Abstract
Catalytic wet air oxidation (CWAO) of o-chlorophenol in wastewater was studied in a stainless steel autoclave using four different Fe catalysts in the temperature range of 100–200 °C. Experimental results showed that high rate of o-chlorophenol and CODCr (Chemical Oxygen Demand, mg/L) removal by CWAO was obtained at relatively low temperature and pressure. The catalysts Fe2(SO4)3, FeSO4, Fe2O3 and FeCl3 all exhibited high catalytic activity. More than 93.7% of the initial CODCr and nearly 100% of o-chlorophenol were removed at 150 °C after 150 min with FeSO4 as catalyst. The CWAO of o-chlorophenol was found to be pseudo-first order reaction with respect to o-chlorophenol, with activation energy of 75.56 kJ/mol in the temperature range of 100–175 °C.
Keywords: Fe, o-chlorophenol, Catalytic wet air oxidation, Kinetic
INTRODUCTION
Chlorophenols (CPs) are chemicals widely used in industry to manufacture insecticides, herbicides, fungicides, biocides, and dye, are also found in the effluent of the pulp and paper mill industry, and are mainly man-made compounds, and have been recognized as organic pollutants of air, groundwater, soil and sediments. They are carcinogenic and stable in water. Most chlorophenols are listed as priority pollutants by the US Environmental Protection Agency (1988) due to their toxicity and persistence. Their fate in the environment is of great importance. Of particular interest is their reactivity by oxidation treatment, including wet air oxidation, ozonation, UV-Fenton oxidation and other advanced oxidation technologies.
Among all the wet oxidation technologies, WAO, which can mineralise pollutants at elevated temperature and pressure, has the ability to convert most organic and inorganic compounds into end products such as minerals, carbon dioxide and water (Shende and Mahajani, 1997; Santos et al., 2001). WAO has been demonstrated as a viable process for o-chlorophenol wastewater treatment (Lin et al., 1999; Lin and Wang, 1999; 2000; Lei and Wang, 2000). Practically, WAO can be implemented in a wide range of temperature and pressure. Temperature is recognized as the most important process parameter. Pressure is required to maintain the water in liquid state and provide an overpressure of oxygen. CWAO has been successfully applied for the treatment of wastewater discharged from various sources such as petroleum and petrochemical industries, and pulp and paper mills, and has become one of the attractive technologies for the treatment of wastewater containing a small amount of pollutants to be incinerated, while still containing high concentration of toxic materials for biological treatment carried out under extreme reaction conditions and involves high operation and installation costs. Therefore, the development of CWAO has been attempted in order to mitigate the severe reactions and enhance the treatment efficiency. CWAO reactions are of interest for reducing the high temperature and pressure requirements of conventional WAO processes. The partial or total replacement of oxygen with a stronger oxidant, such as hydrogen peroxide, also has been applied and is finding increasing use for enhancing the reaction rate (Lei and Wang, 2000). Homogeneous copper salts are known as widely effective catalysts for many kinds of organic compounds and are used in the practical wastewater treatment (Robert and Robert, 1985; Wu et al., 1987; Li et al., 1991; Lei and Wang, 2000; Wu et al., 2003), but the copper salts will cause heavy metal pollution after treatment, so it will be more interesting to choose iron salts or ferric oxide as catalysts in CWAO.
EXPERIMENTS AND METHODS
Chemicals
Fe2(SO4)3, FeSO4, Fe2O3 and FeCl3 (>99%, AR), o-chlorophenol (>98.5%, CR), methanol (reagent for HPLC, >99.9%). These reagents were purchased from Shanghai Chemical Reagents Company.
Batch experimental procedures
Reactions were carried out in a 1 L stainless steel autoclave equipped with automatic temperature control, an adjustable speed stirrer, a tachometer, a valve for sampling and a pressure gauge (Fig.1). Five-hundred ml wastewater and catalysts (when needed) were introduced into the autoclave, which was heated with an electrical heating jacket under the pressure of nitrogen, while a magnetic agitator continuously stirred the solution. After reaching the desired reaction temperature, oxygen was forced into the autoclave and the time was recorded as the starting point of the reaction. At appropriate time intervals, an aliquot of the solution was withdrawn, and samples after filtered and taken for analysis.
