Abstract
Cdc42 and Rac - ancient, highly conserved, small GTPases - mediate extracellular signals, triggering changes in transcription and in the actin cytoskeleton. Although dozens of proteins act downstream of these GTPases, a comparison of effector proteins from evolutionarily diverse organisms suggests that six groups of proteins serve as the core machinery for signaling from Cdc42 and Rac.
Reorganization of the actin cytoskeleton plays crucial roles in many cellular functions, including morphology, motility, and adhesion. Regulation of actin polymerization and depolymerization is orchestrated by small GTPases of the Rho family; these proteins are also important in regulating gene transcription. The basic signaling properties of two major subgroups of Rho GTPases - the Cdc42 and Rac subfamilies - are highly conserved amongst all eukaryotes, but the means by which they act are not well understood. In an effort to understand the fundamental signaling elements, or 'core machinery', required for the function of these GTPases, we describe here the conservation and functional similarities of Cdc42 and Rac effectors in five different species: plant, yeast, fruit fly, roundworm and human. We focus on six 'core' effectors that operate in almost all these species - members of the Pak, WASP/WAVE, formin, lipid-kinase, IQGAP and NADPH oxidase families. Additional modules that have been added during the evolution of particular lineages are not considered in detail here.
Core functions of Rho GTPases
Rho GTPases are small (20-30 kDa) GTP-binding proteins of the Ras superfamily. The Rho subfamily is divided in three main subgroups - Cdc42, Rac, and Rho - examples of which are represented in all eukaryotes from plants to man. The mammalian Rho family consists of at least sixteen distinct members: the Cdc42 subgroup (Cdc42, TC10, Chp, and Wrch-1); the Rac subgroup (Rac1-Rac3); and the Rho subgroup (RhoA-RhoE, RhoG, RhoH, Rnd1, and Rnd2). In this article, we focus on the signaling mechanisms of two of these three subgroups, Cdc42 and Rac, as they are often linked in their physiological functions and have several effectors in common.
Cdc42 and/or Rac homologs are found in fruit flies, round-worms and budding yeast. Interestingly, small GTPases related to Rac are found even in organisms that lack Ras, such as plants. This broad distribution across widely divergent eukaryotic species suggests that Cdc42 and Rac GTPases have an ancient origin, perhaps even predating that of their cousin Ras. The analysis of Cdc42 and Rac function in evolutionarily distant organisms is useful as a tool to uncover the basic activities of these proteins. In all systems, these GTPases are best known for their effects on the polymerization and distribution of actin in the cell cortex [1]. This activity is strongly conserved and probably represents a primordial function of these proteins. By regulating filamentous actin, Cdc42 and Rac exert a profound effect on cell shape, polarity, migration, cell:cell and cell:matrix adhesion, protein traffic, and cytokinesis. In addition, they play important roles in gene transcription (via activation of mitogen activated protein (MAP) kinase pathways and, in higher eukaryotes, the transcription factor NFκB), generation of reactive oxygen species, apoptosis, and cell-cycle progression.
Core GTPase effectors
In mammalian cells, as many as twenty types of protein have been reported to bind to activated Cdc42 [2]. A roughly similar number of proteins are recruited to activated Rac [3]. Among this large group of effectors are protein kinases; lipid kinases, hydrolases, and phosphatases; actin-bundling proteins; coatomers (vesicle coat proteins); adaptor proteins; and proteins that interact with members of the Arp2/3 complex and hence the actin cytoskeleton (Table 1). Many, but not all, of these effectors contain a conserved 18 amino-acid binding motif that has been termed CRIB (Cdc42-Rac interactive binding), PBD (p21-binding domain) or GBD (GTPase-binding domain) [4]. This motif is found in Cdc42/Rac-associated proteins such as the protein kinases Pak, MRCK and Ack, the adaptor proteins Spec and WASP, and, in degenerate form, in the kinases MLK, Mekk4, adaptor Par6, scaffold protein IRSp53, and the Borg proteins. Interestingly, the CRIB motif also makes an appearance in plant proteins, but here it is attached to a GTPase-activating protein, or GAP (reviewed in [5]). The presence of such CRIB-containing proteins in plants suggests that either the CRIB motif has a very ancient origin, and became associated with a variety of signaling proteins over the course of evolution in various organisms, or else it arose independently in plants. The latter possibility seems unlikely, however, as there are several Rac effectors that lack a CRIB domain, indicating that this particular sequence of amino acids is not a unique solution to the problem of how to bind activated Rac.
