Abstract
The origin of 5-HMF (5-hydroxymethyl-2-furaldehyde) in a Shengmaiyin decoction was investigated by the RP-HPLC method below. A C18 column (250 mm×4.6 mm, i.d. 5 μm) with a column temperature of 25 °C was used. The mobile phase was a mixture of ultra-pure water-acetonitrile (95:5, V/V) and the flow rate was 1.0 ml/min. The detection wavelength was 280 nm. The injection volume was 1 μl and the running time was about 20 min. The addition of Schisandra was regulated to assess the contribution of an acid environment to the production of 5-HMF. In order to confirm the role of saccharides in the production of 5-HMF, different amount of fructose was used. The 5-HMF level in decoctions of processed and unprocessed Schisandra was investigated in order to determine the origin of 5-HMF. The results showed that 5-HMF was derived mainly from the decoction of Schisandra only and not the mixed decoction of Ophionpogon and Schisandra. The appearance of 5-HMF is not simply the result of the decomposition of saccharides under the acid environment created by Schisandra, but the processing procedure plays an important role in the production of 5-HMF.
Keywords: Shengmaiyin, 5-HMF, Origin, Schisandra, Process
INTRODUCTION
Shengmaiyin or Shengmaisan, one of the famous herbal recipes in traditional Chinese medicine, is made from Radix ginseng, Ophiopogon and Schisandra. It is used to treat hypotension (Chen et al., 2000; Gui, 2001), cardiovascular system diseases (Qian et al., 2004; Zeng and Zeng, 2004), to increase immunity (Wu et al., 1997; Guo et al., 2004) and cure other diseases (Li et al., 1996; Liu et al., 1998; Fang et al., 2000; Yi et al., 2003; Xu et al., 2003; Wang et al., 2004a; Wang et al., 2004b). Zhu et al.(1998a) found a new component, 5-hydroxymethyl-2-furaldehyde (5-HMF) in the decoction of Shengmaiyin and believed that 5-HMF derives from the decoction of Ophiopogon and Schisandra in the decomposition of saccharide under the acid environment created by Schisandra (Xia et al., 1998). The contribution of 5-HMF to the cure of cardiovascular disease (Zhu et al., 1998b) was also found in many other herbs (Li et al., 1999; Zhang et al., 2001; Meng et al., 2002; Wang et al., 2003; Dong and Wang, 2004; Yuan et al., 2004; Xu et al., 2004).
Normally, 5-HMF is poisonous to the nervous system (Chi et al., 1998), by combining to protein and thus causing the accumulation of poisons in the body; and has potent reproductive toxicity (Zdzienicka et al., 1978; Wang et al., 1994; Qasim et al., 1995), and also damages striated muscles and viscera (Reinald et al., 1995). Many countries strictly limit the content of 5-HMF in honey, beer and glucose injections (Cohen et al., 1994; Lo et al., 1995; Zeng et al., 2002; Li et al., 2004).
It is very important to control the level of 5-HMF for bio-activity and side effects of the herbs. The origin of 5-HMF in the Shengmaiyin was investigated in order to optimize Shengmaiyin production to acheieve quality control and high curative effect with minimal side-effects, and to contribute to better understanding of traditional Chinese medicine.
MATERIALS AND INSTRUMENTS
Materials and reagents
Unprocessed Schisandra is the dry, ripe fruit of Schisandra chinensis (Turcz.) Baill, but medicinal Schisandra is made from both Schisandra chinensis (Turcz.) Baill and Schisandra sphenanthera Rehd. et Wils. by different processes. Ophiopogon is the dry root of Ophiopogon japonicus (Thunb.) Ker-Gawl. All crude herbal and medicinal medicines were authenticated by Professor Chen Kong-rong, Zhejiang College of Traditional Chinese Medicine and met the standards of the Pharmacopoeia of PRC 2005 Edition. Chemicals used included HPLC grade acetonitrile (Merck, Germany), ultra-pure water (milli-Q) and 5-HMF (Sigma, USA).
Instrumentation
PHSJ-4A pH meter and Agilent HP 1100 HPLC systems equipped with a G1322A solvent degasser, a G1354A quaternary gradient solvent pump, a G1313A multiple auto-sampler, a G1316A thermostat column compartment, a G1314A UV-Vis detector and Agilent HP 1100 chromatography workstation were used for analyses.