Fig. 1.

Schematic diagram of the experimental set-up
Analytical method
o-chlorophenol was measured by HPLC. Analysis parameters were as follows: Instrument: Waters High Performance Liquid Chromatography. Column: Nova-Pak C18, 4 μm, 3.9 mm×150 mm. Mobile phase: MeOH/H2O (80/20). Flow rate: 1.0 ml/min. Detector: UV at 254 nm. Column temperature: 35 °C. Sample size: 20 μl.
RESULTS AND DISCUSSION
Effect of addition of catalyst
Catalysts in the form of metal salt solution were added to the reactor to enhance the oxidation. Different Fe catalysts including Fe2(SO4)3, FeSO4, Fe2O3 and FeCl3 were tested. The amount of catalysts in terms of metal ion (Fe) concentration added to the reactor was 100 mg/L. Reaction conditions were kept constant at a temperature of 150 °C, and an oxygen partial pressure of 0.3 MPa throughout the test. The wastewater treatment results are shown in Fig.2 as CODCr and o-chlorophenol removal versus reaction time. Addition of a catalyst significantly enhanced, the efficiency of wastewater treatment. The highest CODCr removal achieved was about 93.7% after 150 min of reaction. Generally, the CODCr removals were approximately three times that obtained without adding catalyst under the same reaction conditions. The o-chlorophenol removals were determined as 86.7%, 75.0%, 63.7%, 55.2% and 30.8% at 5 min with FeSO4, FeCl3, Fe2O3, Fe2(SO4)3 and no other catalyst, respectively, and more than 99.9% o-chlorophenol was removed by CWAO after 30 min reaction. Among these four Fe catalysts, the activity order was FeSO4>FeCl3>Fe2O3>Fe2(SO4)3. However, in the absence of the above catalysts, CODCr removal was only 30.4% at the end of the treatment.
Fig. 2.
Effect of addition of catalysts on CWAO (a) o-CP; (b) CODCr
Initial conditions: CODCr,in, 2000 mg/L; P O2, 0.30 MPa at 25 °C; pH, 6.0; T=150 °C; Catalys: 100 mg/L (total metal content): ○: No other catalyst; □: Fe2(SO4)3; ◊: Fe2O3; ×: FeCl3; Δ: FeSO4
The effect of the amount of catalyst used
To obtain the optimal catalyst for CWAO, experiments were done with various amounts of Fe(II). Fig.3 shows the percentage degradation of CODCr and o-chlorophenol as a function of the amount of Fe(II) (FeSO4 was added) added. As expected, the CODCr and o-chlorophenol removal process was greatly expedited with increasing amount of iron salt. A CODCr removal of 93.7% was achieved after two and a half hour reaction time with Fe(II) level of 100 mg/L while only 53.3% of CODCr was removed when the dosage of Fe(II) dropped to 25 mg/L. The o-chlorophenol removal also could increase from 59.14% to over 99.98% after 30 min for different catalyst added.
Fig. 3.
Effect of catalysts dosage on CWAO (a) o-CP; (b) CODCr
Initial conditions: CODCr,in, 2000 mg/L; P O2, 0.30 MPa at 25 °C; pH, 6.0; T, 150 °C; FeSO4, total metal content: ○: No other catalyst; Δ: 25 mg/L; □: 50 mg/L; ◊: 100 mg/L
The influence of temperature
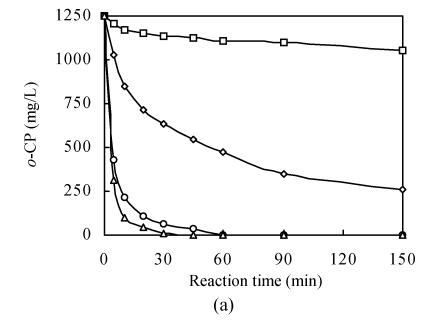
The treatment of o-chlorophenol wastewater by CWAO in the absence of FeSO4 was studied. This was conducted at temperature of 100 °C to 175 °C, and the oxygen partial pressure was kept at 0.30 MPa at a reference temperature of 25 °C. The progression of the oxidation process at various temperatures with CWAO is shown in Fig.4 showing that the catalytic reaction rate can be considerably increased with increasing temperature increasing. The o-chlorophenol removal rate can be increased from less than 10% to over 99% after 30 min, and the final CODCr removal level can be increased from around 10.5% to 73.6% with the reaction temperature of 100 °C to 175 °C.
