Abstract
The ZM-1 tissue microarrayer designed by our groups is manufactured in stainless steel and brass and contains many features that make TMA (tissue microarray) paraffin blocks construction faster and more convenient. By means of ZM-1 tissue microarrayer, biopsy needles are used to punch the donor tissue specimens respectively. All the needles with the punched specimen cylinders are arrayed into the array-board, with an array of small holes dug to fit the needles. All the specimen cylinders arraying and the TMA paraffin block shaping are finished in only one step so that the specimen cylinders and the paraffin of the TMA block can very easily be incorporated and the recipient paraffin blocks need not be made in advance, and the paraffin used is the same as that for conventional pathology purpose. ZM-1 tissue microarrayer is easy to be manufactured, does not need any precision location system, and so is much cheaper than the currently used instrument. Our method’s relatively cheap and simple ZM-1 tissue microarrayer technique of constructing TMA paraffin block may facilitate popularization of the TMA technology.
Keywords: Tissue microarray (TMA), Construction technique, Method
INTRODUCTION
Biochip is developed since the 1990s high-throughput technology tool developed for molecular biology research in the biological technology revolution (Watson and Akil, 1999) that closely followed the advent of Large Scale Integrated circuit. Biochip is usually divided into several different kinds, such as gene chip, protein chip, cell chip, microbiology chip, tissue chip and so on. In 1998, Kononen et al.(1998) reported the technology of tissue chip: tissue microarray (TMA) (Frantz et al., 2001). TMA technology (Kononen et al., 1998) is the miniaturized collection of arrayed tissue spots on a microscope glass slide providing a template for highly parallel localization of molecular targets, either at the DNA, RNA or protein level. It permits high-throughput in situ analysis of specific molecular targets in hundreds or thousands of tissue specimens at a time (Rimm et al., 2001). TMA provides a link between molecular targets and tissue or cell morphology, as well as with the clinical data associated with the specimens (Bubendorf, 2001; Fuller et al., 2002; Mousses et al., 2002; Simon and Sauter, 2002).
The key TMA technology is the construction of its paraffin blocks. Although the equipments such as Manual Tissue Arrayer (MTA-II, Beecher Instrument, Silver Spring, MD, USA) can be an adminicle (Gulmann et al., 2003a; Jubb et al., 2003), the process of TMA paraffin block construction is dependent on manual manipulation. Using a pair of needles, researchers have to repeat sampling a tissue specimen cylinder and put it into the hole in the recipient block that was punched in advance one by one (Gulmann et al., 2003b). This manual procedure has to be repeated many times for large scale study. So that TMA construction is not efficient, the TMA paraffin block is not of reliable quality. In addition, before the construction, a recipient paraffin block should be made ahead of the schedule, and higher paraffin quality is required in case of its chapping. The quality of a TMA paraffin block is affected directly by the performance level of the technician. Current equipment (MTA-II) for TMA paraffin block construction entails a set of exactly precise “location system”, which is very expensive. All the problems mentioned above have been limiting the popularization of the TMA technology. Therefore the efficiency of TMA paraffin block construction is the bottle neck of this technology. In order to overcome the difficulties mentioned above, our group designed a new set of instruments, ZM-1 tissue microarrayer, using a new constructing technique devised by us (Pat. App. No. 200410025645.7; Pat. App. No. 200420037020.8). In this method, the recipient paraffin block need not be made in advance, and the paraffin used to make a TMA block is the same as that for routine pathological purpose. The ZM-1 tissue microarrayer making the TMA block without the “location system” is easier to manufacture and is of lower cost and technician can easily operate it. The needles sample the tissue specimen cylinders individually, and put them into an array-board instead of putting them in high quality paraffin recipient block with array “holes”. The dispensing with all the tissue specimen cylinders and the shaping of the TMA paraffin block are finished in one step. Therefore, using ZM-1 tissue microarrayer, not only reduces the cost of TMA paraffin blocks construction, but the efficiency of the TMA paraffin blocks construction and the quality of its paraffin block are also improved dramatically.