METHODS
Analytical methods
The standards and samples of 5-HMF were all analyzed by RP-HPLC. Chromatographic conditions were as follow: Chromatographic separation was performed on a Diamonsil C18 column (250 mm×4.6 mm i.d., 5 μm; Dikma) with a guard column (4 mm×3 mm i.d., 5 μm; Phenomenex) packed with the same material at 25 °C. The mobile phase was a mixture of ultra-pure water-acetonitrile (95:5, V/V) filtered through a 0.45 μm filter and delivered at a flow rate of 1.0 ml/min and detection wavelength of 280 nm, injection volume was 1 μl and running time was about 20 min.
Preparation of calibration curve
The stock solution of 5-HMF was prepared by dissolving 13.62 mg 5-HMF in a 50 ml volumetric flask with ultra-pure water and stored at 4 °C. Seven concentrations of 5-HMF standard solutions (2.724, 5.448, 8.172, 10.896, 13.620, 16.344, 27.240 mg/100 ml) were obtained by appropriately diluting the stock solution with mobile phase and prepared for evaluating the linearity by HPLC. The injection volume was 1 μl. The calibration curve was obtained by plotting the peak areas against the concentrations of 5-HMF standard solutions.
Samples preparation
Schisandra and fructose samples: 12.5 g medicinal Schisandra and a series of different weights of fructose (0 g, 0.25 g, 0.5 g, 1.0 g, 1.5 g, 2.0 g) were soaked in 250 ml deionized water for 2 h and weighed, then decocted for 1 h at boiling temperature and reweighed. Deionized water was added to the decocted samples to make them reach the weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm filter for HPLC analysis.
Ophiopogon and acid samples: 25 g medicinal Ophiopogon were soaked in a series of different pH value acid solutions regulated by hydrochloric acid, sulfuric acid and acetic acid (HCl: 2.972, 3.142, 3.454; H2SO4: 2.987, 3.058, 3.401; HAc: 2.894, 3.012, 3.331) for 2 h and weighed, then decocted for 1 h at boiling temperature, reweighed, and then deionized water was added to the decocted samples to make them reach the weight of the sample before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm filter for HPLC analysis. Ophiopogon samples were prepared by soaking 25 g medicinal Ophiopogon in 250 ml deionized water for 2 h and then treated the same way as that for the Ophiopogon and acid samples.
Schisandra and Ophiopogon samples preparation: 25 g medicinal Ophiopogon and a series of different weights of medicinal or unprocessed Schisandra (6.25 g, 12.5 g, 18.75 g, 25 g, 50 g) were soaked in 250 ml deionized water for 2 h and weighed, then decocted for 1 h at boiling temperature, reweighed, and then deionized water was added to the decocted samples to make them reach the weight of the sample before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm filter for HPLC analysis.
Schisandra samples: different weights of medicinal or unprocessed Schisandra (6.25 g, 12.5 g, 18.75 g, 25 g, 50 g) were soaked in 250 ml deionized water for 2 h and weighed, then decocted for 1 h at boiling temperature and reweighed, and then deionized water was added to the decocted samples to make them reach the weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm filter for HPLC analysis.
Precision
Both standard solutions and samples were injected at a volume of 1 μl six times repeatedly according to the analytical method mentioned above, and the relative standard deviations were calculated.
Stability
Both standard solutions and sample decoctions at 2 h, 4 h, 6 h, 8 h, 10 h, 12 h and 24 h were analyzed according to the analytical method mentioned above, and the relative standard deviations were calculated.
Accuracy
In 250 ml deionized water 12.5 g processed medicinal Schisandra were soaked for 2 h and weighed, then decocted for 1 h at boiling temperature and reweighed, and then deionized water was added to the decocted samples to make them reach the weight before decoction. The decoction was filtered and the filtrate was passed through a 0.45 μm filter for HPLC analysis. Six Schisandra samples were prepared under the same conditions above, and the relative standard deviation of the six samples was calculated.
