Abstract
During preimplantation development in mammals, distinct epigenetic marks on oocyte and sperm DNA are remodeled to an embryonic pattern. A recent study examining global methylation of repetitive elements in various mammals showed that the reprogramming that occurs during normal preimplantation development is aberrant in cloned embryos.
Cloning of mammals from adult somatic cells has been successful in mice, sheep, cattle and pigs [1,2,3,4]. Despite this recent success, the overall efficiency of producing live cloned offspring is quite low (2-5%), as there is extremely high loss during embryonic and fetal development [5,6]. In addition, many cloned animals display abnormalities at or after birth, which have been collectively termed large-calf or cloned-off-spring syndrome and include increased body weight, placentomegaly, pulmonary hypertension and respiratory problems [7,8]. As a result, much of the research effort surrounding somatic cell cloning has been directed towards trying to improve the rate of production of live clones. Such effort has so far met with little success, and current thinking is that incomplete epigenetic reprogramming of the somatic nucleus may be the cause of developmental failure of cloned animals. It is thought that cloning mammals from differentiated somatic cells requires the erasure or reprogramming of epigenetic imprints that 'mark' the DNA, thereby rendering the somatic nucleus totipotent. Because our knowledge of the biological changes involved in reprogramming the somatic cell nucleus after it is transferred into the enucleated oocyte is limited, it is essential to understand the molecular events that occur after nuclear transfer and the extent to which a somatic nucleus can be reprogrammed to execute normal embryonic development. This article discusses the early efforts that aim to achieve these goals.
Genome reprogramming during preimplantation development
Fertilization in mammals results in the union of two distinct chromatin sets: sperm chromatin, which is highly compacted with protamines in place of histones; and oocyte chromatin, which is more loosely packaged into nucleosomal arrays and arrested in metaphase II of meiosis. Remodeling of these chromatin sets in the zygote and early-cleavage-stage embryo is elegantly choreographed by ooplasmic factors. The independent remodeling of the two sets of parental chromatin is coordinated by the level of activity of one such factor, maturation-promoting factor (MPF, now known to be made up of a p34cdc2-cyclin B kinase), which is present at high levels in oocytes arrested at metaphase II of meiosis [9]. The incorporated sperm nucleus undergoes nuclear envelope breakdown, sperm nucleoprotein remodeling (substitution of protamines with histones), chromatin decondensation and pronuclear formation [10]. The maternal oocyte chromatin proceeds through the second meiotic division and a maternal pronucleus is formed. DNA synthesis and transcription then occur asynchronously within the individual maternal and paternal pronuclei. In the mouse, a minor burst of transcription occurs exclusively from the paternal genome during the first cell cycle and is followed by a major burst during the second cell cycle [11,12]; transcriptional activation in the bovine embryo begins at the 8- to 16-cell stage. During the series of events that accompany remodeling of the chromatin sets, the distinct parental epigenetic marks that are acquired during gametogenesis are transformed into an embryonic epigenetic pattern - with the exception of the marks carried by imprinted genes. A recent study by Dean and colleagues [13] examined one particular epigenetic process in an attempt to gain an understanding of the reprogramming events that occur during preimplantation development.
The methylation of cytosine residues in CpG dinucleotides, which is associated with transcriptional silencing [14], is an example of an epigenetic process that must be reprogrammed during preimplantation development. It has been shown that mammalian genomic DNA undergoes gradual demethylation during preimplantation development and subsequently becomes methylated de novo during postimplantation development (de novo methylation occurs at CpG dinucleotides on a strand of DNA whose complement is not already methylated) [15,16,17,18]. To examine the dynamics of this process in more detail, Dean and colleagues [13] used antibodies against 5-methylcytosine that mainly detect densely methylated cytosine residues within highly and interspersed repetitive sequences. Their data revealed that, in the mouse, DNA within the larger paternal pronucleus is significantly demethylated by a putative demethylase activity after fertilization but before the first S phase (Figure 1). This replication-independent demethylation is conserved in eutherian mammals: cattle, pigs and rats all exhibit active global demethylation of the presumed paternal genome. Asymmetric behavior of the parental genomes is corroborated by the replication-independent demethylation of the paternal allele of several single-copy genes in mouse zygotes [19]. During mouse and bovine preimplantation development, the maternal genome is also demethylated, notably in a replication-dependent manner (Figure 1). Reduction in global methylation is first observed at the 2-cell stage and proceeds through the next cell divisions, suggesting that the maternal genome is somehow protected from the active demethylase activity and instead undergoes passive demethylation [20]. Both mouse and cattle genomes are hypomethylated by the 8- to 16-cell stage. In contrast to the mouse, in which genome-wide de novo methylation begins in the inner cell mass (ICM) of the blastocyst-stage embryo, in bovine embryos this process commences at the 8- to 16-cell stage, before blastocyst formation. As a result, there is divergence in the methylation pattern between mice and cattle, with bovine blastocysts exhibiting methylation both in the ICM and in cells of the outer cell layer (trophectoderm). Similar results were reported in the mouse by Rougier and colleagues [21] and in cattle by Bourc'his et al. [22]. Differential demethylation (and topological separation) of the maternal and paternal genomes continues through to at least the 4-cell stage in the mouse, after which the two parental genomes have attained roughly the same levels of global hypomethylation [23]. Taken together, these data show that the parental genomes undergo extensive changes in global methylation during preimplantation development.
