Abstract
Scrubbing of NOx from the gas phase with Fe(II)EDTA has been shown to be highly effective. A new biological method can be used to convert NO to N2 and regenerate the chelating agent Fe(II)EDTA for continuous NO absorption. The core of this biological regeneration is how to effectively simultaneous reduce Fe(III)EDTA and Fe(II)EDTA-NO, two mainly products in the ferrous chelate absorption solution. The biological reduction rate of Fe(III)EDTA plays a main role for the NOx removal efficiency. In this paper, a bacterial strain identified as Klebsiella Trevisan sp. was used to demonstrate an inhibition of Fe(III)EDTA reduction in the presence of Fe(II)EDTA-NO. The competitive inhibition experiments indicted that Fe(II)EDTA-NO inhibited not only the growth rate of the iron-reduction bacterial strain but also the Fe(III)EDTA reduction rate. Cell growth rate and Fe(III)EDTA reduction rate decreased with increasing Fe(II)EDTA-NO concentration in the solution.
Keywords: Biological reduction, Fe(III)EDTA, NOx, Inhibition
INTRODUCTION
Nitrogen oxides (NOx) are significant airborne contaminants resulting from fossil fuels combustion. The use of metal chelate additives for combined removal of NOx and SO2 in FGD (flue-gas desulfurization) systems was investigated by several groups (Harriott et al., 1993; Tsai et al., 1989; Shi et al., 1996a; 1997). Fe(II)EDTA (EDTA, ethylenediaminetetraacetate) as an additive for removing nitric oxide (NO) from flue gas had been extensively studied, with the absorption of NO occurring according to the following reaction:
| Fe(II)EDTA+NO→Fe(II)EDTA-NO |
One significant drawback of this additive is that Fe(II)EDTA can be easily oxidized by oxygen in the flue gas and be formed Fe(III)EDTA. The oxidation of ferrous EDTA has been described to proceed via a complex multi-step mechanism summarized by the following equation:
| 2Fe(II)EDTA+1/2O2+2H+→2Fe(III)EDTA+H2O |
Fe(III)EDTA, which is not capable of binding NO, would decrease scrubber solution capacity. Consequently, the Fe(III)EDTA reduction rate affects NOx removal efficiency. To circumvent this problem, reducing agents such as sulfite/bisulfite, dithionate, sulfide, ascorbic acid, glyoxal, iron metal, etc. and electrochemical method have been researched to regenerate ferrous chelates (Shi et al., 1996b; 1996c). However, none of these approaches have produced promising results because of the high costs, the production of unwanted by-products, or the low reduction rate. Recently, a new approach to reduce Fe(III)EDTA using cultivated active sludge containing iron-reducing bacteria (Li et al., 2003), is now being researched. The biological process can be expressed by the equation:
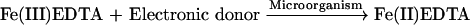 |
In our previous studies, a bacterial strain identified as Klebsiella Trevisan sp. isolated from mixed cultures could be employed effectively to reduce Fe(III)EDTA (Jing et al., 2004a). The ferric ion, serving as a terminal electron acceptor, is reduced to ferrous ion, so that Fe(II)EDTA can be regenerated. In the metal chelate absorption process, the main complex, Fe(II)EDTA-NO, which can also serve as a terminal electron acceptor, may have some effects on the biological reduction of Fe(III)EDTA. However, to the best of our knowledge, there are no reports on the inhibition of Klebsiella Trevisan sp. cell growth and biological reduction of Fe(III)EDTA by Fe(II)EDTA-NO. In order to get better insight into the biological reduction of Fe(III)EDTA, a competitive inhibition study would be conducted in this work.
MATERIALS AND METHODS
Chemicals
Disodium ethylenediaminetetraacetate (Na2EDTA, 99.95%), FeCl3·6H2O (99.5%), D-glucose (99.5%, cell culture tested) were from Shanghai Chemical Reagent Co., China. All other chemicals were analytical grade reagents.
