Abstract
The interaction in two mixtures of a nonionic surfactant AEO9 (C12H25O(CH2CH2O)9H) and different ionic surfactants was investigated. The two mixtures were AEO9/sodium dodecyl sulfate (SDS) and AEO9/cetyltrimethylammonium bromide (CTAB) at molar fraction of AEO9, α AEO9=0.5. The surface properties of the surfactants, critical micelle concentration (CMC), effectiveness of surface tension reduction (γ CMC), maximum surface excess concentration (Γ max) and minimum area per molecule at the air/solution interface (A min) were determined for both individual surfactants and their mixtures. The significant deviations from ideal behavior (attractive interactions) of the nonionic/ionic surfactant mixtures were determined. Mixtures of both AEO9/SDS and AEO9/CTAB exhibited synergism in surface tension reduction efficiency and mixed micelle formation, but neither exhibited synergism in surface tension reduction effectiveness.
Keywords: Nonionic surfactant, Nonionic-ionic mixed surfactants, Molecular interaction parameter, Synergism, Mixed micelle, Mixed adsorption film
INTRODUCTION
Long chain nonionic surfactants such as polyoxyethylenated alcohols C12H25O(CH2CH2O)9H (AEO9), are widely used in the chemical industry in fabric detergency and tertiary oil recovery due to their excellent surface-active properties. In many practical applications, surfactants are used in formulations containing mixtures of different compounds, and synergism can often be observed. Synergism is defined here as the condition in which the properties of a mixture are better than those attainable with the individual components separately. An important mixed system is that including ionic surfactants and nonionic surfactants (Gharibi et al., 2000; Goloub et al., 2000; Hou et al., 2000; Maeda, 1995; Matsubara et al., 2001; Rosen and Zhou, 2001; Shivaji Sharma et al., 2003; Zana et al., 1998).
This work aimed at investigating molecular interaction in two mixtures of nonionic surfactant AEO9 with two different ionic surfactants and at searching for synergism. In this paper, sodium dodecyl sulfate (SDS) and cetyltrimathylammonia bromide (CTAB) were chosen as the components for the two mixtures studied: AEO9/SDS and AEO9/CTAB.
EXPERIMENTAL DETAILS
Materials
AEO9 was obtained from Zhejiang Huangma Co., Ltd. SDS was procured from Fluka Chemical Co. and was recrystallized three times from mixed solvents of water and ethanol before use. CTAB was obtained from Nanjing Robiot Co., Ltd. and was recrystallized three times from mixed solvents of acetone and ethanol before use. Water was deionized and doubly distilled.
Surface tension measurements
The critical micelle concentration (CMC) values of the surfactants were determined from the surface tension isotherms as per the National standard of the PRC “Anionic and non-ionic surface active agents–The critical micellization concentration was determined by measuring surface tension with the ring” GB/T 11278-1989 test method. The surface tension, γ, of aqueous solutions of the surfactants was measured by using a JYW 2000A automatic tensiometer equipped with a du Nouy Pt-Ir ring. The measurements were performed at 20 °C. Sets of measurements were taken until no significant change of tension occurred.
RESULTS AND DISCUSSION
Surface properties of the surfactants mixtures
The relationships of surface tension vs log molar concentration for the two mixtures of AEO9/SDS and AEO9/CTAB at α AEO9=0.5 are shown in Fig.1 and Fig.2, where α AEO9 is the molar fraction of AEO9 in the total surfactant in the solution phase. The surface tension data allow us to calculate the maximum surface excess concentration, Γ max, at the air/water interface based on the Gibbs adsorption equation
| dγ/dlna=−RTΓmax | (1) |
Fig. 1.

Surface tension isotherms for mixture of AEO9/SDS (α AEO9=0.5), AEO9 and SDS
Fig. 2.

Surface tension isotherms for mixture of AEO9/CTAB (α AEO9=0.5), AEO9 and CTAB
where R=8.314 J/(mol·K), γ is the surface tension in mN/m, T is absolute temperature, a is the activity (at low surfactant concentration may be replaced by concentration). Surface excess may be determined from the slope of the surface tension isotherm.
