Abstract
The characteristics of fruit ripening and expression of ripening-related genes were investigated in epi, an ethylene overproduction mutant of tomato (Lycopersicon esculentum Mill.). The epi produces apparently more ethylene than its wild type VFN8 at every stage of vegetative and fruit growth and ripening; compared to VFN8, the epi fruit showed higher CO2 evolution, faster descending of chlorophyll, slightly quicker increase of carotenoid and lycopene, and faster reduction in pericarp firmness during maturation and ripening; and the mRNAs of three ripening-related genes including E8, pTOM5 and pTOM6 were at higher levels in epi. The ripening-related characteristics changing of the fruit are consistent with the increase of ethylene production and ripening-related genes expression. These results suggest that epi mutation possibly did not affect the ethylene perception and signaling during fruit ripening, and that the modified characteristics of fruit ripening possibly resulted from the ethylene overproduction and increased expression of ripening-related genes.
Keywords: Epinastic (epi) mutant, Ethylene overproduction, Ethylene signaling, Fruit ripening
INTRODUCTION
As a gaseous phytohormone, ethylene plays an important role in plant growth and development. Ethylene can alter plant physiology and morphology due to its effect of regulating gene expression (Moctezuma et al., 2003). Such regulation apparently depends on the effect of the normal ability of the plant tissues on ethylene perception and signal transduction. Impairment of the perception and signal transduction pathway(s) would thus ultimately lead to alterations in the plant’s development (Guo and Ecker, 2004).
Epinastic (epi) is a natural mutant from the wild type of VFN8 tomato (Ursin, 1987). It is the first natural tomato mutant overproducing ethylene with strong epinastic growth of petiole. Its vegetative tissues produce ethylene several times that of its wild parent VFN8 (Fujino et al., 1988; Ying and Zeng, 1999; Wang and Ying, 2004). Incomplete triple response was observed in etiolated seedlings of epi with the inhibited apical hook formation (Wang and Ying, 2004). Inhibitors of ethylene synthesis and action were unable to rescue its phenotypes of vegetative growth (Fujino et al., 1989; Ursin and Bradford, 1989), leading to the suggestion that the mutation may result in a blockage of ethylene signaling pathway and constitutive ethylene response. This hypothesis is supported by the similarity in the epi seedling phenotype to that of the Arabidopsis ctr1 mutant seedling whose phenotype also did not revert when grown on ethylene inhibitors (Kieber et al., 1993). Apart from epinasty, the epi phenotype is characterized by dark green leaves, thickened and shortened stems, apparent reduction in anthocyanin production, a shortened and highly branched root system, and a very erect and compact growth habit (Fujino et al., 1988; Wang and Ying, 2004). Barry et al.(2001) obtained a double mutant (epi/epi; Nr/Nr) containing both epi and Nr (never ripe) genes, and found that the double mutant has the same dark-grown seedling and vegetative phenotypes as epi but possesses the senescence and characteristics of never ripening. No significant differences were observed between epi and VFN8 in many aspects, including the leaf and petal senescence or abscission, the rate of fruit ripening and the time from anthesis to the onset of fruit ripening (Barry et al., 2001).
E8, a function unclear gene, expression is regulated during fruit ripening both by ethylene, and by ethylene-independent ripening signals (Deikman et al., 1992). pTOM5 and pTOM6 were confirmed to encode phytoene synthetase and polygalacturonase (PG) respectively, with both being ethylene-regulated (Bird et al., 1991; Grierson et al., 1986). Small increases in the transcript abundance of the three ethylene-regulated genes were observed in epi fruit compared to those in VFN8 throughout development and ripening (Barry et al., 2001).
However, we observed apparent delayed senescence of leaves and petals, accelerated petiole abscission in epi compared to its wild type (Wang and Ying, 2004). Here we aim to illustrate the effects of epi mutation on fruit ripening through the difference of ripening characteristics and ripening-related genes expression between epi and wild type VFN8.
