Abstract
Objectives: To explore the mechanism of development and aggressiveness in gastric carcinomas by investigating the expression and role of CD97 and its cellular ligand CD55 in gastric carcinomas. Methods: Tumor and corresponding normal mucosal tissue, collected from 39 gastric carcinoma patients, were examined by immunohistochemistry and RT-PCR for the expression of CD97 and CD55. Results: CD97stalk was strongly stained on scattered tumor cells or small tumor cell clusters at the invasion front of gastric carcinomas. The expression of CD97stalk was frequently observed in tumors of stage I and T1 gastric carcinoma patients. The expression of CD97stalk between Stage I and Stage II, III, IV specimens showed significant difference (P<0.05), between T1 and T2, T3, T4 specimens also showed significant difference (P<0.05). Specimens with tumor invasion depth limited in mucosa of T1 specimens showed higher positive CD55 expression than specimens with the same tumor invasion depth in T2, T3, T4 specimens, the expression of CD55 between T1 and T2, T3, T4 specimens was significantly different (P<0.05). There was strong correlation between the distribution patterns of CD97stalk and CD55 on tumor tissues (r=0.73, P<0.05). Signet ring cell carcinomas frequently contained strong CD97stalk and CD55-staining. Conclusions: Our results suggest that CD97stalk is probably involved in the growth, invasion and aggressiveness of gastric carcinomas by binding its cellular ligand CD55. CD97stalk and CD55 could be useful as molecular markers for prognosis and therapy of gastric carcinoma patients.
Keywords: CD97stalk, CD55, Gastric carcinoma, Molecular markers
INTRODUCTION
The heterodimeric CD97 protein is a member of the EGF-TM7 (epidermal growth factor seven-transmembrane member) family receptors of 75000~90000. EGF-TM7 proteins possess a unique hybrid structure consisting of various numbers of N-terminal EGF-like domains coupled to a seven-span transmembrane (TM7) domain by a mucin-like stalk (Mcknight and Gordon, 1998; Kwakkenbos et al., 2004). CD97 is abundantly expressed in normal tissues and a series of epithelial cancers (Jaspars et al., 2001; Aust et al., 1997; Hoang-Vu et al., 1999; Steinert et al., 2002; Aust et al., 2002). Various evidence shows that CD97 plays an important role in tumor dedifferentiation, migration, invasiveness and metastasis by binding its cellular ligand CD55 (Hamann et al., 1996; Mustafa et al., 2004). There are some reports that the observed expression of CD97 does not correlate with clinicopathological features of these cancers (Aust et al., 2002; Boltze et al., 2002). Now it is clear that cell type-specific N-glycosylation causes CD97 to show two kinds of different epitopes CD97EGF and CD97stalk, 2 immunodominant CD97 epitopes that are not equally present in the various cell types. Carcinomas frequently expressed CD97stalk within tumor cells, the selectivity of CD97 mAbs significantly influences the prognostic value of CD97 in clinical studies (Wobus et al., 2004). We systematically investigated for the first time the expression and role of CD97stalk and its cellular ligand CD55 in gastric carcinomas.
MATERIALS AND METHODS
Tissue samples
This study used 39 gastric carcinomas obtained from patients undergoing resection in the Department of Surgery, Zhejiang University, between March 2003 and March 2004. Besides tumors, normal mucosal specimens at least 5 cm away from gastric tumor lesions was obtained from all patients. Samples were cryopreserved in liquid nitrogen immediately after the resection. A small portion of each sample was fixed in formalin and stained with hematoxylin and eosin (HE) to confirm the histological diagnosis of gastric carcinoma and count positive stained cells.
No patient received chemotherapy or radiotherapy before surgery. Histological diagnosis and stage were based on the TNM classification proposed by the International Union Against Cancer as evaluated in the Institute of Pathology, Second Affiliated Hospital, Zhejiang University. To compare the relationship between the immunostaining of CD97 and CD55 with known clinicopathologic parameters, patients were divided into two groups respectively, each group was depicted as detailed in the Table 1.