DEVELOPMENT OF ZM-1 TISSUE MICROARRAYRER
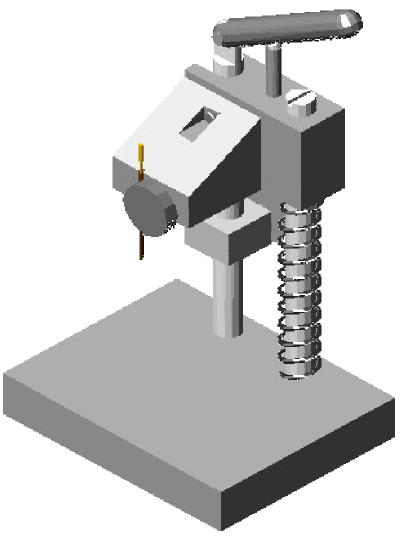
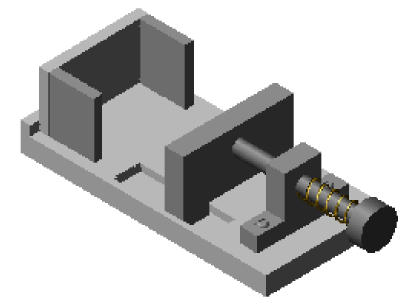
The ZM-1 tissue microarrayer for TMA paraffin blocks construction includes three parts: a series of biopsy needles with stylets, the biopsyer and the TMA paraffin block shaper (Figs.1~6).
Fig. 1.
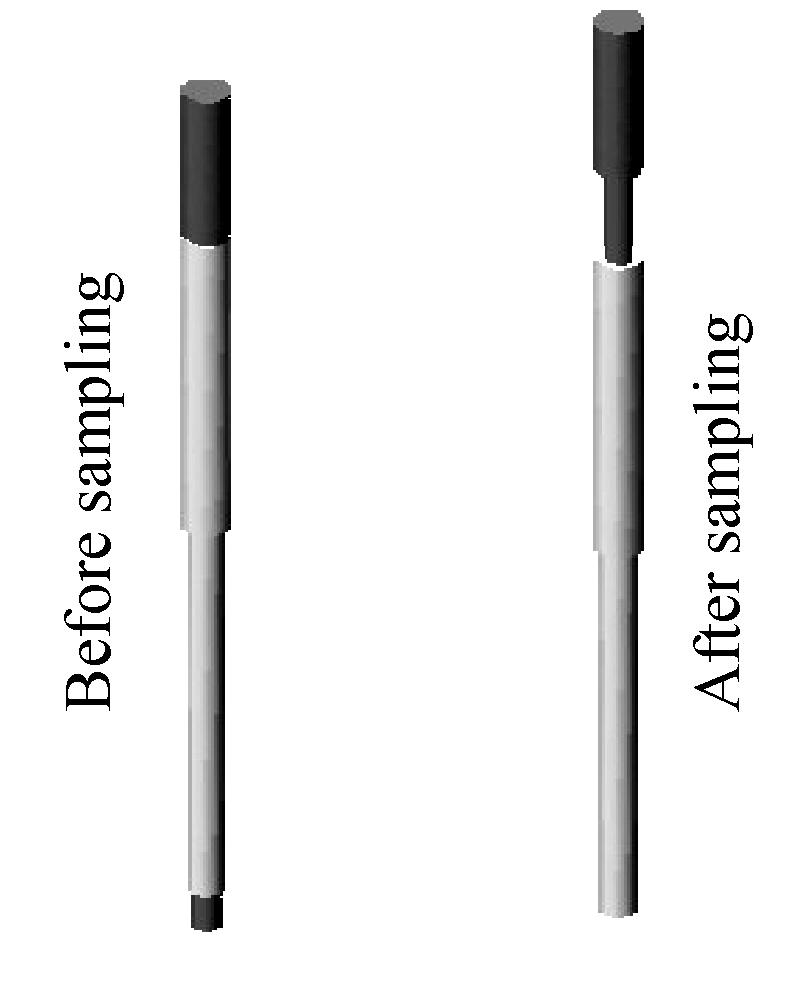
Biopsy needle
Fig. 6.
TMA paraffin block shaper with the array-board
Biopsy needles: A biopsy needle is only used to sample one tissue specimen every time for construction. The number of the biopsy needles is equal to the number of tissues in a TMA paraffin block. All biopsy needles are made of stainless steel. Each biopsy needle (Fig.1) has a stylet, which is 1 mm longer than its needle then the specimen cylinder can be pushed into the paraffin when it is still in semi-liquid state during disposal. During the whole process of sampling and disposal, the stylets always keep together with their needles. After sampling the tissue specimens, the stylets are propped up correspondingly, and the length of the stylets moving up is equal to the length of the sampled tissue specimen cylinders. There are several kinds of biopsy needles designed with different inner diameter (0.5~2.0 mm).