Figure 1.
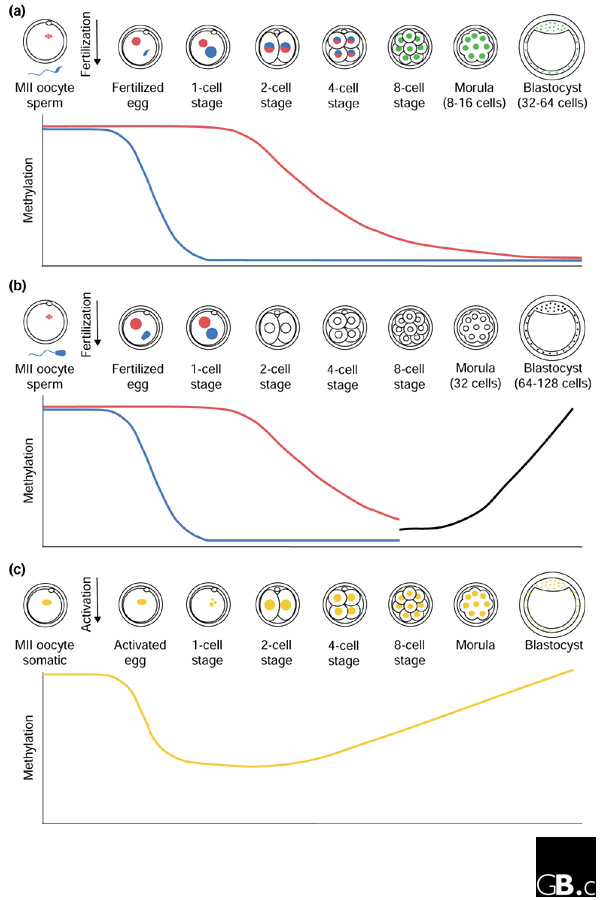
Reprogramming of global methylation in normal and cloned preimplantation embryos. (a) The mouse paternal genome (blue) (in the mouse distinguishable as the larger pronucleus at the 1-cell stage) undergoes active demethylation while the maternal genome (red) is passively demethylated. Differential demethylation and topological separation of the maternal and paternal genomes continues through to at least the 4-cell stage, after which the two parental genomes attain roughly the same levels of global hypomethylation (green). (b) Bovine embryos exhibit the same active and passive demethylation as the mouse up to the 8- to 16-cell stage, after which de novo methylation is observed (black line on the graph; the parental genomes cannot be distinguished to determine whether methylation is acquired at the same rate in the maternal and paternal chromosomes). (c) Cloned bovine embryos (with somatic nuclei in orange) have active but not passive demethylation activity. Precocious de novo methylation is observed in all nuclei beginning at the 4-cell stage. MII, meiotic metaphase II.
Genome reprogramming during somatic cell cloning
Embryogenesis comprises a series of well-orchestrated events that begins with fertilization to form a single-cell zygote and ends with a multicellular organism. The majority of cells within an embryo differentiate into histologically distinct and functionally diverse cell types. As these cells develop their specialized identities, they undergo epigenetic changes to reflect their need to express particular genes. Once fully differentiated, the cellular state is stable and heritable. It is assumed that for somatic cell cloning to be successful, the terminally differentiated somatic cell nucleus that is transferred into the cytoplasm of an enucleated meiotic metaphase II oocyte must undergo reprogramming to erase cell-type-specific epigenetic traits and to gain totipotency [24]. This would require tissue-specific gene expression to be switched off and embryo-specific gene transcription to be turned on, so that embryonic development can be properly initiated and executed. This reversal of differentiation could theoretically be accomplished by reprogramming of genome-wide epigenetic processes such as DNA methylation and chromatin remodeling.