Table 1.
Cdc42 and Rac effectors
| Effectors | Biological functions |
| Actin-associated proteins | |
| Formins | Actin polymerization; cell polarity; cytokinesis |
| IQGAP1 | Actin organization; regulation of cell-cell contacts |
| WASP | Actin polymerization |
| WAVE | Actin polymerization |
| p140Sra-1 | Actin organization |
| Adaptor proteins | |
| POSH | Jnk/NFκB activation |
| Par6 | Cell polarity |
| Lipid kinases | |
| DGK | Phosphatidic acid levels; maybe also actin regulation |
| PI3K | PIP3 levels; actin organization |
| PI5K | PIP2 levels; actin organization |
| Miscellaneous proteins | |
| Borg | Actin organization |
| CIP4 | Actin organization |
| NADPH oxidase | ROS production; apoptosis |
| POR1 | Actin organization |
| SPEC | Actin organization |
| Phospholipases | |
| PLC-β | DAG/IP3 levels |
| PLD | Phosphatidic acid levels; cytoskeletal reorganization |
| Serine/threonine kinases | |
| MEKK | Jnk activation |
| MLK | Jnk activation |
| MRCK | Actin organization |
| Pak | Actin organization; MAPK/Jnk activation; apoptosis |
| p70S6K | Transcription; cell-cycle progression; RNA processing |
| Tyrosine kinases | |
| Ack | Actin organization |
Abbreviations: Ack, activated cdc42-associated tyrosine kinase; Borg, binder of Rho GTPases; CIP4, Cdc42-interacting protein; DAG, diacylglycerol; DGK, diacylglycerol kinase; IP3, inositol trisphosphate; MEKK, mitogen-activated protein kinase (MAPK) kinase; MLK, mixed-lineage kinase; MRCK, myotonic dystrophy kinase-related Cdc42-binding kinase; NADPH, nicotinamide adenine dinucleotide phosphate; Pak, p21-activated kinase; Par, partitioning defective; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PI3K, phosphatidylinositol 3-kinase; PI5K, phosphatidylinositol 4-phosphate-5 kinase; PLC, phospholipase C; PLD, phospholipase D; POR1, partner of Rac; POSH, plenty of SH3 domains; p70S6K, 70 kDa ribosomal S6 kinase; p140Sra-1, specifically Rac1-associated protein; SPEC, small protein effector of Cdc42; WASP, Wiskott Aldrich syndrome protein; WAVE, WASP-like verproline-homologous protein.
Although the total number of Rac and Cdc42 effectors is not yet known for any organism, it is already sufficiently high that an analysis of the role of individual effectors is difficult. For example, in mammalian cells, the identities and relative contributions of the Cdc42/Rac effectors that mediate activation of the Jnk family of kinases are in dispute, with competing claims made for the Pak, MEKK and MLK kinases and for the adaptor protein POSH [6,7]. Similarly, the relative contributions of various Cdc42/Rac-binding protein kinases to actin reorganization are far from settled. Several approaches have therefore been taken in an attempt to elucidate the precise role of Cdc42/Rac effector proteins. For example, gene deletions have been used to analyze the role of Cdc42 effectors in budding yeast. In mammalian cells, loss-of-function studies of Cdc42 and Rac effectors are less complete, and overexpression studies, using constitutively active or dominant-negative mutants, have instead been the predominant methods of analysis. Several groups have also analyzed the signaling properties of Cdc42 and Rac mutants that selectively bind certain sets of effector proteins [8,9,10]. In general, these kinds of studies have been more useful in excluding effectors from a given Cdc42/Rac function than in proving their involvement.
Another way to reduce this complexity to a more manageable level is to consider which small-GTPase effectors are shared among evolutionarily distant organisms. Presumably, effectors that are preserved across long periods of time represent key proteins that are required to carry out the core activities of the GTPase. If we apply such an evolutionary filter to Cdc42 and Rac effectors, including only those effectors that are shared in mammals and yeast or plants, we find that only a small subset of proteins emerge as potential components of a core apparatus. These are p21-activated kinases (Paks), Wiskott-Aldrich syndrome proteins (WASPs) and their relatives the WAVEs, formins, lipid kinases, IQGAPs, and NADPH oxidases (Figure 1).