Biopsyer: As shown in Fig.2, it is made of stainless steel, and includes a framework system and a set for fixing the biopsy needles. The biopsyer is easy to be manufactured and manipulated. Using it, the biopsy needles can easily be loaded and unloaded and the sampling process is easy to implement.
Fig. 2.
Biopsyer
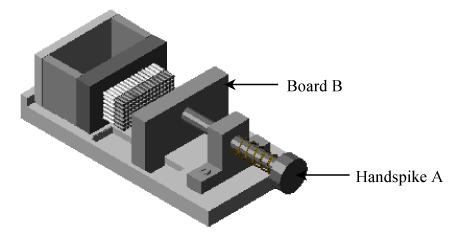
The TMA paraffin block shaper (Figs.3~6) includes a framework system, the “handspike A” for controlling the movement of the “board B”, the “board B” for pushing all the stylets forward together while shaping a TMA paraffin block, the array-board and three pieces of small boards for paraffin block shaping. All parts of the shaper are made of stainless steel except for the array-board, which is made of brass. The array-board is the key part of the TMA paraffin block shaper, in which there is an array of small holes fitting the diameter of the biopsy needles. The array of small holes was dug at the same time when the array-board was made. Different kinds of array-boards with different sizes of small holes corresponded to the kind of needles respectively. As shown in Fig.3, after sampling the tissue specimens, all the needles with the sampled specimen cylinders and the stylets propped up are arrayed into the small holes in the array-board. The thickness of the array-board is equal to the length of the fore part of the needles, and the diameter of the array-board holes fits the fore part of the needles. Thus, all the parts of needles and their stylets are located outside of the array-board except the fore parts of the needles. The array-board is mobile and can be placed flatly so that the needles with sampled specimen cylinders are easily arrayed.
Fig. 3.
The array-board arrayed the biopsy needles with specimens
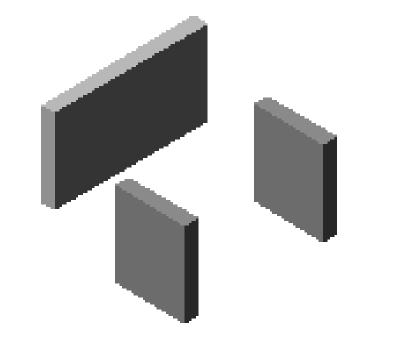
Fig.4 shows the three small stainless steel boards used as the container for TMA paraffin block shaping.
Fig. 4.
The three small stainless steal boards for the TMA paraffin block shaping
Fig.5 shows the TMA paraffin block shaper without the array-board, while Fig.6 shows the TMA shaper, in which the array-board with the sampled specimen cylinders has been fixed already.
Fig. 5.
TMA paraffin block shaper without the array-board
The new technique for constructing the TMA paraffin blocks by ZM-1 tissue microarrayer, step by step is as follows:
1. Select the tissue specimens paraffin blocks.
2. A fresh section was cut from each donor block and stained with hematoxylin and eosin (H & E) to define representative tumor regions and to guide the sampling from morphologically representative regions of the tissues (Kononen et al., 1998; Kallioniemi et al., 2001).
3. A series of needles designed by our group to punch the tissue specimen block cylinders respectively instead of repeatedly doing it only by using one pair of biopsy needles. As shown in Fig.3, every time after the tissue specimens are punched, the series of needles with specimen cylinders are inserted into the small holes located in the array-board as the design of TMA.
4. The array-board is fixed into the TMA paraffin block shaper (Figs.5~6), and melted liquid state paraffin is poured into the container for TMA paraffin block formation in the TMA shaper.
5. The “handspike A” was pushed to make the “board B” drive all the stylets forward at the same time, so that all the tissue specimen cylinders were thus pushed together into the semi-liquid paraffin. After the semi-liquid paraffin cooled down completely to solid, the array-board and the three small metal boards were unloaded, and a TMA paraffin block was then constructed and made ready for sectioning.
APPLIED STUDY OF ZM-1 TISSUE MICROARRAYER
Materials and methods
The tissue paraffin blocks: All the 106 cases of pathological paraffin blocks of colon tumors of the Pathologic Department, Second Affiliated Hospital of Zhejiang University and Tai’an Centre Hospital of Shandong Province were from 1999 to 2001, and included 12 esophagus carcinomas, 12 gastric carcinomas, 15 colon carcinomas, 11 primary liver carcinomas, 10 breast carcinomas, 10 kidney carcinomas, 14 lung carcinomas, 10 thyroid carcinomas and 12 bladder carcinomas.
