Abstract
A hydroponic experiment carried out to study the effect of five Cd levels on growth and photosynthesis of two tomato cultivars showed that the addition of 0.1 μmol/L Cd induced a slight increase in plant height of Hezuo 903 and the SPAD (the Soil–Plant Analyses Development) value of the 2 cultivars. However, at higher Cd levels, i.e., 1 and 10 μmol/L, root length and volume, plant height, and SPAD value were all significantly reduced. On an average of the 2 cultivars, exposure to 1 and 10 μmol/L Cd for 33 d reduced plant height by 18.9% and 46.4% and SPAD value by 11.2% and 31.6%, compared with control, respectively. Similarly, root length was reduced by 41.1% and 25.8% and root volume by 45.2% and 63.7%, respectively. The addition of Cd in the growth medium also had significant deleterious effect on net photosynthetic rate (Pn) and intracellular CO2 concentration (Ci), with Pn being reduced by 27.2% and 62.1% at 1 μmol/L and 10 μmol/L Cd treatments compared to the control, respectively, while Ci increased correspondingly by 28.4% and 39.3%.
Keywords: Cadmium, Growth, Photosynthesis, Tomato
INTRODUCTION
Cadmium (Cd) is a heavy metal toxic to all organisms (Lagerwerff, 1972; Chakravarty and Srivastava, 1992). The accumulation of Cd in biotic systems as a consequence of human activities is becoming a major environmental problem. The application of sewage sludge, city waste, and Cd-containing fertilizers causes the increase of Cd content in soils (Williams and David, 1973). Cd is easily taken up by plants and then enters the food chain, resulting in a serious health issue for humans. For instance, people will suffer from renal tubular disease if they consume rice with relatively high Cd content (Watanabe et al., 1998). Therefore, addition of toxic heavy metals into soil and transference of these metals into the food chain have been a matter of concern to people in arable soils (Grant et al., 1998). The presence of excessive amount of Cd in soil causes many toxic symptoms in plants, such as reduction of growth, especially root growth (Weigel and Jäger, 1980), disturbances in mineral nutrition and carbohydrate metabolism (Moya et al., 1993), and may therefore strongly reduce biomass production. The reduction of biomass by Cd toxicity could be the direct consequence of the inhibition of chlorophyll synthesis (Padmaja et al., 1990) and photosynthesis (Bazzaz et al., 1975; Baszynski et al., 1980). Some studies reported a marked reduction in photosynthetic rate for different plant species under exposure to Cd stress (Sawhney et al., 1990; Sheoran et al., 1990a; 1990b). Vegetables growing in medium with high level of Cd showed deleterious effect in photosynthetic processes, such as chlorophyll content and photosynthesis (Baszynski et al., 1980; Padmaja et al., 1990; Satyakala, 1997), the activities of related enzymes (Ouariti et al., 1997a; 1997b; Ascencio and Cedeno-Maldonado, 1979; Weigel, 1985a; 1985b) and photochemical reaction (Li and Miles, 1975; Skorzynska and Baszynski, 1995). In contrast, Haag-Kerwer et al.(1999) reported that photosynthesis in Brassica juncea was not affected even when it was exposed to 25 μmol/L Cd, while transpiration showed significant decline, in particular, under lower light conditions (≤300 μmol photons/(m2·s)). It is very imperative to determine the effect of Cd toxicity on photosynthesis of tomato as it is one of the most important vegetables in the world.
The present study was undertaken to investigate the effect of Cd on growth, and photosynthesis of two tomato cultivars at seedling stage, in order to clarify physiological effect of Cd stress on photosynthesis in tomato plants.