MATERIALS AND METHEHODS
Fruit growth and ripening
The epi mutant and its wild-type VFN8 were grown in greenhouse conditions. Flowers were tagged at anthesis and the number of fruit was restricted to less than four per cluster. The fruits displaying first sign of colour change were identified as BK (breaker) stage. Average days from anthesis to BK were counted from 30 fruits. The fruits at 20 days before BK was marked as IM (immature green) and 5 d before BK as MG (mature green); The fruits on 3, 5, 7, 10 d after BK were noted as BK1, BK3, BK5, BK7, BK10 respectively. Each item of the measurements was conducted with fresh harvested fruits at every stage except for firmness determination. For RNA extraction the fruit pericarp were cut into 1 cm2 piece, frozen in liquid nitrogen, and stored at −80 °C.
Measurements of respiration and ethylene production
For CO2 determination, an infrared gas analyzer in A CIRAS-1 intellectual photosynthesis parameter analyzer (PP Systems Ltd., UK) and opened gas flow system with 163 ml/min gas flow rate was employed. At least five fruit were individually determined for each ripening stage at 20 °C.
For ethylene determination, at least five single fruits were sealed in an air-tight jar for 1 h at 20 °C, and 1 ml of headspace gas was injected into a gas chromatograph (Unicam 610) equipped with a flame ionization detector (FIA-21) and an activated alumina column kept at 90 °C, using nitrogen as the carrier gas. 10.44 μl/L of ethylene was used as a standard.
Determination of chlorophyll, carotenoid and lycopene in fruit
Cut each fruit into six pieces along the vertical axis. Three interval pieces were cut into very small pieces and mix well. Grind two grams fresh weight fruit slices in precooled mortar with 5 ml hexane and acetone (60:40) and small amount of acid washed sand. Transfer the upper organic layer into a capped tube on ice. Re-extract the remaining aqueous layer with 5 ml of the same solvent repeatedly and transfer the organic layer to the same tube until the aqueous layer becomes colorless. Take 1 ml from the total volume of the organic extract for determining the absorbance at 450 nm, 502 nm, 643 nm and 663 nm respectively (OD450, OD502, OD645 and OD663 respectively) on spectrophotometer (Tomes, 1963; Kirk, 1968; Davies, 1976). Calculate the amount of each pigment in 1 ml sample with the following equations.
Chlorophyll (μg/ml)=(20.2×OD645)+(8.2×OD663)
Carotenoid (μg/ml)=4×OD450
Lycopene (μg/ml)=3.12×OD502
Measurement of fruit firmness
Thirty fruit at the same full-red stage without softening were harvested from each variety and stored at 20 °C, 85% RH (relative humidity) for 15 d. Five fruit were randomly selected for the measurement on day 0, 5, 10, 15 of storage using M series texture analyzer (J.J. Lloyd Instrument, Canada) as described by Jackman et al.(1990). Ten mm×10 mm pericarp samples with epidermal tissue intact were excised from the equatorial region of each tomato fruit and placed exocarp down on a steel plate and compressed with a 500-N load cell in conjunction with an 8-mm flat-ended cylindrical probe at a deformation speed of 5 mm/min. The bioyield force (Fm) (force to rupture) and deformation up to bioyield point (Lm) were obtained from force-deformation profiles. The firmness values were calculated as F m/L m.
RNA isolation and RNase protection assay (RPA)
Isolation of total RNA from tomato fruit was done according to Lashbrook et al.(1994). Template sequences for E8 gene probe transcription were synthesized by PCR from E8 gene cDNA (GenBank, No. X13437) as reported by Lincoln et al.(1987). Left and right Primers were 5′-TAGGAAAGCCCTAGAGTTG and 5′-TTAGATCTTGTAACGGGAC. Amplified 1092 bp fragments were inserted into vector PCR®2.1 with T7 RNA polymerase promoter. The templates for pTOM5 (encoding phytoene) and pTOM6 (encoding polygalacturonase) genes probe transcription were provided by BBSRC (University of Nottingham, UK). The plasmid containing cDNA templates was linearized with appropriate restriction enzymes. RNA probes were synthesized with the linearized plasmid templates, α-32P labelled UTP and in vitro transcript system with T7 RNA polymerase (Amersham). Rnase ONE™ (Promega) was used for RPA performed according to the manufacturer’s procedure.