Bacterial strains
Bacterial strains were isolated from mixed culture with terminal electron acceptors of Fe(III)EDTA. The strain is rod-form and Gram negative, 1.0 µm in diameter and 3.0 µm in length, mono, binary or short catenarin-arrange, nonmotile and without gemma, and was identified as Klebsiella Trevisan sp. Detailed physiological properties can be found in our previous paper (Jing et al., 2004a). Enrichment of bacterial strains was done in 250 ml conical flasks containing 100 ml basal medium at 40 °C and shaked at 140 r/min in a rotary shaker. Cells in the medium were harvested by centrifugation at 5000 r/min for 15 min and washed twice with 0.1 mol/L phosphate buffer (pH 7.0), and then suspended in the phosphate buffer at certain concentration for use. Details of the process can be found in Jing et al.(2004b).
Analytical methods
The concentration of ferrous irons and total irons in solution was determined by the 1,10-phenanthroline colorimetric method at 510 nm. The concentration of Fe(II)EDTA-NO was measured by a model 723A spectrophotometer at 420 nm. The concentration of cells was determined from the linear relationship between the optical density at 610 nm (OD610) and dry cell weight.
Experiments
The complex of Fe(III)EDTA was prepared with equal mol FeCl3·6H2O and Na2EDTA.
Preparation of Fe(II)EDTA-NO solution: NO was bubbled through a solution of ferrous EDTA until full breakthrough of NO was observed in the sparging vessel effluent. The prepared solution was stored in a glass serum vials under N2 positive pressure to avoid oxidation of ferrous EDTA in solution. Details of the process can be found in Jing et al.(2004c).
The inhibition experiment was conducted in 50 ml conical flasks sealed with teflon-coated rubber septa in a gyrating shaker at 140 r/min and temperature of 40 °C. The anaerobic condition was obtained by replacing the air above the solution surface with oxygen-free nitrogen gas. Glucose was added in the amount of 1000 mg/L to supply electron acceptor for biological reduction. Twelve mmol/L Fe(III)EDTA and certain concentration of Fe(II)EDTA-NO were added to the solution. The total solution volume was 50 ml, and 140 mg/L cells were inoculated. The experiments were repeated in the same conditions, and the data in this paper were average ones. The maximum relative standard deviation (RSD max) was showed in the figures.
RESULTS AND DISCUSSION
The growth of cells in flask containing 12 mmol/L Fe(III)EDTA with different concentration of Fe(II)EDTA-NO is shown in Fig.1. It was found that Fe(II)EDTA-NO inhibited the cell growth of Klebsiella Trevisan sp. and that the cell growth rate decreased with the concentration of Fe(II)EDTA-NO. Several distinct phases (lag phase, exponential phase, declining growth phase and stationary growth phase) of cell growth could be observed when there was no Fe(II)EDTA-NO present. There was an initial lag phase, followed by an exponential growth phase when cell concentration increased exponentially, following which was a short phase of declining growth, and then a stationary phase, and the highest cell concentration was obtained finally. The cell growth period changed when Fe(II)EDTA-NO was present. When the concentration of Fe(II)EDTA-NO was 0.75 mmol/L, the phase of cell growth was not distinct. The exponential phase of cell growth was obviously delayed when 1.50 mmol/L Fe(II)EDTA-NO was present. The final cell concentration was also decreased when Fe(II)EDTA-NO was present. The cell concentration only increased 82 mg/L in 9 h in the solution when the initial Fe(II)EDTA-NO concentration was 1.50 mmol/L, but increased about 180 mg/L when there was no Fe(II)EDTA-NO present.
Fig. 1.
Curve of cells growth in Fe(III)EDTA solution containing Fe(II)EDTA-NO (T=313 K, [Fe(III)EDTA]=12 mmol/L)
The cell concentration and Fe(II)EDTA-NO concentration was monitored during inoculation of microbial cells into solution containing Fe(II)EDTA-NO at initial concentration of 1.50 mmol/L. Fig.2 shows the cell growth rate was slow when the concentration of Fe(II)EDTA-NO was higher than 0.4 mmol/L. It was also found that this bacteria could reduce Fe(II)EDTA-NO though the reduction was slow. Fe(II)EDTA-NO could be reduced totally within 12 h when the initial concentration was 1.5 mmol/L. However, a control experiment showed that Fe(II)EDTA-NO would hardly be reduced without bacteria in this experimental condition (data not shown).