Figure 1.
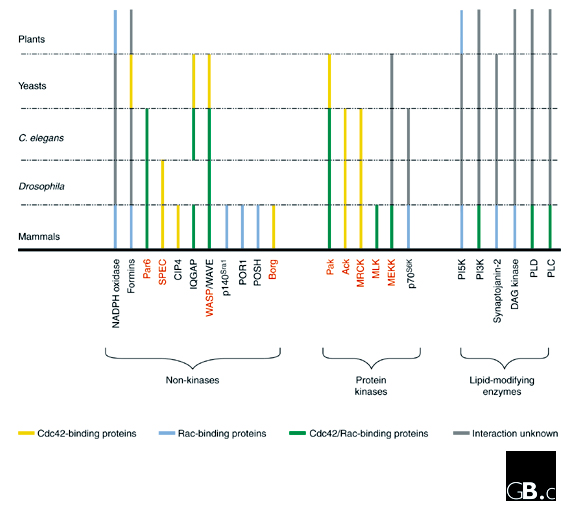
Cdc42- and Rac-interacting proteins in the species discussed in the text. Rac and Cdc42 effectors are grouped into three categories: non-kinases, kinases, and lipid-modifying enzymes. These effectors are represented in the different species by colored lines according to their binding abilities: yellow for Rac, blue for Cdc42, and green for both. The absence of a line means that no homolog is known from current databases, and gray lines indicate that the homologous protein is present in the organism but has not yet been shown to interact with Cdc42 or Rac. The names of CRIB-domain-containing effectors are written in red.
Paks
Paks are serine/threonine protein kinases that associate with Cdc42, and usually also with Rac, via a conventional CRIB motif. The Pak family in mammals has six members, which can be further divided into two groups on the basis of structure [7]. Plant genomes do not encode any Paks, but both the budding and the fission yeast genomes encode multiple Pak homologs that are critical to cell polarity, mating, and cytokinesis. In the budding yeast Saccharomyces cerevisiae, the Pak homolog Ste20p phosphorylates the MEKK-like protein kinase Ste11p, and thus activates the mating and filamentous growth MAP kinase pathways [11]. A similar arrangement is found in the fission yeast Schizosaccharomyces pombe, in which Pak1 and Pak2 act upstream of MAP kinase cascades [12,13]. Given that these two yeasts are only distantly related (having diverged around 800 million years ago), these data support the idea that Paks constitute a conserved and fundamental link from Rho GTPases to MAP kinase cascades. This notion is supported by data from mammals, in which Paks have also been shown to function in MAP kinase activation, albeit in a more baroque manner than in yeast. Here, the linkage is through the kinases Raf1 and Mek1, which Pak phosphorylates [14,15]. Such phosphorylations are not in themselves sufficient for MAP kinase activation, but rather are permissive events: they promote efficient coupling of Raf to Ras and of Mek to Raf, respectively. There is also evidence that Paks may mediate Cdc42 and Rac signaling to stress-activated kinases such as Jnk and p38. The effects of Paks on these stress-kinase cascades are not robust, however, and there is as yet no evidence that Pak acts directly on any MEKKs.
In addition to their effects on MAP kinase cascades, Paks also play an important role in regulating cell morphology. In budding yeast, the Pak Ste20p phosphorylates myosin I and is also associated with the scaffold proteins Ste5p and Bem1p, both of which interact with actin. One other Pak target of note in yeast is the guanine-nucleotide exchange factor (GEF) Cdc24p, which is inactivated as a consequence of phosphorylation. By phosphorylating Cdc24p, the Pak homolog Cla4p provides a feedback inhibition to terminate Cdc42p action as the cell cycle progresses [16]. Thus, Paks in budding yeast link a Rho family GTPase (Cdc42p) to the cell polarity and morphology apparatus via a variety of cytoskeletal targets. In metazoans, the same general rules seem to apply. The fruit fly and worm genomes each encode at least one enzyme from each Pak subgroup, and genetic analysis in Drosophila indicates that these different forms of Pak play distinct roles in embryogenesis: DPak (a group I Pak) is genetically linked to Dock (an adaptor protein homologous to mammalian Nck), Trio (a Rac GEF), and Rac, and is involved in axon guidance [17,18], whereas mbt (which encodes a group II Pak), is required for normal development of the mushroom body, which is thought to be the seat of memory formation in flies [19]. The relevant targets for DPak and Mbt are not known. In man, mutations in the brain-specific Pak3 gene are associated with mental retardation [20,21].