The reagents for immunohistochemistry: The primary antibody (monoclonal mouse anti-human cox-2), Elivision™ plus kit (Kit-9901) and DAB Kit (20×, DAB-0031) were ordered from Maxin Company, USA.
TMA slices preparation and HE staining: Using the TMA paraffin block constructed by ZM-1 tissue microarrayer to prepare 4 μm consecutive sections. Every 50 slides were stained with hematoxylin and eosin (H & E). The method for sectioning and HE staining was the same as the routine procedures.
The immunohistochemistry of TMA: The immunohistochemical staining used S-P (streptavdin-peroxidase-biotin) two-step staining system by Elivision™ plus kit, and the primary antibody was omitted for the negative controls. The result of immunostaining was evaluated as previously described (Chung et al., 2001).
Each step strictly followed the protocol of the test kits.
Result
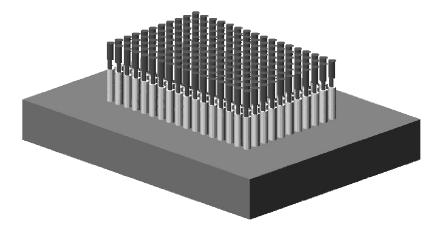
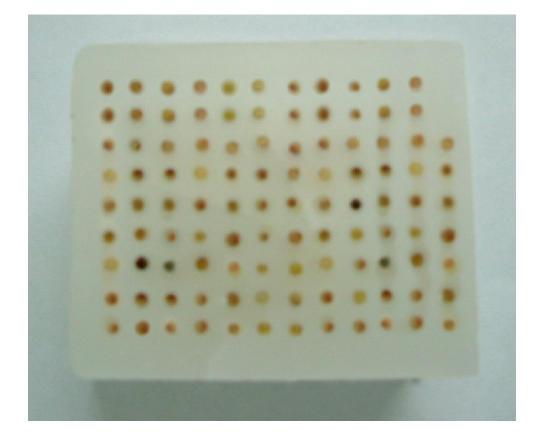
As shown in Fig.7, the TMA paraffin block constructed by ZM-1 tissue microarrayer contains 106 tissues. No more than 30 min in average were required to finish this block construction by only one technician with a biopsyer, and no less than 300 consecutive sections (4 μm) could be obtained.
Fig. 7.
The TMA paraffin block constructed by our ZM-1 tissue microarrayer
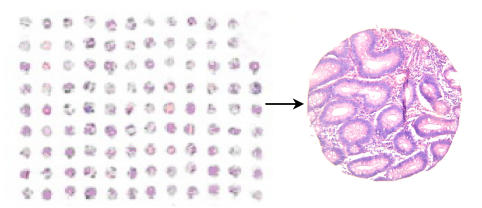
The hematoxylin and eosin (H & E) stained slide, in which the tissue spots array was trimmed in such a way that not any specimen was lost (Fig.8).
Fig. 8.
H & E staining
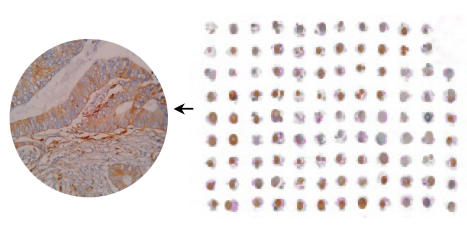
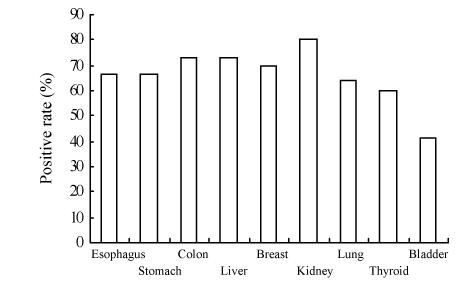
The tissue structure and background of the immunohistochemical staining were clear (Fig.9). The cox-2 protein was detected in 66.67% of esophagus carcinoma, 66.67% of gastric carcinoma, 73.33% of colon carcinoma, 72.73% of primary liver carcinoma, 70.00% of breast carcinoma, 80.00% of kidney carcinoma, 64.29% of lung carcinoma, 60.00% of thyroid carcinoma and 41.67% of bladder carcinoma (Fig.10). The reagents for the TMA slide staining was no more than 1% of that for conventional slides.
Fig. 9.
Cox-2 immunohistochemical staining
Fig. 10.