MATERIALS AND METHODS
Experimental design
The experiment was carried out in October 2003 at Huajiachi campus, Zhejiang University, Hangzhou, China. Two tomato cultivars, Hezuo 903 and Jiangshu 14, widely planted in southeastern China, were used in this study. Tomato seeds were submerged in distilled water for 4 h, surface sterilized with 0.5% (m/V) K2MnO4 for 30 min, rinsed several times with distilled water and then germinated on moist filter paper placed in sterilized petri dishes. Three-day-old seedlings were transferred to sterilized moist sand at (22±0.5) °C (13 h, day)/(22±0.5) °C (11 h, night). When seedlings grew the first true-leaf, the plants were supplied with 25% nutrient solution containing the following chemicals (mg/L) Ca(NO3)2·4H2O 708.45, MgSO4·7H2O 492.94, KH2PO4 136.09, K2SO4 348.50, (NH4)2SO4 396.39, CaCl2·2H2O 441.09, H3BO3 2.868, MnSO4·H2O 1.545, EDTA-Fe 33.0345, ZnSO4·7H2O 0.220, CuSO4·5H2O 0.080, Na2MoO4·2H2O 0.0299. At two leaf stages, seedlings were selected for uniformity and transplanted to a 6-L container containing 5 L 25% (V/V) basic nutrient solution as described above, which was covered with a polystyrol-plate with 5 evenly spaced holes. One plant was fixed in each hole and the containers were placed in a greenhouse under natural light condition at (22±0.5) °C. The pH of the solution was adjusted to 6.5±0.1 with NaOH or HCl as required. At the 14th day after transplanting, cadmium as CdCl2 was added to each container to form 5 concentrations: 0 (control), 0.1, 1, 5, and 10 μmol/L. The experiment was arranged as a split-plot design with Cd concentration as main plot and cultivar as sub-plot, and with five replications. The nutrient solution in the growth container was continuously aerated with pumps and renewed once a week.
Measurements and statistical analysis
Plant height, leaf number per plant, SPAD value of the upper second fully expanded leaves (Wu et al., 1998), and leaf symptoms of Cd toxicity (necrotic patches on leaf blade), were measured at 7, 14, 21, 33 d after Cd exposure. Root length and volume were measured at 33 d after Cd exposure, and plants in each treatment were harvested and rinsed thoroughly with distilled water, separated into leaves, stems and roots, then dried at 80 °C and weighed.
Photosynthetic parameters, including net photosynthetic rate (Pn), stomatal conductance (Gs) and intracellular CO2 concentration (Ci), were determined using an LCi (leaf chamber analysis) portable photosynthesis system (ADC, Analytical Development Company, England). All measurements were performed on the upper second fully expanded leaves.
All data presented are the mean values. Statistical analysis was carried out by one-way ANOVA using Student’s t-test to test significance of the difference between means. Means were considered significantly different for P≤0.05.
RESULTS
Plant growth parameters and biomass
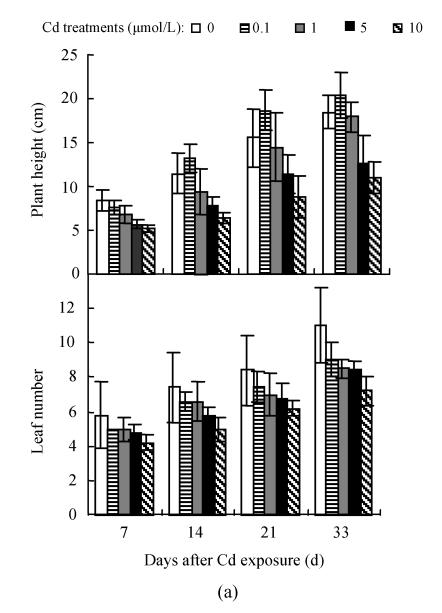
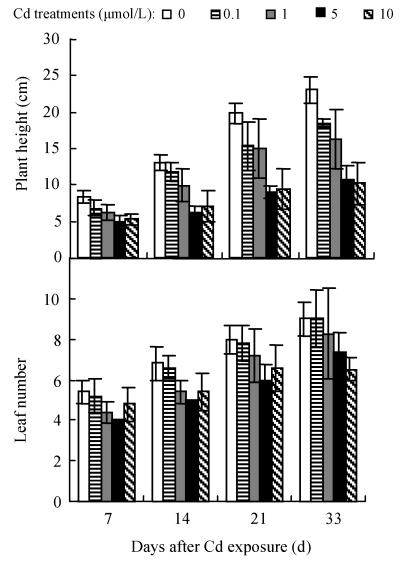
As shown in Fig.1, no significant difference in leaf number and plant height was found between 0.1 μmol/L Cd treatment and control. However, increasing Cd concentration (1~10 μmol/L) in the medium induced a significant decline (P≤0.05) in these 2 measurements and the deleterious effect of Cd became more severe with increasing Cd level and extended time exposure. For example, at the 33 d of 10 μmol/L Cd exposure, the average plant height, and leaf number of the two cultivars decreased by 48.9% and 31.5%, respectively, while, the leaf area per plant was significantly smaller at higher Cd concentration (data not shown), indicating that irreversible damage to tissue formation was induced under higher Cd level.