The sum of the genetic data is consistent with the notion that one of Pak's oldest functions is to regulate actin dynamics. These data are supported by overexpression experiments in mammalian cells, which suggest that Paks play a key role in actin polymerization and in cell motility (reviewed in [6]). In higher eukaryotes, two relevant Pak targets are LIM kinase and myosin light chain kinase (MLCK), which control actin dynamics via their substrates cofilin and myosin light chain, respectively [22]. When activated LIM kinase phosphorylates cofilin, cofilin becomes inactive and promotes stabilization of actin filaments, whereas the phosphorylation of MLCK decreases its ability to phosphorylate and activate the regulatory light chain of myosin. The tumor suppressor Merlin has recently emerged as a third potentially important target that might mediate Pak's cytoskeletal effects in mammalian cells [23]. Merlin belongs to the Ezrin/Radixin/Moesin family of proteins, which are thought to link actin filaments to the plasma membrane. Pak phosphorylates Merlin, downregulating Merlin's activity. Finally, stathmin, a protein that regulates microtubule stability, is also a direct target of Pak [24]. Stathmin may link Cdc42, via Pak, to the tubulin cytoskeleton.
WASPs and WAVEs
WASPs and WAVEs represent another class of ancient small-GTPases effectors and are likely to be essential for small-GTPase function in all eukaryotes. In mammals, there are two forms of these proteins, the WASPs, which are activated by Cdc42, and the WAVEs, which are activated by Rac. WASP/WAVE homologs are found in Drosophila, Caenorhabditis elegans, and yeast. Interestingly, while the WASPs contain a recognizable CRIB motif, the WAVEs do not. Instead, their linkage to small GTPases is thought to be provided by an adaptor protein, IRSp53 [25], although its precise role is controversial [26]. In budding yeast, a similar arrangement may prevail, as the WASP/WAVE homolog Bee1p lacks a CRIB motif but is associated with Cdc42p indirectly via a formin [27]. Disruption of WASP/WAVE function has major consequences for actin organization in every system where it has been studied. In budding yeast, loss of Bee1p leads to a severe defect in cortical actin-patch assembly [28], while in fruit flies WASP has an essential role in lineage decisions mediated by the Notch signaling pathway [29]. In man, mutations in WASP lead to severe immunodeficiency (the Wiskott Aldrich syndrome, from which the protein takes its name); this disorder is characterized at the cellular level by abnormalities of cytoskeletal structure, polarization, and motility. WASP has been shown to regulate actin polymerization by stimulating the actin-nucleating activity of the Arp2/3 complex [30]. The binding to WASP of GTP-Cdc42 (via the CRIB motif) and phosphatidylinositol bisphosphate, PIP2 (via a pleckstrin-homology lipid-interaction domain), induces WASP activation by opening the normally masked and auto-inhibited carboxy-terminal WASP domain that binds Arp2/3. In this way, WASP regulates the position of newly assembled actin filaments.