Ten microgram of total RNA extracted from pericarp of each sample was employed for RPA. The band with the highest radioactive signal from each crossed probe was separately designated as a standard of 100% of each gene; Relative percentages of signal on the other bands were obtained by comparison with the standard of the same probe and calculated by Quantity One software (Bio-Rad).
RESULTS
Respiration and ethylene production
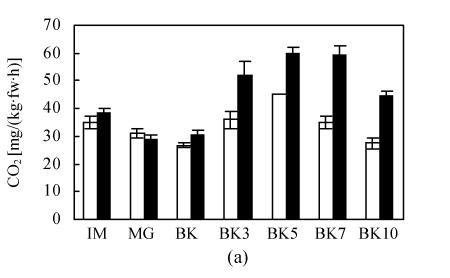
Before BK stage, there was no distinct difference of CO2 production between epi and VFN8 fruits. Once the fruit came into ripening stage, CO2 production of epi was constantly higher than that of VFN8 (Fig.1a). 50.9% more CO2 production at peak stage was observed in epi. However, no advance of climacteric peak was observed in epi compared with its wild type VFN8.
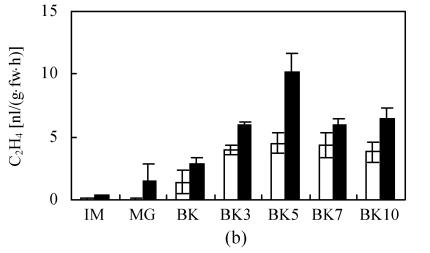
Fig. 1.
The production of CO2 (a) and C2H4 (b) during fruit growth and ripening of epi (black bars) and VFN8 (white bars). Mean±SE values were determined for five fresh harvested fruit
At every stage of fruit growth from IM to BK10, endogenous ethylene production in epi was always higher than that in VFN8 (Fig.1b). Peak level of ethylene production in both varieties was synchronized, occurring at 5 d after BK in consistence with CO2 production. At the peak stage, the epi fruit produced ethylene was two twice that of VFN8. This result integrating previous results further demonstrates that epi mutation overproduces ethylene throughout vegetative growth, fruit development and ripening (Wang and Ying, 2004).
Similar trends of ethylene and CO2 production during fruit development and ripening and a synchronized respiration peak between the two varieties confirmed that ethylene can intensify the ripening response and accelerate the ripening process, but can not trigger the ripening of immature fruit.
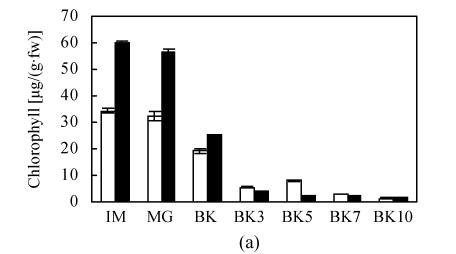
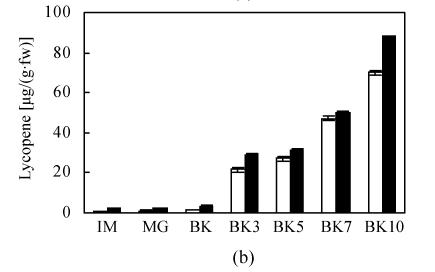
Transformation of pigments in fruits
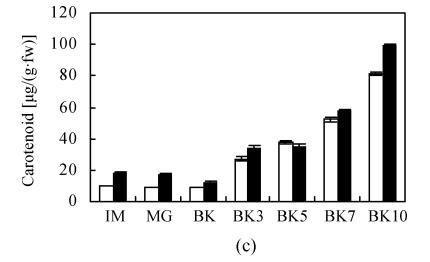
The transformation of main color components in fruit including chlorophyll, carotenoid, and lycopene conformed to the normal trend during development and ripening of epi and VFN8 fruit. The decrease pattern of chlorophyll and increase pattern of carotenoid and lycopene in epi were not significantly different from those of its wild type. The amount of carotenoid and lycopene at every ripening stage was just slightly higher in epi than in VFN8 (Fig.2). However, higher level of chlorophyll in IM fruit of VFN8 decreased at higher rate post MG stage; and carotenoid and lycopene accumulated to higher levels at the last stage investigated in epi than in its wild type.
Fig. 2.