Fig. 1.
Effects of different Cd levels on plant height and leaf number (a) Hezuo 903; (b) Jiangshu 14
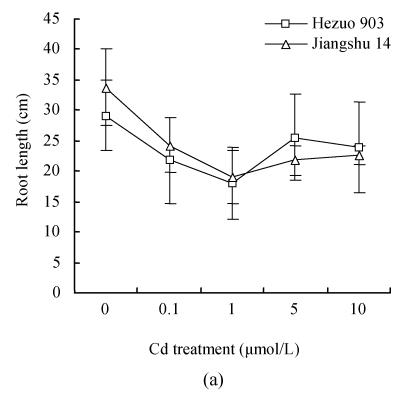
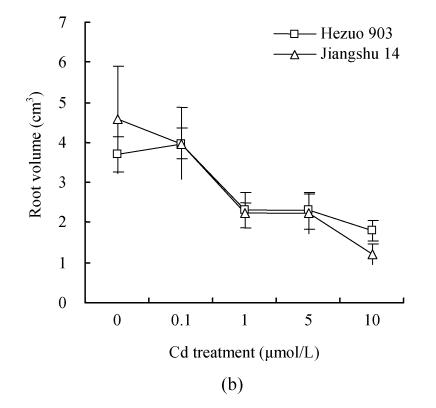
Cd addition significantly decreased root length and volume at 33 d after Cd exposure (Fig.2). The root length and volume, 33 d after 1 μmol/L Cd exposure, reduced by 41.1% and 45.2%, respectively, compared with the control. No significant differences were found between the 2 cultivars for these root parameters.
Fig. 2.
Effects of different Cd levels on (a) root length and (b) root volume after 33 d Cd exposure
Plant biomass of leaves, stems and roots decreased linearly with increasing Cd level in the nutrient solution for the two tomato cultivars after 33 d Cd exposure (Table 1). For example, the addition of 10 μmol/L Cd resulted in leaf, stem and root dry weight decreased by 69.8%, 76.2% and 68.3% respectively. As in root length, no difference was found between the 2 cultivars.
Table 1.
Dry weight (g) of the two tomato cultivars under five Cd levels exposure for 33 d
| Cd treatment (μmol/L) | Dry weight (g/plant) |
|||||
| Root |
Stem |
Leaf |
||||
| Hezuo 903 | Jiangshu 14 | Hezuo 903 | Jiangshu 14 | Hezuo 903 | Jiangshu 14 | |
| 0 | 10.1832 a | 0.1873 a | 0.2254 a | 0.2717 a | 0.9808 a | 1.0219 a |
| 0.1 | 0.1382 b | 0.1540 a | 0.1914 a | 0.2090 b | 0.7576 b | 0.9241 a |
| 1 | 0.0969 c | 0.0881 b | 0.1146 b | 0.1188 c | 10.5261 bc | 0.5326 b |
| 5 | 0.0847 c | 10.0799 bc | 10.0858 bc | 10.0811 cd | 0.4418 c | 0.4215 b |
| 10 | 0.0638 c | 0.0535 c | 0.0617 c | 0.0566 d | 0.3202 c | 0.2847 b |
Different letters within the same column represent significant difference at 0.05 probability levels
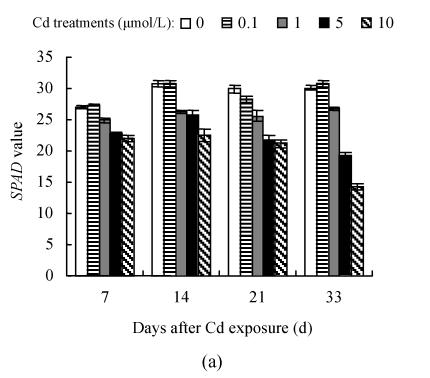
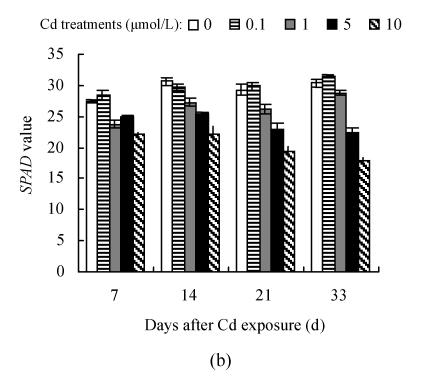
SPAD value and leaf color
The dose- and time-responses of SPAD value to Cd are summarized in Fig.3. The plants exposed to 0.1 μmol/L Cd showed slight but not statistically significant increase in SPAD value, compared with controls except for the 21 d of Hezuo 903 and the 14 d of Jiangshu 14, whereas exposure to 1 μmol/L or higher Cd caused a marked decline, and the deleterious effect of Cd became more pronounced with extended time exposure. For example, the reduction of SPAD values of Hezuo 903 and Jiangshu 14 were 29.2% and 33.9% after 21 d exposure to 10 μmol/L Cd, and 52.8% and 41.6% after 33 d exposure to 10 μmol/L Cd.