Table 1.
Relationship between the expression of CD97 and CD55 in tumor cells and clinicopathological features of gastric carcinoma patients. Patients were classified according to the positive (+) or negative (−) staining of tumor tissues for each antigen. Statistical analysis and correlations of data were performed by determining the P value (P<0.05)
| Clinicopathological features | Number of patients |
|||||
| CD97 |
CD55 |
|||||
| (−) | (+) | P value | (−) | (+) | P value | |
| Age (years) | ||||||
| <50 | 3 | 8 | 0.274 | 4 | 7 | 0.568 |
| ≥50 | 13 | 15 | 13 | 15 | ||
| Gender | ||||||
| Male | 12 | 18 | 0.812 | 13 | 17 | 0.953 |
| Female | 4 | 5 | 4 | 5 | ||
| TNM Stage | ||||||
| Stage I | 1 | 9 | 0.021 | 2 | 8 | 0.081 |
| Stage II, III, IV | 15 | 14 | 15 | 14 | ||
| Histologic type | ||||||
| Differentiated | 3 | 9 | 0.175 | 3 | 9 | 0.119 |
| Undifferentiated | 13 | 14 | 14 | 13 | ||
| Lymph node metastasis | ||||||
| (−) | 3 | 9 | 0.175 | 5 | 7 | 0.872 |
| (+) | 13 | 14 | 12 | 15 | ||
| Depth of invasion | ||||||
| T1 | 0 | 8 | 0.008 | 1 | 7 | 0.047 |
| T2, T3, T4 | 16 | 15 | 16 | 15 | ||
| Metastasis | ||||||
| (−) | 15 | 19 | 0.306 | 16 | 18 | 0.255 |
| (+) | 1 | 4 | 1 | 4 | ||
Methods
1. Detection of the CD97 and CD55 mRNA
Total tissue RNA in samples was isolated using Trizol Reagent (Gibco BRL, N.Y., USA) in accordance with the manufacturer’s instructions. Complementary DNA (cDNA) was prepared by reverse transcription (RT) of 2 µg total RNA using oligo dT18 and 200 U superscript II reverse transcriptase (Invitrogen, California, USA) at 42 °C for 70 min according to the manufacturer’s instructions.
PCRs were carried out in a final volume of 50 µl with 2 µl cDNA and 2.5 U Taq DNA polymerase (Invitrogen, California, USA). The specific oligonucleotide primers were from Sangong (Shanghai, China), CD97: 5′-TCC TGC CGG CAG CTC CAA-3′ (sense) and 5′-GGC AGC GGC AGC TGT ATG AAC-3′ (antisense), resulting in an amplicon of 452 bp; CD55: 5′-TTC AGG CAG CTC TGT CCA GTG-3′ (sense) and 5′-GAG GCT GAA GTG GAA GGA TCG-3′ (antisense) resulting in an amplicon of 562 bp; β-actin: 5′-GCT GGA AGT GGA CAG CGA-3′ (sense) and 5′-GGC ATC GTG ATG GAC TCC G-3′ (antisense) resulting in an amplicon of 608 bp. The amplification was performed for 30 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min before a 10 min extension was carried out at 72 °C. The amplified PCR products were resolved by electrophoresis on 1.5% (w/V) agarose gels and identified by ethidium bromide staining. After 30 cycles were completed, visible bands were determined under UV-lights as positive band.
The correlation between positive expression of CD97stalk and CD55 at mRNA and clinicopathological features of patients was treated by U test, P<0.05 was considered significant.