Fig. 2.
Relationship between concentration of Fe(II)EDTA-NO and cells growth (T=313 K, [Fe(III)EDTA]=12 mmol/L)
Fig.3 shows that the Fe(III)EDTA reduction rate was about 70% in 13.5 h when there was no Fe(II)EDTA-NO present, but the reduction rate decreased to 54.8% in the presence of 0.75 mmol/L Fe(II)EDTA-NO, and decreased to 45.2% in the presence of 1.5 mmol/L Fe(II)EDTA-NO. The main reason was that the quantities of cells decreased when there was Fe(II)EDTA-NO present, but the final concentration of cells was almost same when there was different concentration of Fe(II)EDTA-NO present. It was also found that Fe(II)EDTA-NO could weaken the ability of Klebsiella Trevisan sp. to reduce Fe(III)EDTA.
Fig. 3.
Effect of Fe(II)EDTA-NO on the Fe(III)EDTA reduction (T=313K, [Fe(III)EDTA]=12 mmol/L)
From the above experimental results, we know that Fe(II)EDTA-NO significantly inhibits the growth of the iron-reduction bacteria and the Fe(III)EDTA reduction. Further study will focus on the inhibition kinetics of Fe(III)EDTA reduction.
Footnotes
Project (No. 20176052) supported by the National Natural Science Foundation of China and the Scientific Research Foundation for Returned Overseas Chinese Scholars, Ministry of Education
References
- 1.Harriott P, Smith K, Benson LB. Simultaneous removal of NO and SO2 in packed scrubbers or spray towers. Environ Prog. 1993;12(2):110–113. [Google Scholar]
- 2.Jing GH, Li W, Shi Y, Ma BY, Tan TE. Regeneration of nitric oxide chelate absorption solution by two heterotrophic bacterial strains. J Zhejiang Univ SCI. 2004;5(4):432–435. doi: 10.1631/jzus.2004.0432. [DOI] [PubMed] [Google Scholar]
- 3.Jing GH, Li W, Shi Y, Ma BY, Tan TE. Isolation and its properties of bacterial strain for Fe3+EDTA reduction. China Environmental Science. 2004;24(4):447–451. (in Chinese) [Google Scholar]
- 4.Jing GH, Li W, Shi Y, Ma BY, Tan TE. Properties of Pseudomonas sp. DN-1 in reduction of nitric oxide chelate absorption solution. Chinese Journal of Environmental Science. 2004;25(4):163–166. (in Chinese) [PubMed] [Google Scholar]
- 5.Li W, Shi Y, Jing GH, Ma BY, Chang SG. A novel approach for simultaneous reduction of Fe(II)EDTA-NO and Fe(III)EDTA using micro-organisms. J Chem Ind Eng (China) 2003;54(9):1340–1342. [Google Scholar]
- 6.Shi Y, Littlejohn D, Chang SG. Integrated tests for removal of nitric oxide with iron thiochelate in wet flue gas desulfurization systems. Environmental Science and Technology. 1996;30(11):3371–3376. [Google Scholar]
- 7.Shi Y, Littlejohn D, Chang SG. Kinetics of NO absorption in aqueous iron(II) bis(2,3-dimercapto-1-propanesulfonate) solutions using a stirred reactor. Ind Eng Chem Res. 1996;35(5):1668–1672. [Google Scholar]
- 8.Shi Y, Littlejohn D, Kettler PB, Chang SG. Removal of nitric oxide from flue gas with iron thiochelate aqueous solution in a turbulent contact absorber. Environ Prog. 1996;15(3):153–158. [Google Scholar]
- 9.Shi Y, Wang H, Chang SG. Kinetics of NO absorption in aqueous iron(II) thiochelate solutions. Environ Prog. 1997;16(4):301–306. [Google Scholar]
- 10.Tsai SS, Bedell SA, Kirby LH, Zabick DJ. Field evaluation of nitric oxide abatement with ferrous chelates. Environ Prog. 1989;8:126–129. [Google Scholar]