Formins
Formins are a diverse group of proteins defined by the presence of two regions of sequence homology, an approximately 100 amino-acid, proline-rich region termed FH1 (formin-homology 1), and an approximately 130 amino-acid region termed FH2 [31]. Many members of the formin family affect both the actin and the tubulin cytoskeletons, but it is not clear if all proteins with FH domains retain these functions. For example, Arabidopsis encodes at least eight proteins with FH domains, but their unusual structures (for example, including both signal peptides that indicate secretion and transmembrane motifs) suggests that these proteins have functions that are unique to plants [32]. In fungi, including yeast, and in animal cells, several formins are known to bind to activated Rho-family GTPases and are implicated in cytoskeletal regulation. In budding yeast, the formin Bni1p is associated, via its amino terminus, with Cdc42p, and, via its FH1 domain, with profilin and Bud6p, both of which regulate actin dynamics [33]. Mutations in BNI1 lead to defects in cortical actin patch assembly. In Drosophila, the gene encoding the formin protein Diaphanous is required for cytokinesis [34]. Embryos lacking Diaphanous show fatal defects in actin-filament organization that result in abnormalities in formation of the metaphase furrow, in cellularization, and in the formation of pole cells [35]. In C. elegans, the formin Cyk-1 co-localizes with actin filaments at the cleavage furrow of dividing cells, and loss of Cyk-1 function leads to abnormal polar-body extrusion during meiosis and aberrant cytokinesis during embryonic mitosis [36]. There is no biochemical or genetic evidence, however, that either Diaphanous or Cyk-1 is linked to small GTPases. In mammals, formins have been firmly associated with both Rho and Rac.
Mammalian formins, such as the Diaphanous homologs mDia1 and mDia2, play a key role in the formation of stress fibers (a Rho function) as well as cytokinesis and Rho-mediated transcriptional activation of the serum response factor [37]. How these effects are achieved is not known, but the mDia proteins, like yeast Bnip, do associate with profilin [38]. Recently, another member of the formin family, termed FHOS, has been shown to bind activated Rac1 rather than Rho [39]. Like the mDia proteins, PHOS regulates the activation of the serum response factor by a GTPase (Rac). Unlike budding yeast formins, however, mammalian formins have not been found to associate with Cdc42. A formin-binding protein is closely related to the Cdc42 effector protein CIP4, raising the interesting possibility that some formins could be indirectly linked to Cdc42 [40]. Thus, formins are ancient, highly conserved effectors for Rho-family GTPases that, like Paks, mediate both cytoskeletal and transcriptional responses.
Lipid kinases
Two lipid kinases, phosphatidylinositol 3-kinase (PI 3-kinase) and phosphatidylinositol-4-phosphate 5-kinase (PI 5-kinase), have been found to act downstream of Rho GTPases. Whether PI 3-kinase represents a core Cdc42/Rac effector, as defined in this article, is debatable, as the evidence for a relationship between PI 3-kinase and Cdc42/Rac is confined to mammalian systems, but PI 5-kinase is known to act as a cytoskeletal effector for Rac homologs in both man and plants [41,42,43]. PI 5-kinase generates the lipid PIP2 which is an important second messenger in the regulation of actin cytoskeletal dynamics; it uncaps actin filaments (primarily by binding to actin-capping proteins) and thus allows for their further polymerization. PI 5-kinase activity is physically associated with the Rac-related Rop proteins in plants and is thought to regulate calcium fluxes and actin dynamics [42]. In S. cerevisiae, the PI 5-kinase Mss4p is required for normal actin dynamics and is genetically linked to RHO2, but the nature of this relationship is undefined [44]. Homologous PI 5-kinases are expressed in fruit flies and roundworms, but they have not been linked to Cdc42, Rac, or Rho GTPases in these organisms. In mammalian cells, Rac and Rho interact directly with PI 5-kinase. Blockade of PI 5-kinase activity by overexpressing a kinase-dead PI 5-kinase mutant blocks Rac-induced actin assembly, and Rac mutants that cannot bind PI 5-kinase fail to assemble actin in vitro [45]. These results demonstrate that PI 5-kinase is a critical mediator of Rac-dependent actin assembly.
IQGAPs
In spite of their name, IQGAPs have no GTPase-activating activity. Instead, these highly-conserved proteins are bona fide cytoskeletal effectors for Cdc42 and Rac. IQGAPs are broadly distributed: homologs have been found in yeast, Hydra, Dictyostelium, C. elegans, and many other eukaryotes, though not, surprisingly, in Drosophila. In S. cerevisiae Iqg1p is involved in actin recruitment to the bud neck [46,47]; it promotes cytokinetic actin ring formation and is required for cytokinesis and viability. Iqg1p acts as a scaffold to recruit and localize a protein complex involved in actin-based cellular functions, and thus mediates the regulatory effects of Cdc42p on the actin cytoskeleton [48]. A similar actin-filament bundling function has been proposed for mammalian IQGAPs [49]. In mammalian cells, IQGAPs interact with both GTP-Rac1 and GTP-Cdc42, and localize to membrane ruffles. IQGAP1 has been detected in a complex with filamentous actin and Cdc42, and has been shown to localize to cell-cell adhesion sites. It has also been detected in a complex with Cdc42 and the Golgi apparatus [50]. The combined genetic and biochemical data on IQGAPs suggest that these proteins are Cdc42/Rac effectors that contribute to proper actin organization, particularly during cytokinesis.