2. Immunostaining of CD97stalk and CD55 on gastric tumor tissues
Serial frozen 4 µm thick sections were mounted on poly-lysine-coated glass slides and air-dried overnight. For IHC (immunohistochemistry), labeled streptoavidin-biotin (LSAB) was used. The frozen sections were first immersed in phosphate-buffered saline (PBS) for 30 min. Endogenous peroxidase was quenched with 3% (V/V) hydrogen peroxide in methanol for 15 min and then nonspecific antibody binding was blocked with normal goat serum for 30 min. The sections were incubated overnight at 4 °C with the primary antibody at 1:100 dilution. Primary antibodies used were MEM-180 (anti-CD97) and BRIC110 (anti-CD55) purchased from RDI, New Jersey, USA and Biosource, Camarillo, USA, respectively. The sections were rinsed in PBS before the detection step. Bound antibody was detected with a detection kit (DakoCytomation, Copenhagen, Denmark. Code No. K5001) including biotinylated anti-mouse Ig (Bottle A), horseradish-peroxidase-conjugated-streptoavidin (Bottle B) and diaminobenzidine (Bottle C+D). Sections that were treated in PBS without the primary antibodies were used as negative controls.
The immunoreactivity was evaluated semi-quantitatively with the use of a light microscope by two independent investigators who were blind to the histological results. Tissue from a patient with a dedifferentiated thyroid tumor was used for each staining procedure as a positive control. The product of positive cells and staining intensity gave the score (0 to 12). We considered samples positive if the score was higher than 2. A score of 3 to 6 was regarded as moderate, and >6 as strong staining (Remmele and Stegner, 1987). The comparison between these two antigen (CD97stalk and CD55) expressions and clinical pathological features was assessed with the Mann-Whitney U Test. P<0.05 was considered significant. The Spearman test was used for correlation analysis.
RESULTS
mRNA expression of CD97 and CD55 in tumor tissues
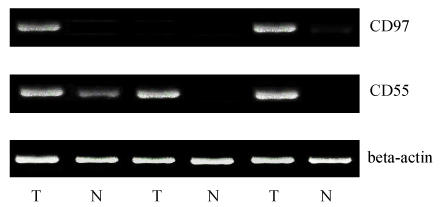
The specific amplicon size of CD97 was 452 bp, the specific amplicon size of CD55 was 562 bp (Fig.1). Thirty of 39 gastric tumors showed positive expression of CD97 at mRNA level, 7 out of 39 in the normal gastric glandular epithelium showed positive expression of CD97, the positive expression of CD97 at mRNA level in tumor tissues (77%) was much higher than that of normal tissues (18%); 34 of 39 gastric tumors showed positive expression of CD55 at mRNA level, 9 out of 39 in the normal gastric glandular epithelium showed positive expression of CD55, the positive expression of CD55 at mRNA level in tumor tissues (87%) was much higher than that of normal tissues (23%). However, the mRNA positive expression of CD97 and CD55 in tumor tissues was not related to any clinicopathological indicators obtained from gastric carcinoma patients.
Fig. 1.
The expression of CD97 and CD55 at mRNA levels on tumors (T) and normal tissues (N) of gastric carcinomas
CD97 and CD55 mRNA expression tumors (T) and normal tissues (N) of gastric carcinomas were examined with RT-PCR. PCR products were separated in 1.5% agarose gels and analyzed after ethidium bromide staining. β-actin was used as housekeeping gene for internal control
Distribution pattern of CD97stalk and CD55
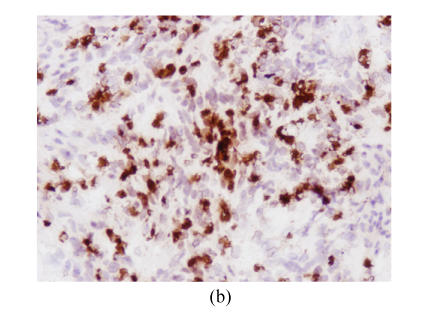
In normal glandular epithelia of gastric carcinoma patients, CD97stalk staining was absent or only weakly positive. In the tumor tissues there were two cases: CD97stalk is strongly localized in the scattered tumor cells or small tumor cell clusters at the invasion front (Fig.2a), on the other hand, CD97 staining was weak or absent in tumor cells located in the center of solid tumor formations. Twenty-three of 39 gastric tumors showed CD97stalk positive staining, including 13 moderate and 10 strong staining. Nine of 10 stage I patients showed CD97stalk positive staining on the tumor cells, 4 moderate and 5 strong staining, and among 5 cases with strong staining, 4 were present in signet ring cell carcinomas (Fig.2b). Thirteen of 29 stage II, III, and IV patients showed positive staining, including 8 moderate and 5 strong staining. The expression of CD97stalk between stage I and stage II, III, IV patients showed significant difference (P<0.05). All of the 8 patients with tumor invasion depth limited in mucosa T1 showed positive CD97stalk staining, including 4 moderate and 4 strong staining. Among those 4 patients that showed strong CD97 staining on tumor cells, 3 of 4 patients had signet ring cell carcinomas. The expression of CD97stalk between T1 and T2, T3, T4 was significantly different (P<0.05). CD97stalk staining was frequently observed on the cytoplasm of tumor cells in gastric carcinoma stage I patients and the depth of tumor invasion T1, especially on signet ring cells.