Recoveries
Different weight 5-HMF was added to the Schisandra decoction samples and the content of 5-HMF in the decoctions was analyzed by the method mentioned above. The recoveries were calculated by using the ratio of the detected content to added content of 5-HMF.
RESULTS AND DISCUSSION
Performance of HPLC system
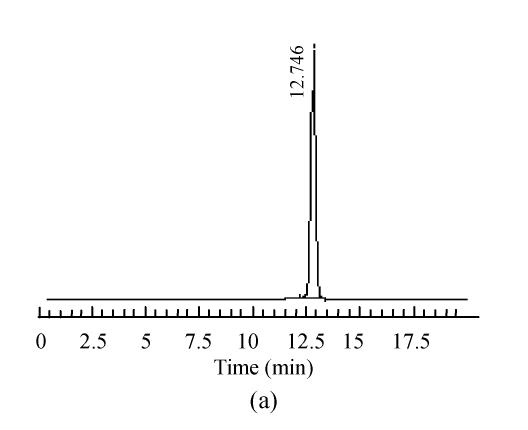
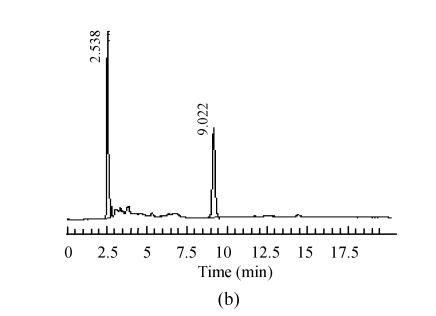
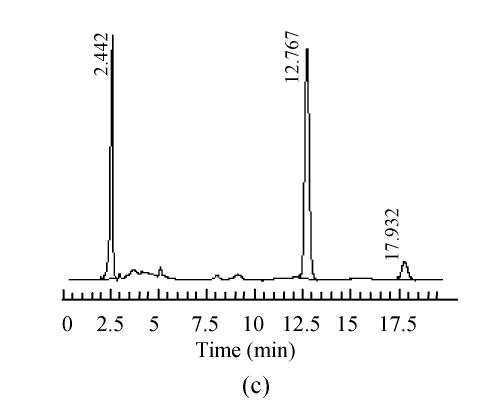
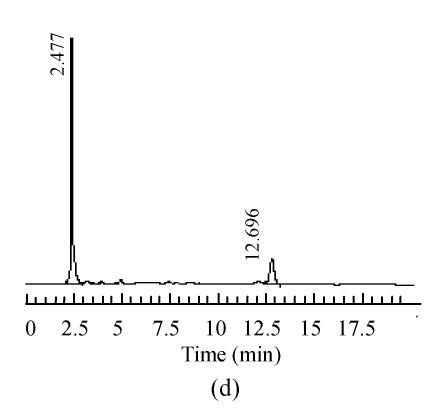
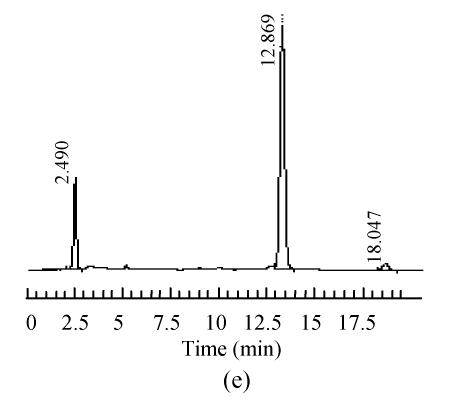
The mobile phase containing different proportions of ultra-pure water and acetonitrile was tested to find the optimal combination. After several trials, a mobile phase consisting of ultra-pure water and acetonitrile (95:5, V/V) was used which could achieve effective separation and acceptable sensitivity, as well as symmetric peak shape and short analytical period. Representative chromatograms are shown in Fig.1. The retention time of 5-HMF was about 13 min, no interferences were observed.
Fig. 1.