The positive expression of cox-2 in different tissue carcinomas
DISCUSSION
In the post-genomics era, genetic information accumulated at a rapid pace from new genomic technologies and approaches. A challenge for this era will be the unscrambling and validating of this rich resource of data (Collins et al., 1998). Though tens of thousands of candidate genes and protein targets have readily been identified, they tell us very little about the biological function of the gene, its potential clinical impact or suitability as a drug target. It is therefore essential to functionally validate target genes identified by microarray screening (Mousses et al., 2002). TMA technology meets the need mentioned above exactly. It is comprised of a miniaturized collections of arrayed tissue spots on a microscope glass slide serving as a template for highly parallel localization of molecular targets, either at the DNA, RNA or protein level (Kononen et al., 1998). Though it is already successfully utilized in many cases, there is still some to be consummated furthermore of this technology. This study attempts to provide a better solution.
We tried to devise and make a new set of instruments to simplify the technique of constructing the TMA paraffin block. The MTA-II type instrument requires a set of precision “location system”, which is very expensive. Use of the MTA-II requires advance preparation of a recipient block and high quality paraffin for one by one premaking of the recipient block in of its chapping, and the sampling and disposing process has to be performed one by one also. On the other hand, the MTA-II device for TMA paraffin block construction requires a moderate degree of dexterity, and the results improve with experience. The ZM-1 tissue microarrayer which overcomes almost all the disadvantages mentioned above does no need any precision “location system”, and is convenient for handling. Using ZM-1 tissue microarrayer, the recipient paraffin blocks need not be prepared in advance. The paraffin for TMA blocks construction is identical to that for conventional pathological purpose, while no chapping is guaranteed. A technician without any special training can operate ZM-1 tissue microarrayer excellently. There are a series of designed needles needed for sampling instead of only one sampling needle. Thus the possibility of the adulteration among different tissues is avoided completely. In addition, the job of specimen sampling can be finished either by only one researcher with one biopsyer, or by several researchers with corresponding biopsyers respectively. Therefore, not only reduces the cost of TMA paraffin blocks construction, but the efficiency of the TMA paraffin blocks construction and their quality are also improved dramatically. In our experiences, it took no more than 30 min for one technician to construct a TMA paraffin block with 106 tissue specimens. Of course ZM-1 tissue microarrayer needs to prepare a group of specially designed needles for sampling. Actually, the designed needles can be easily economically ordered from injection needle producer. Generally speaking, no more than 200~400 needles can meet all the demands. The ZM-1 tissue microarrayer facilitates the TMA technology’s popularization.
We tested the quality of the TMA paraffin block made by our new ZM-1 tissue microarrayer. Since the MTA-II device requires preparation of the recipient paraffin block ahead of schedule, the disposed tissue specimen and the recipient block are hard to be incorporated together completely. That is why the loss of tissue specimen is unavoidable. Using ZM-1 tissue microarrayer, the tissue specimen disposal and the shaping of the TMA paraffin block were finished in a single step so that the specimen cylinders and the TMA block paraffin were incorporated together completely. Over 300 consecutive slides (4 μm) were sectioned, and each of the 50 slides was stained with H & E. As showed in Fig.8, almost no any specimen spots were lost. Our test showed that the ZM-1 tissue microarrayer was feasibility and that the TMA paraffin blocks constructed by it was reliable.
Finally, we investigated the expression of cox-2 in 9 kinds of carcinoma by means of the TMA paraffin block constructed by ZM-1 tissue microarrayer. Since the histo-spots in TMA were only about 0.6 mm in diameter, it tended to be homogenous. Thus, no area variable was included in the assessment of the scores (Chung et al., 2001). Fig.9 shows that the tissue structure and background of the immunohistochemical staining were much clearer. The cox-2 protein was detected in different kinds of carcinomas, and the positives ranged from 41.67%~80.00% (Fig.10). Immunohistochemical staining of the TMA slides showed that cox-2 expression was localized to the colon carcinoma cells and was not detectable in the stroma compartment of the cancers. The cox-2 immunostaining pattern was predominantly homogenous, and perinuclear cytoplasmic within the tumors. Normal colonic epithelium adjacent to the tumor showed no staining for cox-2 (Fig.9). The cox-2 protein was detected in 73.33% of colorectal carcinoma tissues. The result accorded with that reported (Sheehan et al., 2004; Sinicrope and Gill, 2004). The TMA paraffin block constructed by ZM-1 tissue microarrayer was again shown to be reliable.