Fig. 2.
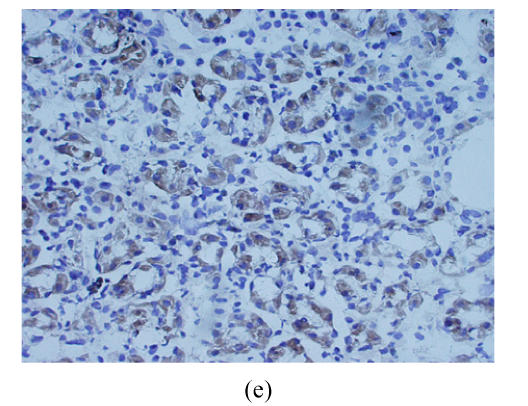
Cryostat section of gastric carcinoma tissue (magnification 200×). Positive staining of CD97 and CD55 was observed on cytoplasm and membrane of gastric carcinoma cells and showed brown-yellow, the cellular nuclears generally showed blue. When the staining was too strong, the cellular nuclears was covered by brown-yellow (a) Strong CD97stalk-staining was localized in scattered tumor cells surrounded in matrix or small tumor cell clusters; (b) Strong CD97stalk-staining was observed on cytoplasm of signet ring cell carcinomas; (c) Strong CD55-staining was located on cytoplasm of signet ring cell carcinomas; (d) CD55-staining was located on glandular luminal sides, and was expressed over the entire surface where the ducts were present; (e) Positive-staining of CD55 was frequently observed in normal gastric glandular epithelium
The positive expression for CD55 was found in 22 out of 39 tumor cases, including 16 moderate and 6 strong staining. CD55 was predominantly expressed in the cytoplasms of tumor cells. Signet ring cell carcinoma showed the strongest CD55 staining (Fig.2c). CD55 was also expressed on the glandular luminal sides, particularly, the staining was observed on the entire surface at sites where the ducts were present (Fig.2d). Seven out of the 8 patients with tumor invasion depth limited in mucosa T1 showed positive CD55 staining, and the expression of CD55 between T1 and T2, T3, T4 was significantly different (P<0.05). On the other hand, strong CD55-positive staining was frequently displayed in the normal gastric glandular epithelium (Fig.2e). Comparison analysis of the expression of CD97stalk and CD55 and the clinicopathological features of gastric carcinoma patients are described in detail in Table 1. Spearman test (r=0.73, P<0.05) revealed strong correlation between the distribution patterns of CD97stalk and CD55 on tumor tissues.
DISCUSSION
The positive-expression of CD97 and CD55 at mRNA level in tumor tissue was not consistent with the clinicopathologic features in gastric carcinoma patients. This result in pancreatic ductal adenocarcinoma and medullary thyroid carcinomas was discussed in (Mustafa et al., 2004; Boltze et al., 2002).