Chromatograms of standard and samples
(a) Chromatogram of 5-HMF standard solution; (b) Chromatogram of unprocessed Schisandra decoction from Schisandra chinensis (Turcz.) Baill; (c) Chromatogram of medicinal Schisandra decoction from Schisandra chinensis (Turcz.) Baill processed by factory A; (d) Chromatogram of medicinal Schisandra decoction from Schisandra sphenanthera Rehd. et Wils. processed by factory B; (e) Chromatogram of medicinal Schisandra decoction from Schisandra sphenanthera Rehd. et Wils. processed by factory C
System suitability
The number of theoretical plates of column (N) and the tailing factor (T) to analyze 5-HMF were about 14 300 and 0.96, respectively. Both conformed to the Pharmacopoeia of PRC.
Linearity
The peak area (Y) and concentration (X) of 5-HMF standard solutions were subjected to regression analysis to calculate the calibration equation and correlation coefficient. The calibration equation was Y=7376.8X+6.8016, R 2=0.9999. The results showed excellent correlation between the peak area and the concentration of 5-HMF within the range of concentrations from 0.02724 to 0.2724 mg/ml.
Precision
The relative standard deviations of six injections for standard and samples were 0.14% and 0.71%, respectively. The system yielded excellent performance for analysis of 5-HMF content.
Stability
5-HMF in standard solution and in samples showed excellent stability according to the results. The relative standard deviations of standard solution and sample were 0.15% and 1.23%, respectively.
Repeatability
The content of 5-HMF in 6 Schisandra samples prepared under the same conditions showed no marked difference. The relative standard deviation of the six data was 1.58%.
Recoveries
The average extraction recovery of 5-HMF in decoction was 98.89% and the relative standard deviation was 2.78%. The average recoveries were sufficiently high to reach the normal analytical criterion. The extraction recovery data of 5-HMF are presented in Table 1.
Table 1.
The extraction recovery data on 5-HMF
| Sample | Found (mg) | Added (mg) | Extraction recovery (%) | Average (%) | RSD (%) |
| 1 | 13.46 | 13.62 | 98.83 | 98.89 | 2.78 |
| 2 | 13.04 | 13.62 | 95.77 | ||
| 3 | 13.64 | 13.62 | 100.12 | ||
| 4 | 13.30 | 13.62 | 97.66 | ||
| 5 | 13.25 | 13.62 | 97.31 | ||
| 6 | 14.12 | 13.62 | 103.65 |
The influence of fructose addition on the 5-HMF level
The effect of adding fructose is presented in Table 2. The results showed that additional fructose had no evident effects on the 5-HMF level of the decoctions (Table 2).
Table 2.
The influence of adding fructose on the 5-HMF level
| Fructose (g) | 5-HMF concentration (mg/100 ml) | Average (mg/100 ml) | RSD (%) |
| 0 | 17.6 | 17.5 | 2.18 |
| 0.2500 | 17.7 | ||
| 0.7253 | 17.3 | ||
| 1.0249 | 17.9 | ||
| 1.2650 | 17.8 | ||
| 1.6482 | 16.9 | ||
| 2.3189 | 17.3 |
The influence of pH value variation on 5-HMF level
Although variation in the quantity of Schisandra had very little effect on the pH value, there was nonetheless evident effect on the 5-HMF level in the decoctions. The small change of pH value caused by different acids had no evident effect on the production of 5-HMF when compared to the effects produced by Schisandra. Table 3 shows the influence of the quantity of Schisandra on pH value and the pH value variation on 5-HMF level. The results showed that it was not simply the decomposition of saccharide under the acid environment created by Schisandra but that Ophiopogon alone can also produce 5-HMF at a very low level when decocted without Schisandra or acid. Maybe 5-HMF originated mainly from Schisandra, not in combination with Schisandra/Ophiopogon decoction and there are other factors affecting the producing of 5-HMF besides the acid environment created by Schisandra. The result conflicts with Zhu et al.(1998b)’s report. Straightforward detection of 5-HMF in the sample without any treatment in this experiment makes the result more scientific. Some changes in Schisandra components, properties inevitably occur in the course of sample treatment.
Table 3.