The average minimum area per molecule (A min) occupied by one compound molecule in the surface layer (assuming the layer is monomolecular) can be obtained from the equation
| Amin=1/(ΓmaxNA) | (2) |
where NA is Avogadro constant. The values of CMC, γ CMC (the surface tension at the CMC), Γ max and A min of these mixtures obtained in this way are presented in Table 1 together with those of individual surfactants. Larger surface activity (values of larger Γ max, smaller CMC) of the AEO9/SDS mixture than that of the AEO9/CTAB mixture is apparent.
Table 1.
Surface properties of AEO9/SDS and AEO9/CTAB mixtures (α AEO9=0.5) and individual surfactants
| System | CMC (mol/m3) | γCMC (mN/m) | Γmax (10−6 mol/m²) | Amin (10−20 m2) | Ideal Amin (10−20 m2) |
| AEO9/SDS | 0.11 | 29.8 | 3.0 | 56 | 71 |
| AEO9/CTAB | 0.135 | 30.1 | 2.5 | 66 | 72 |
| AEO9 | 0.10 | 29.7 | 2.3 | 73 | |
| SDS | 7.0 | 32.3 | 3.1 | 53 | |
| CTAB | 0.90 | 35.1 | 2.6 | 63 |
Surfactant-surfactant interaction
The nature and extent of the interaction between the two different surfactant molecules can be measured by the molecular interaction parameters β, which can be evaluated using Eqs.(3)–(6), which are based upon the application of nonideal solution theory to the thermodynamics of the system (Rosen, 1989). Negative β values indicate attractive interaction and positive values, repulsive interaction. The molecular interaction parameter for mixed adsorption film formation at the air/water interface, βσ, is calculated by using the following equations (Li and Zhao, 2003; Rosen, 1989; Zhao, 1991).
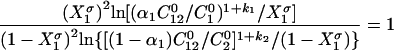 |
(3) |
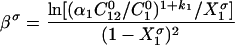 |
(4) |
where α 1 is the molar fraction of surfactant 1 in the total surfactant in the solution phase; X 1 σ is the molar fraction of surfactant 1 in the total surfactant in the mixed adsorption film; k 1 and k 2 are the counterion association degree on the micelle of surfactants 1 and 2; C 1 0, C 2 0, and C 12 0 are the solution phase molar concentrations of surfactants 1, 2 and their mixture, respectively, required to produce a given surface tension value.
Similarly, the value of βm, the interaction parameter for mixed micelle formation in an aqueous solution, is calculated from the following two equations (Rosen, 1989; Li and Zhao, 2003; Zhao, 1991)
 |
(5) |
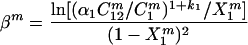 |
(6) |
where X 1 m is the molar fraction of surfactant 1 in the total surfactant in the mixed micelle; C 1 m, C 2 m, and C 12 m are the critical micelle concentrations of individual surfactants 1 and 2, and their mixture, respectively, at a given value of α 1. Eq.(3) (or Eq.(5)) is solved iteratively for X 1 σ (or X 1 m) and substitution of this in Eq.(4) (or Eq.(6)) yields the value of βσ (βm).
In this paper, surfactant 1 is AEO9 and surfactant 2 is CTAB or SDS. From literature (Rosen, 1989; Li and Zhao, 2003; Zhao, 1991), we had k SDS=0.67, k AEQ9=0 and k CTAB=0.67. Data of the β values for the two mixtures are listed in Table 2. As seen from the tabulated data, both βσ and βm values are negative, showing attractive interaction between these surfactant molecules. The larger βσ and βm values for the AEO9/SDS mixture than those for the AEO9/CTAB mixture indicate attractive interaction in the AEO9/SDS mixture larger than that in the AEO9/CTAB mixture.
Table 2.