In conclusion, the ZM-1 tissue microarrayer, a new instrument developed for constructing TMA paraffin blocks does not need any precision location system. Using it, the recipient paraffin blocks need not be made in advance, and the paraffin used is the same as that for conventional pathology purpose. TMA technology’s improved efficiency and reduced cost may facilitate its popularization.
Footnotes
Project (No. G1998051200) supported by the National Basic Research Program (973) of China
References
- 1.Bubendorf L. High-throughput microarray technologies: From genomics to clinics. Eur Urol. 2001;40(2):231–238. doi: 10.1159/000049777. [DOI] [PubMed] [Google Scholar]
- 2.Chung GG, Provost E, Kielhorn EP. Tissue microarray analysis of beta-catenin in colorectal cancer shows nuclear phospho-beta-catenin is associated with a better prognosis. Clin Cancer Res. 2001;7(12):4013–4020. [PubMed] [Google Scholar]
- 3.Collins FS, Patrinos A, Jordan E, Chakravarti A, Gesteland R, Walters L. New goals for the U.S. Human Genome Project: 1998–2003. Science. 1998;282(5389):682–689. doi: 10.1126/science.282.5389.682. [DOI] [PubMed] [Google Scholar]
- 4.Frantz GD, Pham TQ, Peale FVJr, Hillan KJ. Detection of novel gene expression in paraffin-embedded tissues by isotopic in situ hybridization in tissue microarrays. J Pathol. 2001;195(1):87–96. doi: 10.1002/1096-9896(200109)195:1<87::AID-PATH932>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Fuller CE, Wang H, Zhang W, Fuller GN, Perry A. High-throughput molecular profiling of high-grade astrocytomas: The utility of fluorescence in situ hybridization on tissue microarrays (TMA-FISH) J Neuropathol Exp Neurol. 2002;61(12):1078–1084. doi: 10.1093/jnen/61.12.1078. [DOI] [PubMed] [Google Scholar]
- 6.Gulmann C, Grace A, Leader M, Butler D, Patchett S, Kay E. Adenomatous polyposis coli gene, beta-catenin, and E-cadherin expression in proximal and distal gastric cancers and precursor lesions: An immunohistochemical study using tissue microarrays. Appl Immunohistochem Mol Morphol. 2003;11(3):230–237. doi: 10.1097/00129039-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Gulmann C, Butler D, Kay E, Grace A, Leader M. Biopsy of a biopsy: Validation of immunoprofiling in gastric cancer biopsy tissue microarrays. Histopathology. 2003;42(1):70–76. doi: 10.1046/j.1365-2559.2003.01556.x. [DOI] [PubMed] [Google Scholar]
- 8.Jubb AM, Landon TH, Burwick J, Pham TQ, Frantz GD, Cairns B, Quirke P, Peale FV, Hillan KJ. Quantitative analysis of colorectal tissue microarrays by immunofluorescence and in situ hybridization. J Pathol. 2003;200(5):577–588. doi: 10.1002/path.1371. [DOI] [PubMed] [Google Scholar]
- 9.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10(7):657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 10.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 11.Mousses S, Kallioniemi A, Kauraniemi P, Elkahloun A, Kallioniemi OP. Clinical and functional target validation using tissue and cell microarrays. Curr Opin Chem Biol. 2002;6(1):97–101. doi: 10.1016/s1367-5931(01)00283-6. [DOI] [PubMed] [Google Scholar]
- 12.Rimm DL, Camp RL, Charette LA, Costa J, Olsen DA, Reiss M. Tissue microarray: A new technology for amplification of tissue resources. Cancer J. 2001;7(1):24–31. [PubMed] [Google Scholar]
- 13.Sheehan KM, Cahill RA, McGreal G, Steele C, Byrne MF, Kirwan WO, Kay EW, Fitzgerald DJ, Redmond HP, Murray FE. Cyclooxygenase-2 expression in primary human colorectal cancers and bone marrow micrometastases. Dig Liver Dis. 2004;36(6):392–397. doi: 10.1016/j.dld.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Simon R, Sauter G. Tissue microarrays for miniaturized high-throughput molecular profiling of tumors. Exp Hematol. 2002;30(12):1365–1372. doi: 10.1016/s0301-472x(02)00965-7. [DOI] [PubMed] [Google Scholar]
- 15.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23(1-2):63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 16.Watson SJ, Akil H. Gene chips and arrays revealed: A primer on their power and their uses. Biol Psychiatry. 1999;45(5):533–543. doi: 10.1016/s0006-3223(98)00339-4. [DOI] [PubMed] [Google Scholar]