Fig. 4.
Effect of temperature on CWAO (a) o-CP; (b) CODCr
Initial conditions: CODCr,in, 2000 mg/L; P O2, 0.30 MPa at 25 °C; pH, 6.0; FeSO4, total metal content 50 mg/L; T (°C): □: 100; ◊: 125; ○: 150; ∆: 175
Reaction kinetics
If the initial oxygen concentration is much greater than initial o-chlorophenol concentration, the oxygen concentration can be considered practically invariable during the reaction, so it is possible to approximate the expression to pseudo-first order given the equation:
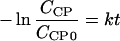 |
(1) |
As shown in Table 1, in all the experiments, initial oxygen concentration was fixed at great excess over the stoichiometric concentration needed to oxidize o-chlorophenol completely. Thus, the rate constant (k) can be calculated from the slope of the straight line obtained when ln(C CP /C CP0) is represented versus time in the period in which rapid oxidation occurs.
Table 1.
Summary of the experiment
| Run | Catalyst | Fe (mg/L) | T (°C) | k (min−1) |
| 1 | No catalyst | 0 | 150 | 0.0278 |
| 2 | FeSO4 | 100 | 150 | 0.2926 |
| 3 | FeCl3 | 100 | 150 | 0.2468 |
| 4 | Fe2O3 | 100 | 150 | 0.2505 |
| 5 | Fe2(SO4)3 | 10 | 150 | 0.2367 |
| 6 | FeSO4 | 25 | 150 | 0.0643 |
| 7 | FeSO4 | 50 | 150 | 0.0936 |
| 8 | FeSO4 | 50 | 100 | 0.0030 |
| 9 | FeSO4 | 50 | 125 | 0.0219 |
| 10 | FeSO4 | 50 | 175 | 0.1441 |
Following this procedure, k values were calculated for all temperature studied. These values are presented in Table 1, showing that k increases with the increasing temperature.
Assuming that k has an Arrhenius behaviour, the activation energy, E a, can be calculated by linear fitting of lnk versus 1/T:
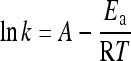 |
(2) |
where E a is the activation energy (kJ/mol), A the pre-exponential factor, k the observed pseudo-first-order reaction rate constant (min−1), R the universal gas constant (8.314 J/(mol·K)), and T is the reaction temperature (K).
The effect of reaction temperature on the effectiveness of the removal efficiency was studied by varying the reaction temperature from 373 to 448 K. By rearranging the experiment data shown in Fig.4, a linear decrease of ln(C CP/C CP0) with time is obtained. This linear relationship reveals a pseudo-first-order reduction reaction regarding the removal efficiency. As a result, the reaction rate constants (k) were determined to be 0.003, 0.0219, 0.0936, and 0.1441 min−1, respectively, with a reaction temperature of 100, 125, 150 and 175 °C (Table 1).
As seen in Table 1, an increase in the reaction temperature can enhance the reaction rates significantly. The k values calculated at various temperatures were correlated by Eq.(1) and Eq.(2) and shown in Fig.5 indicating an estimated activation energy of 75.56 kJ/mol at the temperature range of 373–448 K.
| lnk=18.16−8847.9/T | (3) |
Fig. 5.