Molecular interaction and synergism parameters for AEO9/SDS and AEO9/CTAB mixtures (α AEO9=0.5)
| Mixture | βσ | Xσ | ln(C10 /C20), A | βm | Xm | ln(C1m /C2m), B | βσ−βm | A−B |
| AEO9/SDS | −7.2 | 0.92 | 5.1 | −10.0 | 0.805 | 4.2 | 2.8 | 0.9 |
| AEO9/CTAB | −4.0 | 0.91 | 3.3 | −4.3 | 0.8 | 2.2 | 0.3 | 1.1 |
For the mixed system, if there is no interaction between the two components composing the mixed adsorption film, the ideal molecular cross-sectional area of the mixed adsorption film can be calculated by the following equation (Rosen and Sulthana, 2001)
 |
(7) |
where A min,1 and A min,2 are the cross-sectional areas per molecule of the first individual surfactant and the second surfactant, respectively, and X 1 σ is the molar fraction of surfactant 1 in the mixed adsorption film. The observed value (A min) and ideal mixing value (ideal A min) are given in Table 1. The data revealed that the mixtures of AEO9/SDS and AEO9/CTAB both have an A min value considerably lower than the value obtained upon ideal mixing. The lowered A min value indicates a significant attractive interaction between the two components in the two mixtures. This fact is reflected in the βσ and βm values obtained for the two mixtures. The deviation from ideal mixing, in general, increases with increasing difference between the head group cross-sectional areas (Bergström and Eriksson, 2000). Thus, the interaction in the AEO9/SDS mixture being greater than that in the AEO9/CTAB mixture reflects that the difference in the cross-sectional area between the two components in the former is larger than that in the latter.
Table 2 shows that the molar fraction of AEO9 in bulk solution is smaller than that in the mixed micelles and the mixed adsorption film. From the structure of the two surfactants (AEO9 and SDS or CTAB) and the micellar character, it appears that incorporation of SDS or CTAB into AEO9 micelles is favored over incorporation of AEO9 into SDS or CTAB micelles.
The compositions of the mixed adsorption film and the mixed micelle for the two mixtures obtained by Eq.(3) and Eq.(5) are approximately kept at 9:1 and 4:1 molar ratio for AEO9:SDS or CTAB, indicating that the major portion of higher surface active AEO9 molecules is continuously rearranged in these aggregates.
Synergism
The existence of synergism in mixtures containing two surfactants has been shown to depend not only on the strength of the interaction between them (measured by the values of the β parameter) but also on the relevant properties of the individual surfactant components of the mixture. Thus, the conditions for synergism in surface tension reduction efficiency (when the total concentration of the mixed surfactant required to reduce the surface tension of the solvent to a given value is less than that of either individual surfactant) are as follows: (a) βσ must be negative and (b) |βσ|>|ln(C 1 0/C 2 0)| (Rosen, 1989). From data in Table 2, it is apparent that the AEO9/SDS mixture exhibits synergism in surface tension reduction efficiency, since the βσ value is −7.2 and the |ln(C 1 0/C 2 0)| value is 5.1. The AEO9/CTAB mixture also exhibits synergism in surface tension reduction efficiency, since the βσ value is −4.0 and the |ln(C 1 0/C 2 0)| value is 3.3.
Synergism in the mixed micelle formation exists when the CMC of a mixture is less than that of individual surfactants among the mixture. The conditions for synergism to exist in the mixture are as follows: (a) βm must be negative; (b) |βm|>|ln(C 1 m/C 2 m)| (Rosen, 1989). The mixture of AEO9/SDS exhibits synergism in mixed micelle formation, since the βm value is −10.0 and the |ln(C 1 m/C 2 m)| value is 4.2. The mixture of AEO9/CTAB also exhibits synergism, since the βm value is −4.3 and the |ln(C 1 m/C 2 m)| value is 2.2.
Synergism in surface tension reduction effectiveness (when the surface tension of the mixture at its CMC is lower than that of the individual surfactants at their respective CMC) depends upon the value of both βσ and βm, in addition to the C 1 0, C 2 0, C 1 m and C 2 m values of the two surfactants. The conditions for this type of synergism to exist are (a) (βσ−βm) must be negative; (b) |βσ−βm|>|ln(C 1 0 C 2 m/C 2 0 C 1 m)| (Rosen, 1989). The two mixtures of AEO9/SDS and AEO9/CTAB does not exhibit this type of synergism, since (βσ−βm) is positive (Table 2).
