. nematophila in broth caused greater mortality (97.5%) compared to that in water (92.5%). Control treatments (broth and water only) showed relatively lower insect mortality results.
Fig. 8.
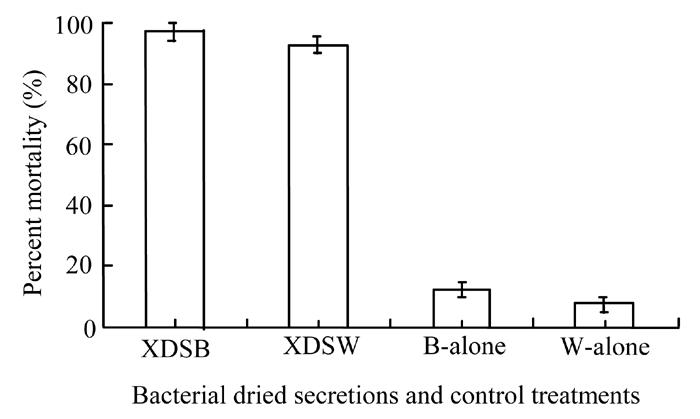
Effect of Xenorhabdus dried secretion in broth (XDSB) and water (XDSW), broth alone (B-alone) and water alone (W-alone) on mortality percentage of Galleria larvae. Vertical bars (where larger than the points) represent the standard error (s.e.) of variability of replicates
DISCUSSION
Entomopathogenic nematode, S. carpocapsae carries specific symbiotic bacterium, X. nematophila which is pathogenic to a wide range of agriculturally important insect pests (Poinar, 1979). Toxic secretion of X. nematophila in broth worked rapidly and caused 95% mortality after 96 h of application while the cells suspension took 48 more days to kill 93% larvae. The difference between the two treatments is due to the direct effect of toxic secretion on the pest’s larvae rather than the indirect effect of cells which released that toxin afterward. Many researchers also approved the use of bacterial toxin obtained from X. nematophila to control various insect pests (Bowen and Ensign, 1998; ffrench-Constant and Bowen, 1999; Ensign et al., 2002). The present findings are in accord with the suggestions of previous scientists.
The bacterial symbionts of X. nematophila and its secretion are lethal to G. mellonella larvae when applied in sand media rather than filter substrate, thus confirming the results of Gotz et al.(1981). Similar results were also reported in fire ant (Dudney, 1997) and the beet army worm (Elawad et al., 1999) when X. nematophila bacterium was applied in sand media to control these pests. These studies considered that symbiotic bacteria may effectively act without the need of vector. Bacterial concentration of 4×106 cells/ml in broth caused 100% mortality of Galleria and indicated the harmful effect of Xenorhabdus nematode (Steinernema carpocapsae) penetration into the haemocoel of this insect. Similar bacterial dose caused 100% mortality in root-knot nematode, Meloidogyne javanica within 24 h of application (Samaliev et al., 2000).
When bacterial cells in broth were applied against the Galleria, they penetrated into the insect body within 4 h, whereas bacterial cells in water gave similar results after 16 h of application. In another study, 100% cells penetration of X. nematophila in broth and water suspension was observed in larvae and pupae of diamondback moth after 4 h and 8 h, respectively (Mahar, 2003). The role of the bacterial cell, as a parasite, is to produce the toxins which break down the immunity of the insect. As shown in the experiment 3, it is the toxin secreted by the bacterial cells, which is responsible for the lethal effects observed. Xenorhabdus nematophila recovered from the insect body indicated that the bacteria entered into the haemocoele of the G. mellonella larvae from different body openings (spiracles, cuticle, mouth or anus). However, the passage through which these bacterial cells gain entry to the haemocoele is unclear but both species of bacterial symbiont exhibit swarming motility when grown on suitable solid media (Mohan et al., 2003; Forst and Nealson, 1996; Boemare et al., 1997; Elawad, 1998). A possible point of entry for motile cells under moist conditions is the spiracle, the only organ other than the mouth or anus directly open to the external environment. Pupae of S. exigua (Elawad et al., 1999) and Plutella xylostella have been shown to be susceptible to cells of the bacterial symbionts and in pupae the spiracle is the only opening in the cuticle (Mahar, 2003). However, further research is required to elucidate this matter.