Fig. 3.
Effects of different Cd levels on SPAD value of (a) Hezuo 903 and (b) Jiangshu 14
Visual symptoms for heavy metal phyto-toxicity are often indicated as leaf necrosis, chlorosis reddish-brown discoloration of the leaf blade, browning of root system, etc. (Greger and Lindberg, 1987; Vazquez et al., 1989). In our experiment, no symptom of phyto-toxicity in the aboveground parts of plants was observed in 0.1 μmol/L Cd treatment. Necrotic patches with browning of root system and leaf necrosis were observed in both cultivars when the plants were exposed to 1 μmol/L Cd, and became more severe with increasing Cd level and extended time exposure. However, initial appearance and severity of the Cd toxicity symptom were almost the same in the 2 cultivars (Table 2).
Table 2.
Leaf symptoms of Cd toxicity of tow tomato cultivars under five Cd levels
| Days of Cd exposure | Cd treatment (μmol/L) | Leaf symptoms |
|
| Hezuo 903 | Jiangshu 14 | ||
| 7 | 0 | V | V |
| 0.1 | V | V | |
| 1 | IV | IV | |
| 5 | III | III | |
| 10 | II | II | |
| 14 | 0 | V | V |
| 0.1 | V | V | |
| 1 | IV | IV | |
| 5 | III | III | |
| 10 | II | II | |
| 21 | 0 | V | V |
| 0.1 | V | V | |
| 1 | IV | IV | |
| 5 | III | III | |
| 10 | I | I | |
| 33 | 0 | V | V |
| 0.1 | V | V | |
| 1 | IV | IV | |
| 5 | III | III | |
| 10 | I | I | |
Leaf symptoms of Cd toxicity (necrotic patches on leaf blade) for Cd treatments: I–very severe; II–severe; III–mild; IV–slight; V–very slight/absent
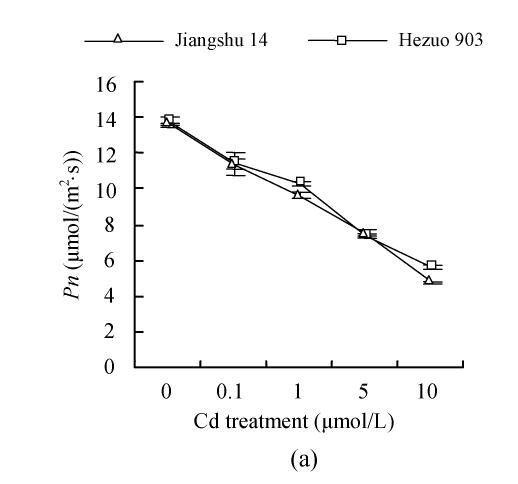
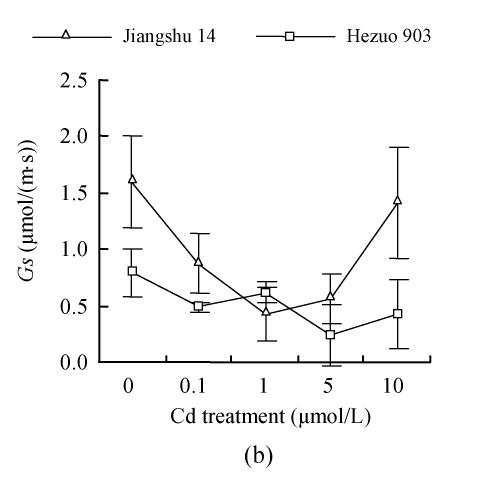
Photosynthesis parameters
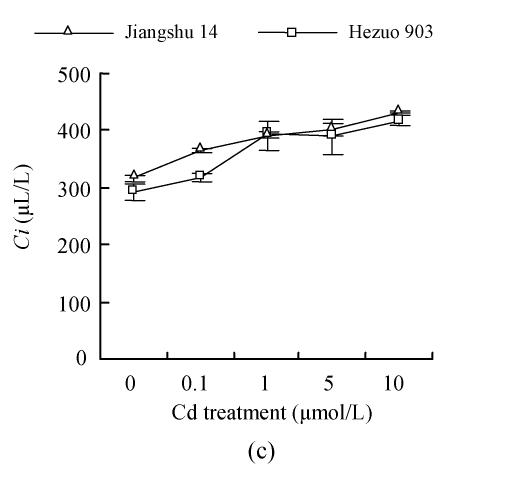
As shown in Fig.4, 21 d after Cd exposure, Pn decreased nearly linearly with increasing Cd in nutrient solution for both cultivars. In plants treated with 10 μmol/L Cd, Pn was lowered by 59.2% for Hezuo 903 and 65.1% for Jiangshu 14, compared with controls. In contrast, the intracellular CO2 concentration (Ci) increased with increasing Cd concentrations in nutrient solution, irrespective of cultivar. As to stomatal conductance (Gs), plants treated with 0.1~5 μmol/L Cd showed significant decrease and the deleterious effect of Cd became more pronounced with increasing Cd level, while a slight increase was observed in the plants exposed to 10 μmol/L Cd.
Fig. 4.
Effects of different Cd levels on (a) Pn, (b) Gs and (c) Ci of tomato after 33 d Cd exposure
DISCUSSION
In the present work, when the plants were exposed to 0.1 μmol/L Cd, plant height of Hezuo 903 and SPAD values of both cultivars showed a slight increase. The results are consistent with the previous finding that there is some potentially positive impact of Cd on barley growth at lower concentration (Wu et al., 2003). The same phenomena were found for Al in that it frequently stimulates plant growth at low concentrations although it is regarded as a toxic element (Kinraide, 1993). The main explanations for this enhancement in growth include increased Fe solubility and availability in calciolous plants, prevention of P toxicity or promotion of P uptake, reduction in growth rate and prevention of Ca depletion, alternation of growth regulators and protection against Cu/Mn toxicity (Barcelò and Poschenrieder, 2002). But verification of the explanation needs more evidences. However, at higher Cd level, e.g., 5~10 μmol/L as used in this study, a significant decrease in root length, plant height, leaf number and SPAD values, and corresponding decrease in biomass of tomato plants was observed. Visual symptoms due to Cd toxicity, such as leaf necrosis, chlorosis reddish-brown discoloration of the leaf blades, browning of root system also became more severe with increasing Cd level and exposure time, indicating that Cd toxicity caused damage to tissue development and function. This accorded with earlier reports (Weigel and Jäger, 1980; Woolhouse, 1983).