The production of chlorophyll (a), lycopene (b), carotenoid (c) in epi (black column) and VFN8 (white column) during fruit ripening. Mean±SE values were determined for five fresh harvested fruit
Decreasing of firmness
The faster (compared to VFN8) decrease of the bioyield point of full-red epi fruit to a lower level during storage at 20 °C indicated that the firmness of epi fruit decreased more rapidly. If decrease by 50% of bioyield point was designated as the end of standard shelf life, the shelf life of epi was 4.5 d, which is less than half that of VFN8 (more than 10 d) (Fig.3). The rate of firmness drop significantly demonstrated that the accelerated ripening of epi fruit was caused by the mutation.
Fig. 3.
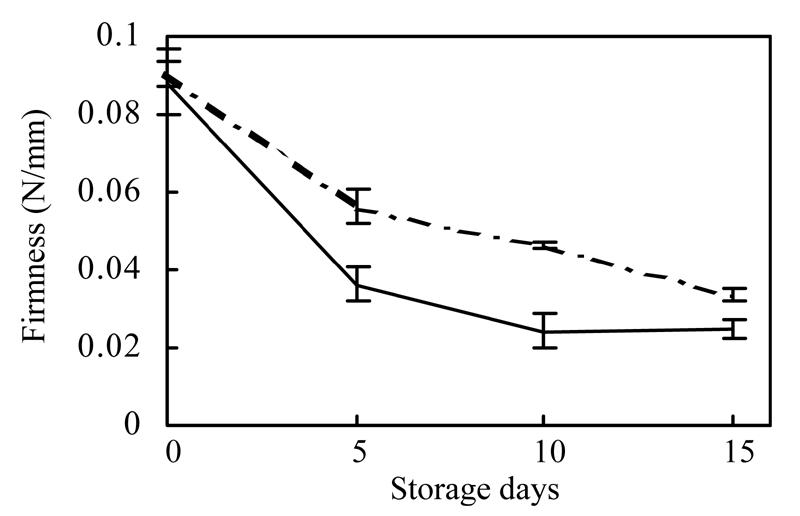
The firmness of epi (solid line) and VFN8 (broken line) fruits during 15 d of ripening at 22 °C. Mean±SE values were determined for five stored fruit
Expression of ripening-related gene
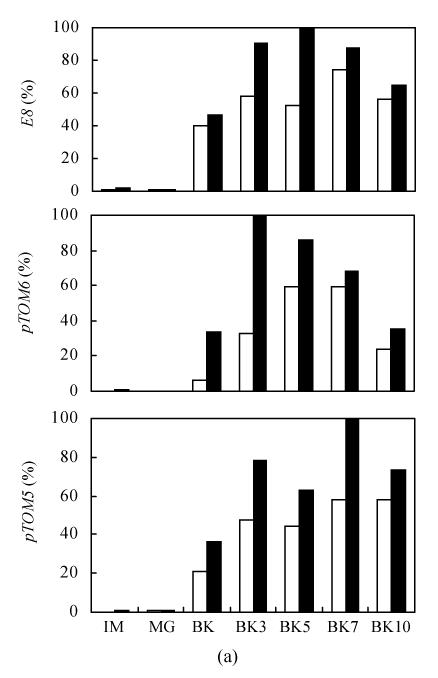
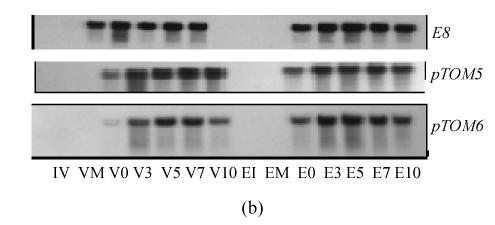
The expression of E8, pTOM5 and pTOM6 genes at mRNA level in epi and VFN8 showed similar pattern of rapid rise to a peak level from that before BK and then descending during fruit ripening. However, the timing of the peak level of mRNAs for each gene was not identical. The expression peaks of E8 and pTOM6 genes in epi and VFN8 were not synchronized while those of pTOM5 in the two varieties were synchronized. Three genes’ expressions at mRNA level in epi were all more than those in VFN8 at every ripening stage except IM and MG (Fig.4). The expression pattern of fruit ripening-related genes changes with fruit development and ripening and that of different genotypes are very consistent with the ethylene production (Fig.1b).