The methodology for somatic cell cloning was designed to approximate the events that occur during fertilization and early embryogenesis. The procedure begins with the removal of the meiotic metaphase II chromosome-spindle complex from the ovulated oocyte, followed by microinjection or fusion of the somatic donor cell or nucleus with the enucleated cytoplast [5,6]. Exposure of the somatic cell nucleus to high MPF activity probably results in nuclear envelope breakdown and premature chromosome condensation. Following oocyte activation and suppression of extrusion of the second polar body, MPF activity probably declines, chromatin decondenses and pseudopronuclei are formed. DNA replication and cellular division follow, and the clone may proceed through preimplantation development.
Given that the major loss (around 95%) of cloned embryos occurs during preimplantation and early postimplantation stages [5], it is likely that cloned embryos are ineffective at reprogramming the somatic donor nucleus. One possible cause of the low rate of cloning success is an incomplete reestablishment of embryonic DNA methylation patterns. To address this possibility, Dean and colleagues [13] used antibodies to 5-methylcytosine to examine genome-wide methylation patterns in cloned bovine preimplantation embryos. Pseudopronuclei formed from the somatic donor nucleus in the reconstructed zygote were found to undergo some degree of active demethylation: pronuclei displayed a reduction in anti-5-methylcytosine immunofluorescence to approximately half that observed in the original somatic nucleus (Figure 1). In contrast to normal embryos, however, there appeared to be no passive demethylation during the following cell divisions and instead precocious de novo methylation was reported in some nuclei at the 4- and 8-cell stages. In cloned morulae, methylation patterns more closely approximated those of the donor somatic nucleus than those of normal fertilized 16-cell embryos.
These results contrast with those reported by Bourc'his and colleagues [22], who observed no active demethylation in cloned bovine zygotes and found that the somatic pattern in donor nuclei was preserved through to the 4-cell stage. In a proportion of 8-cell embryos, and in morula- and blastocyst stage embryos, euchromatin was demethylated while centromeric heterochromatin was de novo methylated. Although the disparity between the results for early active demethylation may reflect the difference in the microscopy assays used by these two groups, it may also be explained by the age of the donor cell used for the studies. In the study by Dean et al. [13], non-starved fetal fibroblast nuclei were used as the donor, whereas in the study by Bourc'his et al. [22] quiescent adult skin fibroblast cells were used, suggesting that older cells are more resistant to reprogramming. The de novo methylation at the 4- to 8-cell stage observed by Dean and co-workers [13] may correspond to the de novo methylation of centromeric heterochromatin in 8-cell clonal embryos reported by Bourc'his and colleagues [22].
Aberrant methylation in cloned bovine preimplantation embryos was corroborated by an independent experimental approach, assaying methylation by bisulfite mutagenesis and sequencing [25]. Cloned bovine embryos fail to demethylate the repetitive sequence elements satellite I and Bov-B, but instead maintain methylation levels similar to those found in donor nuclei [25]. Normal fertilized embryos, by contrast, exhibit gradual demethylation of these sequences during preimplantation development. A detailed examination of methylation levels during the first cell cycle, to determine whether active demethylation of these sequences occurs, was not conducted in this study [25].
The results discussed here point to an impaired ability of clones to appropriately reprogram the somatic genome. The retention of a donor-like methylation profile in reconstituted embryos may be explained by the possible presence of the somatic form of DNA methyltransferase 1 that may be introduced by the original donor cells [26]. In the presence of this enzyme the genome-wide demethylation characteristic of preimplantation embryos may fail to be recapitulated. Impaired epigenetic reprogramming capabilities of the somatic donor DNA during the earliest stage of clone embryogenesis may be one of the factors contributing to the lethality and abnormal development of cloned animals. Continuing investigations that examine the reprogramming events during normal and clonal embryonic preimplantation development will yield further insights into the molecular mechanisms that regulate the transformation of gametic epigenetic marks into the appropriate embryonic patterns in various mammals and the extent to which cloned embryos can reprogram their somatic genome.