It was found that Cd toxicity caused notable reduction in photosynthetic rate in different plant species (Baszynski et al., 1980; Sawhney et al., 1990). These quoted authors showed that Cd concentrations of 56 and 112 mg/L inhibited net photosynthesis to about 50% at early development stages of pigeonpea (30-day-old plants) and did not exert any significant effect on that process at later stages (70-day-old plants). The Cd concentrations used in their experiment were very high and rarely occur naturally. Sheoran et al.(1990b) also reported that at the early stage of pigeonpea, CO2 exchange rate was quite susceptible to Cd stress. In the current study, the results in Pn of the two tomato cultivars exposed to 1 to 10 μmol/L Cd confirmed these observations. The observed decrease of photosynthetic rate in Cd-stressed plants could be partly attributed to lower chlorophyll content, as expressed by SPAD value in this study (Fig.3). On the other hand, intracellular CO2 (Ci) in both cultivars was significantly increased with increasing Cd level in the nutrient solution. Sheoran et al.(1990b) reported that reduction of CO2-exchange rate could not be completely explained by any single factor and appeared to be due to the integrated effect on stomatal conductance, chlorophyll content and on function of photosynthetic apparatus. Malik et al.(1992) showed that reduced CO2 fixation in Cd-treated wheat seedlings was not accompanied by decreased stomatal conductance. In contrast, Barcelò and Poschenrieder (1990) reported that the disturbed water relations to plants comprised one of the main reasons for the heavy metal phytotoxicity. So it may be assumed that the decrease in Pn is not only ascribable to a decline of SPAD value, but also to the inhibition of various reaction steps in the Calvin cycle, the Hill reaction and CO2 fixation (Prasad and Strzalka, 1999). However, further experiment should be done in order to have a better understanding of the mechanism of the effect of Cd toxicity on photosynthesis.
Acknowledgments
We are deeply indebted to the two anonymous reviewers for their valuable suggestions and comments.
Footnotes
Project (No. M303355) supported by the Natural Science Foundation of Zhejiang Province, China
References
- 1.Ascencio CL, Cedeno-Maldonado A. Effects of cadmium on carbonic anhydrase and activities dependent on electron transport of isolated chloroplasts. J Agric Univ Puerto Rico. 1979;63:195–201. [Google Scholar]
- 2.Barcelò J, Poschenrieder C. Plant water relations as affected by heavy metal stress: a review. J Plant Nutri. 1990;13:1–37. [Google Scholar]
- 3.Barcelò J, Poschenrieder C. Fast root growth responses, root exudates, and internal detoxication as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot. 2002;48:75–92. [Google Scholar]
- 4.Baszynski T, Wajda L, Krol M, Wolinska D, Krupa Z, Tuken-Dorf A. Photosynthetic activities of cadmium-treated tomato plants. Physiol Plant. 1980;48:365–370. [Google Scholar]
- 5.Bazzaz FA, Carlson RW, Rolfe GL. Inhibition of corn and sunflower photosynthesis by lead. Physiol Plant. 1975;34:326–329. [Google Scholar]
- 6.Chakravarty B, Srivastava S. Toxicity of some heavy metals in vivo and in vitro in Helianthus annuus . Mut Res. 1992;283:287–294. doi: 10.1016/0165-7992(92)90061-l. [DOI] [PubMed] [Google Scholar]
- 7.Grant CA, Buckley WT, Bailey LD, Selles F. Cadmium accumulation in crops. Canadian J Plant Sci. 1998;78:1–17. [Google Scholar]
- 8.Greger M, Lindberg S. Effects of Cd2+ and EDTA on young sugar beets (Beta vulgaris). II. Net uptake and distribution of Mg2+, Ca2+ and Fe2+/Fe3+ . Physiologia Plantarum. 1987;69:81–86. [Google Scholar]
- 9.Haag-Kerwer A, Schafer HJ, Heiss S, Walter C, Rausch T. Cadmium exposure in Brassica juncea causes a decline in transpiration rate and leaf expansion without effect on photosynthesis. J Exp Bot. 1999;50(341):1827–1835. [Google Scholar]