NADPH oxidases
In mammalian cells, Rac is well-known as a regulator of the NADPH oxidase complex, a specialized enzyme of phagocytic cells that generates oxygen radicals to kill internalized microorganisms. A cytoplasm-derived component of the oxidase complex, p67Phox, binds directly to Rac1 and Rac2 in neutrophils [51]. These data suggest that Rac acts as an allosteric regulator by inducing a conformational change in the preformed NADPH complex to promote catalytic activity. The pathogen-defense function of Rac has ancient origins. In plants, the Rac-related Rop proteins are involved in the elicitor-induced production of reactive oxygen species (ROS), products of an oxidative burst reaction that induces the death of infected host cells. As in the oxidative burst in mammalian neutrophils, ROS production in plants is carried out by an NADPH oxidase enzyme complex that contains a Rho-family GTPase (reviewed in [5]). In rice, over-expression of a dominant-negative Rop (OsRac1) inhibits ROS production and cell death in cells treated with the protein phosphatase inhibitor calyculin A, while constitutively active OsRac1 induces resistance of rice plants against a virulent blast fungus. These findings suggest a role for Rop in general disease resistance in rice. Rac-regulated H2O2 production is also involved in a second, developmental process, namely cell-wall formation in cotton fibers (and probably in other plants as well). In mammalian cells, Rac-regulated ROS production is not limited to pathogen-defense by neutrophils: it also has a key function in cell growth and transformation. Activated Cdc42 or Rac proteins that lack the 'insert domain' (a carboxy-terminal motif that is found in all three Rho GTPase subgroups, but not in other small GTPases) fail to elicit ROS production in fibroblasts, and, unlike the full-length proteins, fail to support neo-plastic transformation [52,53]. The targets for ROS that mediate these effects have not been identified, but protein tyrosine phosphatases, most of which negatively regulate mitogenic signaling and which are known to be exquisitely sensitive to oxidation, are prime candidates.
In conclusion, Cdc42 and Rac are evolutionarily old proteins. The six groups of GTPase effector proteins discussed here are also old. They are present in nearly all eukaryotes and, in most cases, retain their links to GTPases, and so are likely to represent fundamental links between Cdc42, Rac, and their effects on cell morphology and transcription. We do not, however, mean to downplay the importance of other effectors that appeared later in evolution. Clearly, proteins that we have not discussed - in particular Par6, MRCK, and Ack - are not only well-conserved by almost any standard but also known to carry out vital tasks in the organisms that express them. It is probable that the increased complexity of higher eukaryotes demanded additional GTPase effectors, and that these will need to be understood in detail to make sense of signal transduction in these systems. It is still useful to consider the GTPase machine at its most basic, however, as defined by the strict evolutionary filter used here (see Figure 2). The six groups of effectors we have discussed may have coevolved with Cdc42 and Rac GTPases from the time of their earliest appearance. The effectors perform vital tasks, and these tasks have been retained, though often modified, over evolutionary time. Understanding the interplay between the GTPases and these core effectors is important in determining how Cdc42 and Rac are linked to the actin cytoskeleton and to the transcriptional apparatus in eukaryotic cells.
Figure 2.