Fig. 4.
The expression of E8, pTOM5 and pTOM6 genes during VFN8 (white) and epi (black) fruit development and ripening (a) Relative percentage of expression (%); (b) Picture of hybridized bands exposure to X-film (V: VFN8; E: epi; I: Immature; M: Mature green; Number after V or E means days after BK stage)
DISCUSSION
Indicative phenotypes of a whole-plant response to ethylene have previously been reported in tomato. For example, transgenic plants constitutively expressing high levels of the ACC synthase gene, LeACS2, produce elevated levels of ethylene, resulting in leaf epinasty and rapid senescence and abscission of flowers (Lee et al., 1997). Similarly, antisense inhibition of the ethylene receptor LeETR4 results in a constitutive ethylene response phenotype that includes leaf epinasty, premature senescence, abscission of flowers, and early fruit ripening (Tieman et al., 2000). However, the phenotype of epi differs from that of other ethylene-related mutants in that only a subset of ethylene responses appears to be modified.
In this study we found that some ripening-related processes of epi fruit were accelerated in contrast to its wild type VFN8. Climacteric peak represented by ethylene and carbon dioxide production wasn’t advanced, but was significantly heightened (Fig.1). Coloration indicated by carotenoid and lycopene were speeded up (Fig.2) and firmness dropping represented by bioyield point (Fig.3) were notably accelerated. These changing trends were well consistent with the increase of ethylene production (Fig.1b). Furthermore, expression of some ripening-related genes, which were typically ethylene-regulated, were also enhanced (Fig.4). The expression peaks of E8 and pTOM6 genes in epi were advanced and pTOM5 expression of the two varieties synchronized. The earlier expression of pTOM6 gene is well correlated with the advance dropping of firmness. The lycopene and carotenoid accumulated to a higher level in epi than that in VFN8 until BK10, the last stage investigated, which is possibly related to the expression time of pTOM5 in epi. The synthesis of lycopene and carotenoid possibly lag behind the expression of their encoding genes.
Two possible causes are possibly responsible for the accelerated ripening characteristics of epi fruit. One is the increased endogenous ethylene production enhancing the ethylene response. Another is impaired ethylene signaling branches leading to constitutive ethylene response.
Our previous study revealed that leaf senescence was significantly delayed, and apical hook formation in dark grown seedlings was partially inhibited (Wang and Ying, 2004). These phenotypes are not consistent with normal ethylene response caused by increased ethylene, which suggests a reduced ability of ethylene signaling. Some sub-branches in ethylene signal transduction pathways in these vegetative tissues were possibly blocked by the epi mutation. However, the epi fruit ripening was not delayed but accelerated to some degree, which indicates at least that the ability of ethylene perception and signaling was not affected by the mutation.
Accumulated evidences suggested that the epi mutation possibly produced pleiotropic effects on tomato responses to ethylene. Some were constitutively activated, some were inhibited, and some were not affected. So that several subsets of ethylene response pathway might exist throughout the tomato vegetative growth and fruit development and ripening, and different subsets of ethylene responses may be regulated through different mechanisms. However, more work is needed to elucidate the hypothesis.