- 10.Kinraide TB. Aluminium enhancement of plant growth in acid rooting cations: a case of reciprocal alleviation of toxicity by two toxic cations. Physiologia Plantarum. 1993;88:619–625. doi: 10.1111/j.1399-3054.1993.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 11.Lagerwerff JV. Lead, Mercury and Cadmium as Environmental Contaminants. In: Mortvedt JJ, Giordano PM, Lindsay WL, editors. Micronutrient in Agriculture. Madison, WI: Soil Sci. Society of America; 1972. p. 666. [Google Scholar]
- 12.Li EH, Miles CD. Effects of cadmium on photoreactions of chloroplasts. Plant Sci Lett. 1975;5:33–70. [Google Scholar]
- 13.Malik D, Sheoran IS, Singh R. Carbon metabolism in leaves of cadmium treated wheat seedlings. Plant Physiol Biochem. 1992;30(2):223–229. [Google Scholar]
- 14.Moya JL, Ros R, Picazo I. Influence of cadmium and nickel on growth, net photosynthesis and carbohydrate distribution in rice plants. Photosynthesis Res. 1993;36:75–80. doi: 10.1007/BF00016271. [DOI] [PubMed] [Google Scholar]
- 15.Ouariti O, Gouia H, Ghorbal MH. Responses of bean and tomato plants to cadmium: growth, mineral nutrition and nitrate reduction. Plant Physiol Biochem. 1997;35:347–354. [Google Scholar]
- 16.Ouariti O, Boussama N, Zarrouk M, Cherif A, Ghorbal MH. Cadmium- and copper-induced changes in tomato membrane lipids. Phytochem. 1997;45:1343–1350. doi: 10.1016/s0031-9422(97)00159-3. [DOI] [PubMed] [Google Scholar]
- 17.Padmaja K, Prasad DDK, Prasad ARK. Inhibition of chlorophyll synthesis in Phaseolus vulgaris Seedlings by cadmium acetate. Photosynthetica. 1990;24:399–405. [Google Scholar]
- 18.Prasad MNV, Strzalka K. Impact of Heavy Metals on Photosynthesis. In: Prasad MNV, Hagemeyer J, editors. Heavy Metal Stress in Plants. Berlin: Springer; 1999. pp. 117–138. [Google Scholar]
- 19.Satyakala G. Studies on the effect of heavy metal pollution on Pistia stratiotes (water lettuce) Indian J Environ Health. 1997;39:1–7. [Google Scholar]
- 20.Sawhney V, Sheoran IS, Singh R. Nitrogen fixation, photosynthesis and enzymes of ammonia assimilation and ureide biogenesis in nodules of mungbean (Vigna radiata) grown in presence of cadmium. Indian J Exp Biol. 1990;28:883–886. [Google Scholar]
- 21.Sheoran IS, Agarwal N, Singh R. Effect of cadmium and nickel on in vivo carbon dioxide exchange rate of pigeonpea (Cajanus cajan L.) Plant Soil. 1990;129:243–249. [Google Scholar]
- 22.Sheoran IS, Singal HR, Singh R. Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.) Photosynth Res. 1990;23:345–351. doi: 10.1007/BF00034865. [DOI] [PubMed] [Google Scholar]
- 23.Skorzynska E, Baszynski T. Photochemical activities of primary leaves in cadmium stressed Phaseolus coccineus depends on their growth stages. Acta Soc Bot Pol. 1995;64:273–279. [Google Scholar]
- 24.Vazquez MD, Poschenrieder C, Barcelò J. Pulvinus structure and leaf abscission in cadmium-treated bean plants (Phaseolus vulgaris) Canadian J Bot. 1989;67:2756–2764. [Google Scholar]
- 25.Watanabe Y, Zhang ZW, Qu JB, Xu GF, Song LH, Wang JJ, Shimbo S, Nakatsuka H, Higashikawa K, Ikeda M. Urban–rural comparison on cadmium exposure among general populations in Shandong Province, China. Sci Total Environ. 1998;217:1–8. doi: 10.1016/s0048-9697(98)00151-x. [DOI] [PubMed] [Google Scholar]
- 26.Weigel HJ. The effect of Cd2+ on photosynthetic reactions of mesophyll protoplasts. Physiol Plant. 1985;63:192–200. [Google Scholar]
- 27.Weigel HJ. Inhibition of photosynthetic reactions of isolated intact chloroplast by cadmium. J Plant Physiol. 1985;119:179–189. [Google Scholar]
- 28.Weigel HJ, Jäger HJ. Subcellular distribution and chemical forms of cadmium in bean plants. Plant Physiol. 1980;65:480–482. doi: 10.1104/pp.65.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams CH, David DJ. The effect of superphosphate on the cadmium content of soils and plants. Aust J Soil Res. 1973;11:43–56. [Google Scholar]
- 30.Woolhouse HW. Toxicity and tolerance of plants to heavy metals. Encycl Plant Physiol. 1983;12:246–300. [Google Scholar]
- 31.Wu FB, Wu LH, Xu FH. Chlorophyll meter to predict nitrogen sidedress requirement for short-season cotton. Field Crops Res. 1998;56:309–314. [Google Scholar]
- 32.Wu FB, Zhang GP, Dominy P. Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot. 2003;50:67–78. [Google Scholar]