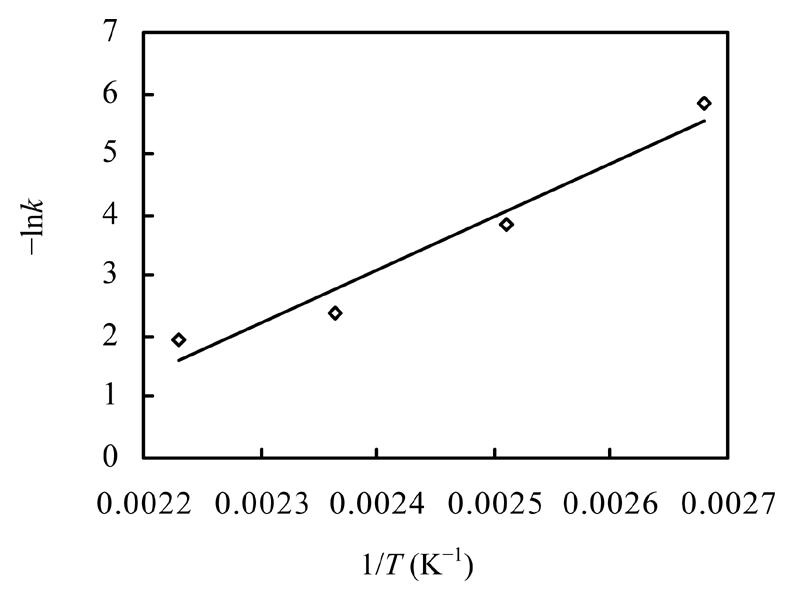
Correlation for reaction rate constant k
CONCLUSION
The experimental results of CWAO of o-chlorophenol showed that at low iron concentration exhibited high catalytic activity at relatively low temperature and pressure; and that FeSO4 has higher catalytic efficiency than other Fe catalysts, Fe2(SO4)3, Fe2O3 and FeCl3. More than 93.7% of the initial CODCr and nearly 100% of o-chlorophenol were removed at 150 °C in 150 min with FeSO4 as catalyst. The CWAO of o-chlorophenol was found to be pseudo-first order reaction with respect to o-chlorophenol. The activation energy was found to be 75.56 kJ/mol at the temperature range of 100–175 °C.
Footnotes
Project (No. 20407015) supported by the National Natural Science Foundation of China
References
- 1.Lei L, Wang D. Wet oxidation of PVA-containing desizing wastewater. Chinese J of Chem Eng. 2000;8(1):52–56. (in Chinese) [Google Scholar]
- 2.Li L, Chen P, Earnest F. Generalized kinetic model for wet oxidation of organic compounds. AIChE Journal. 1991;37(11):1687–1697. [Google Scholar]
- 3.Lin KS, Wang HP. Rate enhancement by cations in supercritical water oxidation of 2-chlorophenol. Environ Sci Technol. 1999;33(18):3278–3280. [Google Scholar]
- 4.Lin KS, Wang HP. Byproduct shape selectivity in supercritical water oxidation of 2-chlorophenol effected by CuO/ZSM-5. Langmuir. 2000;16(6):2627–2631. [Google Scholar]
- 5.Lin KS, Wang HP, Yang YW. Supercritical water oxidation of 2-chlorophenol effected by Li+ and CuO/zeolites. Chemosphere. 1999;39(9):1385–1396. [Google Scholar]
- 6.Robert K, Robert BC. Toxicity to daphnia of the end products of wet oxidation of phenol and substituted phenols. Water Res. 1985;19(6):767–772. [Google Scholar]
- 7.Santos A, Yustos P, Durban B, Carcia-Ochoa F. Catalytic wet air oxidation of phenol: Kinetics of the mineralization rate. Industrial Engineering and Chemical Research. 2001;40:2773–2781. [Google Scholar]
- 8.Shende RV, Mahajani VV. Kinetics of wet oxidation of formic acidandacetic acid. Industrial Engineering and Chemical Research. 1997;36:4809–4814. [Google Scholar]
- 9.US Environmental Protection Agency. National Pollutant Discharge Elimination System, Code of Federal Regulations, 40, Part 122. Washington, DC: US Government Printing Office; 1988. [Google Scholar]
- 10.Wu YC, Hao OJ, Olmstead DG, Hsieh KP, Scholze RJ. Wet air oxidation of anaerobically digested sludge. J WPCF. 1987;59(11):39–45. [Google Scholar]
- 11.Wu Q, Hu XJ, Yue PL. Kinetics study on catalytic wet air oxidation of phenol. Chemical Engineering Science. 2003;58:923–928. [Google Scholar]