CONCLUSION
Interaction in two mixtures of a nonionic surfactant AEO9 (C12H25O(CH2CH2O)9H), and sodium dodecyl sulfate (SDS) or cetyltrimethylammonium bromide (CTAB) at α AEO9=0.5 were investigated. The conclusions were as follows:
1. The AEO9/SDS and AEO9/CTAB mixtures both exhibit synergism in surface tension reduction efficiency and mixed micelle formation, whereas neither exhibits synergism in surface tension reduction effectiveness.
2. The AEO9/SDS and AEO9/CTAB mixtures both show packing contraction at the air/water interface, and the former mixture has larger surface activity.
Footnotes
Project (No. 2004C31058) supported by the National Natural Science Foundation of China
References
- 1.Bergström M, Eriksson JC. A theoretical analysis of synergistic effects in mixed surfactant systems. Langmuir. 2000;16:7173–7181. [Google Scholar]
- 2.Gharibi H, Razavizadeh BM, Hashemianzaheh M. New approach for the studies of physicochemical parameters of interaction of Triton X-100 with cationic surfactants. Colloids Surf A Physicochem Eng Asp. 2000;174:375–386. [Google Scholar]
- 3.Goloub TP, Pugh RJ, Zhmud BV. Micellar interactions in nonionic/ionic mixed surfactant systems. J Colloid Interface Sci. 2000;229:72–81. doi: 10.1006/jcis.2000.6954. [DOI] [PubMed] [Google Scholar]
- 4.Hou ZS, Li ZP, Wang HQ. The interaction of sodium dodecyl sulfonate and petroleum sulfonate with nonionic surfactants (Triton X-100, Triton X-114) Colloids Surf A Physicochem Eng Asp. 2000;166:243–249. [Google Scholar]
- 5.Li XZ, Zhao JX. Effect of quaternary ammonium gemini surfactant C12-6-C12 2Br on the micellization of SDS in aqueous solution. Chin J Appl Chem. 2003;20(7):690–692. (in Chinese) [Google Scholar]
- 6.Maeda H. A simple thermodynamic analysis of the stability of ionic/nonionic mixed micelles. J Colloid Interface Sci. 1995;172:98–105. [Google Scholar]
- 7.Matsubara H, Muroi S, Kameda M, Ikeda N, Ohta A, Aratono M. Interaction between ionic and nonionic surfactants in the adsorbed film and micelle. 3. sodium dodecyl sulfate and tetraethylene glycol monooctyl ether. Langmuir. 2001;17:7752–7757. [Google Scholar]
- 8.Rosen MJ. Surfactants and Interfacial Phenomena. 2nd Ed. New York: Wiley-Interscience; 1989. pp. 393–419. [Google Scholar]
- 9.Rosen MJ, Zhou Q. Surfactant-surfactant interactions in mixed monolayer and mixed micelle formation. Langmuir. 2001;17:3532–3537. [Google Scholar]
- 10.Rosen MJ, Sulthana SB. The interaction of alkylglycosides with other surfactants. J Colloid Interface Sci. 2001;239:528–534. doi: 10.1006/jcis.2001.7537. [DOI] [PubMed] [Google Scholar]
- 11.Shivaji Sharma K, Rodgers C, Palepu RM, Rakshit AK. Studies of mixed surfactant solutions of cationic dimeric (gemini) surfactant with nonionic surfactant C12E6 in aqueous medium. J Colloid Interface Sci. 2003;268:482–488. doi: 10.1016/j.jcis.2003.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Zana R, Lévy H, Kwetkat K. Mixed micellization of dimeric (gemini) surfactants and conventional surfactants. I. Mixtures of an anionic dimeric surfactant and of the nonionic surfactants C12E5 and C12E8 . J Colloid Interface Sci. 1998;197:370–376. doi: 10.1006/jcis.1997.5248. [DOI] [PubMed] [Google Scholar]
- 13.Zhao GX. Surfactants Physical Chemistry. Beijing: Peking University Press; 1991. pp. 32–47. (in Chinese) [Google Scholar]