Footnotes
Project (No. 30371001) supported by the National Natural Science Foundation of China
References
- 1.Barry CS, Fox EA, Yen HC, Lee SH, Ying TJ, Grierson D, Giovannoni JJ. Analysis of the ethylene response in the epinastic mutant of tomato. Plant Physiol. 2001;127:58–66. doi: 10.1104/pp.127.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird CR, Ray JA, Fletcher JD, Boniwell JM, Bird AS, Teulieres C, Blain I, Bramley PM, Schuch W. Using antisense RNA to study gene function: inhibition of carotenoid biosynthesis in transgenic tomatoes. Bio Technology. 1991;9:635–639. [Google Scholar]
- 3.Davies BH. Carotenoids. In: Goodwin TW, editor. Chemistry and Biochemistry of Plant Pigments. Volume 2. New York, San Francisco: Academic Press London; 1976. pp. 38–165. [Google Scholar]
- 4.Deikman J, Kline R, Fischer RL. Organization of ripening and ethylene regulatory regions in a fruit-specific promoter from tomato (Lycopersicon esculentum) Plant Physiol. 1992;100:2013–2017. doi: 10.1104/pp.100.4.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujino DW, Burger DW, Yang SF, Bradford KJ. Characterization of an ethylene overproducing mutant of tomato (Lycopersicon esculentum Mill. cultivar VFN8) Plant Physiol. 1988;88:774–779. doi: 10.1104/pp.88.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujino DW, Burger DW, Bradford KJ. Ineffectiveness of ethylene biosynthetic and action inhibitors in phenotypically reverting the Epenastic mutant of tomato (Lycopersicon esculentum Mill.) J Plant Growth Regul. 1989;8:53–61. [Google Scholar]
- 7.Grierson D, Maunders MJ, Slater A, Ray J, Bird CR, Schuch W, Holdsworth GA, Knapp JE. Gene expression during tomato ripening. Philos Trans R Soc Lond-Biol Sci. 1986;314:399–410. [Google Scholar]
- 8.Guo H, Ecker JR. The ethylene signaling pathway: New insights. Current Opinion in Plant Biology. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Jackman RL, Marangoni AG, Stanley DW. Measurement of tomato fruit firmness. HortiScience. 1990;25:781–783. [Google Scholar]
- 10.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 11.Kirk JTO. Studies on the dependence of chlorophyll synthesis on protein synthesis in Euglena gracilis together with a nomognam for determination of chlorophyll concentration. Planta. 1968;78:200–207. doi: 10.1007/BF00406651. [DOI] [PubMed] [Google Scholar]
- 12.Lashbrook CC, Gonzalez-Bosch C, Bennett AB. Two divergent endo-b-1,4-glucanase genes exhibit overlapping expression in ripening fruit and abscising flowers. Plant Cell. 1994;6:1485–1493. doi: 10.1105/tpc.6.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KY, Baden C, Howie WJ, Bedbrook J, Dunsmuir P. Post-transcriptional gene silencing of ACC synthase in tomato results from cytoplasmic RNA degradation. Plant J. 1997;12:1127–1137. [Google Scholar]
- 14.Lincoln JM, Cordes S, Read E, Fischer RL. Regulation of gene expression by ethylene during Lycopersicon esculentum (tomato) fruit development. Proc Natl Acad Sci USA. 1987;84:2793–2797. doi: 10.1073/pnas.84.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moctezuma E, Smith DL, Gross KC. Effect of ethylene on mRNA abundance of three β-galactosidase genes in wild type and mutant tomato fruit. Postharvest Biology and Technology. 2003;28:207–217. [Google Scholar]
- 16.Tieman DM, Taylor MG, Ciardi JA, Klee HJ. The tomato ethylene receptors NR and LeETR4 are negative regulators of the ethylene response and exhibit functional compensation within a multigene family. Proc Natl Acad Sci USA. 2000;97:5663–5668. doi: 10.1073/pnas.090550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomes ML. Temperature inhibition of carotene synthesis in tomato. Bot Gaz. 1963;124:180–185. [Google Scholar]
- 18.Ursin VM. Morphogenetic and Physiological Analysis of Two Developmental Mutant of Tomato (Lycopersicon Esculentum Mill.), Epinastics and Iageotropic. Davis: University of Califorlia; 1987. [Google Scholar]
- 19.Ursin VM, Bradford KJ. Auxin and ethylene regulation of petiole epinasty in two developmental mutants of tomato, diageotropic and epinastic. Plant Physiol. 1989;90:1341–1346. doi: 10.1104/pp.90.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ZF, Ying TJ. Phenotypes analysis of ethylene response in tomato mutant Epinastics. J Plant Physiol and Mol Biol. 2004;30:27–33. (in Chinese) [PubMed] [Google Scholar]
- 21.Ying TJ, Zeng GW. Differential expression of ACC synthase and ACC oxidase gene families in furit of Epinastics, a tomato mutant overproducing ethylene. J Zhejiang University (Agricultural and life Sciences) 1999;25:458–490. (in Chinese) [Google Scholar]