Bacterial secretions when applied still afresh caused more damage to insect pests than the stored secretion. Application of bacterial secretion in dried form when mixed in broth and water also increased the insect death. This is another possibility of using bacterial secretion in dried form which has also many other advantages. The results obtained here suggest that secretions from bacterial symbionts can be applied to plants to protect them from the attack of Galleria. Similarly, X. nematophila is judged to be the most appropriate bacteria whose secretions could be used as bio-pesticide.
References
- 1.Akhurst RJ, Boemare NE. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. Journal of General Microbiology. 1988;134:1835–1845. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- 2.Akhurst RJ, Boemare NE. Biology and Taxonomy of Xenorhabdus . In: Gaugler R, Kaya HK, editors. Entomopathogenic Nematodes in Biological Control. Boca Raton, Florida: C.R.C. Press; 1990. pp. 75–90. [Google Scholar]
- 3.Akhurst RJ, Dunphy GB. Tripartite Interaction between Symbiotically Associated Bacteria (Xenorhabdus spp) and Nematodes (Steinernenatidae and Heterorhabditidae) and Their Insect Hosts. In: Beckage N, Thomson S, Federici B, editors. Entomopathogenic Nematodes in Biological Control. New York: Academic Press, Inc; 1993. pp. 75–87. [Google Scholar]
- 4.Boemare NE, Givaudan A, Brehelin M, Laumond C. Symbiosis and pathogencity of nematode bacterium complexes. Symbiosis. 1997;22:21–45. [Google Scholar]
- 5.Bowen DJ, Ensign JC. Purification and characterization of the high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium, Photorhabdus luminescens . Applied and Environmental Microbiology. 1998;64:3029–3035. doi: 10.1128/aem.64.8.3029-3035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudney RA. Use of Xenorhabdus Nematophilus Im/l and 1906/1 for Fire Ant Control. No. 5616318. US Patent. 1997
- 7.Elawad SA. Department of Agriculture, University of Reading, UK; 1998. Studies on the Taxonomy and Biology of a Newly Isolated Species of Steinernema (Steinernematidae: Nematoda) from the Tropics and Its Associated Bacteria. [Google Scholar]
- 8.Elawad SA, Gowen SR, Hague NGM. Efficacy of bacterial symbionts from entomopathogenic nematodes against the beet army worm (Spodoptera exigua) Test of Agrochemicals and Cultivars No. 20, Annals of Applied Biology (Supplement) 1999;134:66–67. [Google Scholar]
- 9.Ensign JC, Bowen DJ, Tenor JL, et al. Proteins from the Genus Xenorhabdus are Toxic to Insects on Oral Exposure. No. 0147148 A1. US patent. 2002
- 10.ffrench-Constant R, Bowen D. Photorhabdus toxins: novel biological insecticides. Current Opinion in Microbiology. 1999;2:284–288. doi: 10.1016/s1369-5274(99)80049-6. [DOI] [PubMed] [Google Scholar]
- 11.Forst S, Nealson K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiological Reviews. 1996;60:21–43. doi: 10.1128/mr.60.1.21-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotz P, Boman A, Boman HG. Interactions between insect immunity and an insect-pathogenic nematode with symbiotic bacteria. Proceedings of Royal Society London. 1981;212:333–350. [Google Scholar]
- 13.Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- 14.Mahar AN. Department of Agriculture, University of Reading, UK; 2003. The Efficacy of Bacteria Isolated from Entomopathogenic Nematodes against the Diamondback Moth Plutella xylostella L. (Lepidoptera: Yponomeutidae) [Google Scholar]
- 15.Mohan S, Raman R, Gaur HS. Foliar application of Photorhabdus luminescens, symbiotic bacteria from entomopathogenic nematode Heterorhabditis indica, to kill cabbage butterfly Pieris brassicae . Current Science. 2003;84:1397. [Google Scholar]
- 16.Poinar JrGO. Nematodes for Biological Control of Insects. Boca Raton, Florida: C.R.C. Press; 1979. [Google Scholar]
- 17.Poinar JrGO, Thomas GM. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobactereace: Eubacteriale), in the development of the nematode, DD-136 (Neoplectanta sp., Steinernematidae) Parasitology. 1966;56:385–388. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- 18.Samaliev HY, Andreoglou FI, Elawad SA, Hague NGM, Gowen SR. The nematicidal effects of the bacteria Pseudomonas oryzihabitans and Xenorhabdus nematophilus on the root-knot nematode Meloidogyne javanica . Nematology. 2000;2:507–514. [Google Scholar]