In this study, a strong CD97stalk staining was observed in isolated tumor cells as well as in small tumor clusters at the gastric carcinoma invasion front, whereas CD97stalk-staining appeared weakly homogenous, or even negative, at the center of the solid tumor tissue. The pattern of CD97stalk-staining on gastric tumors is consistent with previous reports on gastric, colorectal and thyroid carcinomas (Aust et al., 1997; Steinert et al., 2002; Aust et al., 2002). However, there are distinguishing discrepancies in the relation between the expression of CD97stalk and the clinicopathological features of the gastric carcinoma patients. Steinert et al.(2002) reported that carcinomas with strong CD97-staining showed a higher clinical stage as well as increased lymph vessel invasion compared to carcinomas with weak CD97-staining in colorectal carcinomas. However, our study showed that stage I and T1 gastric carcinoma patients, with the exception of gastric cancer with higher stage, frequently showed the strongest CD97stalk-staining, particularly in signet ring cell carcinoma. It is well known that newborn tumor tissue is highly vital, and so, have strong capability for invasion, aggressiveness and metastasis. The expression of CD97stalk varies in different lesions of the same tumor and with different developmental stage of the gastric carcinoma. This variation may be similar to the situation described in β-catenin and CD97 localization in colorectal carcinomas (Steinert et al., 2002; Brabletz et al., 1998; 2001; Maruyama et al., 2000). These data suggest that the accumulation of CD97stalk in scattered tumor cells or tumor cells at the invasion front of gastric carcinomas enables the tumor cells or cell clusters to develop, migrate and invade the surrounding tissue.
In this study, the expression of CD55 in T1 gastric carcinoma tissues usually showed high and strong positive staining. It suggests that early stage gastric carcinomas are easily exposed to the environment of complement attack, and that gastric carcinoma cells express CD55 protein to protect malignant cells from complement lysis as CD55 in endometrial adenocarcinoma (Nowicki et al., 2001). Moreover, the expression of CD55 was strong in normal gastric mucosa and was consistent with the result of previous studies (Shimo et al., 2004; Niehans et al., 1996; Li et al., 2001) on the expression of CD55 in other carcinomas. Given that CD55 plays a role in tumor immune escape, the strong expression of CD55 in normal gastric glandular epithelia may be considered as a predicting factor in gastric carcinoma patients, further investigation must be carried out.
In gastric tumor tissues, the expression of CD55 was well correlated with that of CD97stalk. Particularly, strong staining of CD97stalk and CD55 was observed in the cytoplasm of the signet ring cell carcinoma of patients. These findings indirectly support the previous report that CD97 may be involved in the remodelling of adhesive contacts of the invading tumor cells by direct receptor-ligand interactions (Steinert et al., 2002; Mustafa et al., 2004; Li et al., 2001). Although the mechanisms for the interaction of CD97 and CD55 remains unclear, both molecules may be used as good targets for diagnosis and treatment of gastric carcinomas.