References
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, Awata T, Hanada H, Perry AC. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000;289:1188–1190. doi: 10.1126/science.289.5482.1188. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–373. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R. Cloning the laboratory mouse. Semin Cell Dev Biol. 1999;10:253–258. doi: 10.1006/scdb.1998.0267. [DOI] [PubMed] [Google Scholar]
- Solter D. Mammalian cloning: advances and limitations. Nat Rev Genet. 2000;1:199–207. doi: 10.1038/35042066. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Cross JC. Factors affecting the developmental potential of cloned mammalian embryos. Proc Natl Acad Sci USA. 2001;98:5949–5951. doi: 10.1073/pnas.111182398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KH. Nuclear transfer in farm animal species. Semin Cell Dev Biol. 1999;10:245–252. doi: 10.1006/scdb.1999.0310. [DOI] [PubMed] [Google Scholar]
- Wright SJ. Sperm nuclear activation during fertilization. Curr Top Dev Biol. 1999;46:133–178. doi: 10.1016/s0070-2153(08)60328-2. [DOI] [PubMed] [Google Scholar]
- Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol. 1997;181:296–307. doi: 10.1006/dbio.1996.8466. [DOI] [PubMed] [Google Scholar]
- Ram PT, Schultz RM. Reporter gene expression in G2 of the 1-cell mouse embryo. Dev Biol. 1993;156:552–556. doi: 10.1006/dbio.1993.1101. [DOI] [PubMed] [Google Scholar]
- Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, Reik W. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci USA. 2001;98:13734–13738. doi: 10.1073/pnas.241522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AP, Wolffe AP. Methylation-induced repression - belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- Howlett SK, Reik W. Methylation levels of maternal and paternal genomes during preimplantation development. Development. 1991;113:119–127. doi: 10.1242/dev.113.1.119. [DOI] [PubMed] [Google Scholar]
- Monk M, Boubelik M, Lehnert S. Temporal and regional changes in DNA methylation in the embryonic, extraembryonic and germ cell lineages during mouse embryo development. Development. 1987;99:371–382. doi: 10.1242/dev.99.3.371. [DOI] [PubMed] [Google Scholar]
- Razin A, Shemer R. DNA methylation in early development. Hum Mol Genet. 1995;4:1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. [DOI] [PubMed] [Google Scholar]
- Sanford JP, Clark HJ, Chapman VM, Rossant J. Differences in DNA methylation during oogenesis and spermatogenesis and their persistance during early embryogenesis in the mouse. Genes Dev. 1987;1:1039–1046. doi: 10.1101/gad.1.10.1039. [DOI] [PubMed] [Google Scholar]
- Oswald J, Engemann S, Lane N, Mayer W, Olek A, Fundele R, Dean W, Reik W, Walter J. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–478. doi: 10.1016/s0960-9822(00)00448-6. [DOI] [PubMed] [Google Scholar]
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–502. doi: 10.1038/35000654. [DOI] [PubMed] [Google Scholar]
- Rougier N, Bourc'his D, Gomes DM, Niveleau A, Plachot M, Paldi A, Viegas-Pequignot E. Chromosome methylation patterns during mammalian preimplantation development. Genes Dev. 1998;12:2108–2113. doi: 10.1101/gad.12.14.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourc'his D, Le Bourhis D, Patin D, Niveleau A, Comizzoli P, Renard JP, Viegas-Pequignot E. Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr Biol. 2001;11:1542–1546. doi: 10.1016/s0960-9822(01)00480-8. [DOI] [PubMed] [Google Scholar]
- Mayer W, Smith A, Fundele R, Haaf T. Spatial separation of parental genomes in preimplantation mouse embryos. J Cell Biol. 2000;148:629–634. doi: 10.1083/jcb.148.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414:122–128. doi: 10.1016/S0014-5793(97)00960-5. [DOI] [PubMed] [Google Scholar]
- Kang YK, Koo DB, Park JS, Choi YH, Kim HN, Chang WK, Lee KK, Han YM. Aberrant methylation of donor genome in cloned bovine embryos. Nat Genet. 2001;28:173–177. doi: 10.1038/88903. [DOI] [PubMed] [Google Scholar]
- Mertineit C, Yoder JA, Taketo T, Laird DW, Trasler JM, Bestor TH. Sex-specific exons control DNA methyltransferase in mammalian germ cells. Development. 1998;125:889–897. doi: 10.1242/dev.125.5.889. [DOI] [PubMed] [Google Scholar]