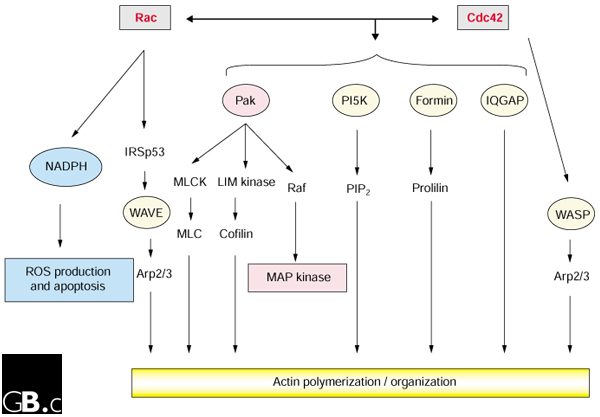
Cdc42/Rac core effectors. Cdc42, Rac and their primary common conserved effectors are represented on shaded background. Rac-specific core effectors include NADPH oxidase and WAVE, while Cdc42-specific effectors include WASP. With the exception of Pak, all the effectors common to Rac and Cdc42 are involved only in actin reorganization. IQGAP and NADPH are the only effectors to act directly on actin or apoptosis (via ROS), with no secondary effectors required. MAPK pathways are shown in pink, actin polymerization pathways in yellow, and ROS pathways in blue.
References
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. doi: 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Burbelo PD, Dreschel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–29074. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- Valster AH, Hepler PK, Chernoff J. Plant GTPases: the Rhos in bloom. Trends Cell Biol. 2000;10:141–146. doi: 10.1016/s0962-8924(00)01728-1. [DOI] [PubMed] [Google Scholar]
- Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997;7:162–167. doi: 10.1016/S0962-8924(97)01003-9. [DOI] [PubMed] [Google Scholar]
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. Rac regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenström P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65Pak and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Westwick JK, Lambert QT, Clark GJ, Symons M, van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drogen F, O'Rourke SM, Stucke VM, Jaquenoud M, Neiman AM, Peter M. Phosphorylation of the MEKK ste11p by the PAK-like kinase ste20p is required for MAP kinase signaling in vivo. Curr Biol. 2000;10:630–639. doi: 10.1016/s0960-9822(00)00511-x. [DOI] [PubMed] [Google Scholar]
- Ottilie S, Miller PJ, Johnson DI, Creasy C, Sells MA, Bagrodia S, Forsburg SL, Chernoff J. Fission yeast pak1+ encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO J. 1995;14:5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla A, Johnson DI. The Schizosaccharomyces pombe Cdc42p GTPase signals through Pak2p and the Mkh1p-Pek1p-Spm1p MAP kinase pathway. Curr Genet. 2001;39:205–209. doi: 10.1007/s002940100210. [DOI] [PubMed] [Google Scholar]
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, Cobb MH. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli M, Jaquenoud M, Shimada Y, Niederhauser G, Wiget P, Peter M. Phosphorylation of the cdc42 exchange factor cdc24 by the PAK-like kinase cla4 may regulate polarized growth in yeast. Mol Cell. 2000;6:1155–1167. doi: 10.1016/s1097-2765(00)00113-1. [DOI] [PubMed] [Google Scholar]
- Hing H, Xiao J, Harden N, Lim L, Zipursky SL. Pak functions downstream of Dock to regulate photoreceptor axon guidance in Drosophila. Cell. 1999;97:853–863. doi: 10.1016/s0092-8674(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, Dickson BJ. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–294. doi: 10.1016/s0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- Melzig J, Rein KH, Schafer U, Pfister H, Jackle H, Heisenberg M, Raabe T. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr Biol. 1998;8:1223–1226. doi: 10.1016/s0960-9822(07)00514-3. [DOI] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, Cerione RA, Mulley JC, Walsh CA. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. doi: 10.1038/1675. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, des Portes V, McDonell N, Carrie A, Zemni R, Couvert P, Ropers HH, Moraine C, van Bokhoven H, Fryns JP, et al. Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation. Am J Med Genet. 2000;93:294–298. doi: 10.1002/1096-8628(20000814)93:4<294::AID-AJMG8>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chernoff J. Close encounters of the LIM kinase. Nat Cell Biol. 1999;1:E115–E117. doi: 10.1038/12942. [DOI] [PubMed] [Google Scholar]
- Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to Merlin. J Biol Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1677–1680. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- Miki H, Yamaguchi H, Suetsugu S, Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–735. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- Lechler T, Jonsdottir GA, Klee SK, Pellman D, Li R. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J Cell Biol. 2001;155:261–270. doi: 10.1083/jcb.200104094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. Bee1, a yeast protein with homology to Wiscott-Aldrich syndrome protein, is critical for the assembly of cortical actin cytoskeleton. J Cell Biol. 1997;136:649–658. doi: 10.1083/jcb.136.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED. Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J Cell Biol. 2001;152:1–13. doi: 10.1083/jcb.152.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Field CM. Actin cytoskeleton: are FH proteins local organizers? Curr Biol. 1997;7:R414–R417. doi: 10.1016/s0960-9822(06)00205-3. [DOI] [PubMed] [Google Scholar]
- Cvrcková F. Are plant formins integral membrane proteins? Genome Biol. 2000;1:research001.1–001.7. doi: 10.1186/gb-2000-1-1-research001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127:1887–1897. doi: 10.1242/dev.127.9.1887. [DOI] [PubMed] [Google Scholar]
- Swan KA, Severson AF, Carter JC, Martin PR, Schnabel H, Schnabel R, Bowerman B. cyk-1: a C. elegans FH gene required for a late step in embryonic cytokinesis. J Cell Sci. 1998;111:2017–2027. doi: 10.1242/jcs.111.14.2017. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/s1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- Krebs A, Rothkegel M, Klar M, Jockusch BM. Characterization of functional domains of mDia1, a link between the small GTPase Rho and the actin cytoskeleton. J Cell Sci. 2001;114:3663–3672. doi: 10.1242/jcs.114.20.3663. [DOI] [PubMed] [Google Scholar]
- Westendorf JJ. The formin/diaphanous-related protein, FHOS, interacts with Rac1 and activates transcription from the serum response element. J Biol Chem. 2001;276:46453–46459. doi: 10.1074/jbc.M105162200. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr Biol. 1997;7:479–487. doi: 10.1016/s0960-9822(06)00219-3. [DOI] [PubMed] [Google Scholar]
- Ren XD, Schwartz MA. Regulation of inositol lipid kinases by Rho and Rac. Curr Opin Genet Dev. 1998;8:63–67. doi: 10.1016/s0959-437x(98)80063-4. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua N-H. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;145:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CL, Tolias KF, Couvillon AC, Hartwig JH. Signal transduction pathways involving the small G proteins rac and Cdc42 and phosphoinositide kinases. Adv Enzyme Regul. 1997;37:377–390. doi: 10.1016/s0065-2571(96)00005-2. [DOI] [PubMed] [Google Scholar]
- Desrivieres S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J Biol Chem. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type Ialpha phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol. 2000;10:153–156. doi: 10.1016/s0960-9822(00)00315-8. [DOI] [PubMed] [Google Scholar]
- Lippincott J, Li R. Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol. 1998;140:355–366. doi: 10.1083/jcb.140.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JA, Chant J. An IQGAP-related protein controls actin-ring formation and cytokinesis in yeast. Curr Biol. 1997;7:921–929. doi: 10.1016/s0960-9822(06)00411-8. [DOI] [PubMed] [Google Scholar]
- Osman MA, Cerione RA. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JW, Cerione RA, Hart MJ. Identification of an actin cytoskeletal complex that includes IQGAP and the Cdc42 GTPase. J Biol Chem. 1997;272:24443–24447. doi: 10.1074/jbc.272.39.24443. [DOI] [PubMed] [Google Scholar]
- McCallum SJ, Erickson JW, Cerione RA. Characterization of the association of the actin-binding protein, IQGAP, and activated Cdc42 with Golgi membranes. J Biol Chem. 1998;273:22537–22544. doi: 10.1074/jbc.273.35.22537. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Regulation of phagocyte respiratory burst by small GTP-binding proteins. Trends Cell Biol. 1995;5:109–113. doi: 10.1016/s0962-8924(00)88960-6. [DOI] [PubMed] [Google Scholar]
- Joneson T, Bar-Sagi D. A Rac1 effector site controlling mitogenesis through superoxide production. J Biol Chem. 1998;273:17991–17994. doi: 10.1074/jbc.273.29.17991. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Lin R, Cerione RA, Manor D. Transformation activity of Cdc42 requires a region unique to Rho-related proteins. J Biol Chem. 1998;273:16655–16658. doi: 10.1074/jbc.273.27.16655. [DOI] [PubMed] [Google Scholar]