Footnotes
Project (No. 2004C34010) supported by the Science and Technology Bureau of Zhejiang Province, China
References
- 1.Aust G, Eichler W, Laue S, Lehmann I, Heldin NE, Lotz O, Scherbaum WA, Dralle H, Hoang-Vu C. CD97: A dedifferentiation marker in human thyroid carcinomas. Cancer Res. 1997;57:1798–1806. [PubMed] [Google Scholar]
- 2.Aust G, Steinert M, Schutz A, Boltze C, Wahlbuhl M, Hamann J, Wobus M. CD97, but not its closely related EGF-TM7 family member EMR2, is expressed on gastric, pancreatic, and esophageal carcinomas. Am J Clin Pathol. 2002;118(5):699–707. doi: 10.1309/A6AB-VF3F-7M88-C0EJ. [DOI] [PubMed] [Google Scholar]
- 3.Boltze C, Schneider-Stock R, Aust G, Mawrin C, Dralle H, Roessner A, Hoang-Vu C. CD97, CD95 and Fas-L clearly discriminate between chronic pancreatitis and pancreatic ductal adenocarcinoma in perioperative evaluation of cryocut sections. Pathol Int. 2002;52:83–88. doi: 10.1046/j.1440-1827.2002.01324.x. [DOI] [PubMed] [Google Scholar]
- 4.Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract. 1998;194:701–704. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 5.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamann J, Vogel B, van Schijndel GM, van Lier RA. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF) J Exp Med. 1996;184(3):1185–1189. doi: 10.1084/jem.184.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang-Vu C, Bull K, Schwarz I, Krause G, Schmutzler C, Aust G, Dralle H. Regulation of CD97 protein in thyroid carcinoma. Clin Endocrinol Metab. 1999;84:1104–1109. doi: 10.1210/jcem.84.3.5557. [DOI] [PubMed] [Google Scholar]
- 8.Jaspars LH, Vos W, Aust G, van Lier RA, Hamann J. Tissue distribution of the human CD97 EGF-TM7 receptor. Tissue Antigens. 2001;57:325–331. doi: 10.1034/j.1399-0039.2001.057004325.x. [DOI] [PubMed] [Google Scholar]
- 9.Kwakkenbos MJ, Kop EN, Stacey M, Gordon S, Lin HH, Hamann J. The human EGF-TM7 family: A postgenomic view. Immunogenetics. 2004;55:655–666. doi: 10.1007/s00251-003-0625-2. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Spendlove I, Morgan J, Durrant LG. CD55 is over-expressed in the tumour environment. Br J Cancer. 2001;84(1):80–86. doi: 10.1054/bjoc.2000.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maruyama K, Ochiai A, Akimoto S, Nakamura S, Baba S, Moriya Y, Hirohashi S. Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology. 2000;59:302–309. doi: 10.1159/000012187. [DOI] [PubMed] [Google Scholar]
- 12.Mcknight AJ, Gordon S. The EGF-TM7 family: Unsual structures at the leukocyte surface. Leukoc Biol. 1998;63:271–280. doi: 10.1002/jlb.63.3.271. [DOI] [PubMed] [Google Scholar]
- 13.Mustafa T, Klonisch T, Hombach-Klonisch S, Kehlen A, Schmutzler C, Koehrle J, Gimm O, Dralle H, Hoang-Vu C. Expression of CD97 and CD55 in human medullary thyroid carcinomas. Int J Oncol. 2004;24(2):285–294. [PubMed] [Google Scholar]
- 14.Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin) Am J Pathol. 1996;149(1):129–142. [PMC free article] [PubMed] [Google Scholar]
- 15.Nowicki S, Nowicki B, Pham T, Hasan R, Nagamani M. Expression of decay accelerating factor in endometrial adenocarcinoma is inversely related to the stage of tumor. Am J Reprod Immunol. 2001;46(2):144–148. doi: 10.1111/j.8755-8920.2001.460205.x. [DOI] [PubMed] [Google Scholar]
- 16.Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 17.Shimo K, Mizuno M, Nasu J, Hiraoka S, Makidono C, Okazaki H, Yamamoto K, Okada H, Fujita T, Shiratori Y. Complement regulatory proteins in normal human esophagus and esophageal squamous cell carcinoma. Gastroenterol Hepatol. 2004;19(6):643–647. doi: 10.1111/j.1440-1746.2003.03328.x. [DOI] [PubMed] [Google Scholar]
- 18.Steinert M, Wobus M, Boltze C, Schutz A, Wahlbuhl M, Hamann J, Aust G. Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am J Pathol. 2002;161:1657–1667. doi: 10.1016/S0002-9440(10)64443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wobus M, Vogel B, Schmucking E, Hamann J, Aust G. N-glycosylation of CD97 within the EGF domains is crucial for epitope accessibility in normal and malignant cells as well as CD55 ligand binding. Int J Cancer. 2004;112:815–822. doi: 10.1002/ijc.20483. [DOI] [PubMed] [Google Scholar]