The influence of Schisandra quantity on pH value and 5-HMF level
| Acid environment creating material |
Ophiopogon (g) | pH | 5-HMF content (mg/100 ml) | |
| Schisandra (g) | Acid | |||
| 6.25 | - | 25 | 3.094 | 8.70 |
| 12.50 | - | 25 | 3.046 | 16.40 |
| 18.75 | - | 25 | 3.024 | 24.20 |
| 25.00 | - | 25 | 2.993 | 31.60 |
| 50.00 | - | 25 | 2.930 | 59.90 |
| - | HCl | 25 | 2.972 | 0.39 |
| - | HCl | 25 | 3.142 | 0.46 |
| - | HCl | 25 | 3.454 | 0.46 |
| - | H2SO4 | 25 | 2.987 | 0.44 |
| - | H2SO4 | 25 | 3.058 | 0.50 |
| - | H2SO4 | 25 | 3.401 | 0.40 |
| - | HAC | 25 | 2.894 | 1.15 |
| - | HAC | 25 | 3.012 | 0.67 |
| - | HAC | 25 | 3.331 | 0.52 |
| - | - | 25 | 6.576 | 0.34 |
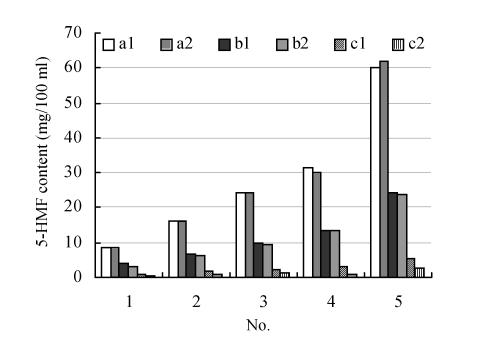
The influence of different Schisandra on the 5-HMF level of Ophiopogon existence or inexistence
There were great differences in 5-HMF level among the three decoctions of medicinal or unprocessed Schisandra and Ophiopogon. The 5-HMF level between the decoctions of unprocessed Schisandra only and Schisandra/Ophiopogon group was different but there was no evident difference between the decoctions of medicinal Schisandra only and Schisandra/Ophiopogon group. 5-HMF content differed with the processing methods of Schisandra. The results showed that the presence of Ophiopogon had no evident effect on the production of 5-HMF when Schisandra was processed but that the processing method had great effect on the 5-HMF level in the decoctions (Table 4, Fig.2). The changing 5-HMF level in the decoction was mainly affected by Schisandra processing methods. The results was confirmed in the report that 5-HMF appeared in the course of Ephedra sinica Stapf processing (Xu et al., 2004). The processing time, processing temperature and herb species probably all affect 5-HMF level of medicinal Schisandra during the course of the processing.
Table 4.
The influence of the Ophiopogon and non-Ophiopogon existence on the level of 5-HMF in decoctions
| No. | Schisandra content (g) | 5-HMF content (mg/100 ml) |
|||||
|
Ophiopogon+Schisandra group |
Schisandra group |
||||||
| a1 | b1 | c1 | a2 | b2 | c2 | ||
| 1 | 6.25 | 8.67 | 3.98 | 1.06 | 8.32 | 3.28 | 0.52 |
| 2 | 12.50 | 16.35 | 6.72 | 1.62 | 16.08 | 6.22 | 0.81 |
| 3 | 18.75 | 24.23 | 9.95 | 2.15 | 24.44 | 9.53 | 1.22 |
| 4 | 25.00 | 31.55 | 13.26 | 3.01 | 29.95 | 13.37 | 1.07 |
| 5 | 50.00 | 59.91 | 24.25 | 5.18 | 62.11 | 23.67 | 2.48 |
Fig. 2.
The influence of Ophiopogon addition on the production of 5-HMF
CONCLUSION
The composition of traditional Chinese medicine is very complex and every procedure of processing may change the results. The method of analysis established can reduce the operational error by a straight analysis of the components of herbal decoctions without extra treatment and provide more accurate results.
It was concluded that 5-HMF of Shengmaiyin was mainly derived from the decoction of Schisandra and not from the combined Ophiopogon/Schisandra decoction, because there was no evident difference in the 5-HMF level between the medicinal Schisandra/Ophiopogon group and Schisandra alone group and Schisandra content or Schisandra processed methods had great effect on the 5-HMF level. The factors affecting 5-HMF level in Schisandra will be reported in succession.
The addition of fructose has no effect on the production of 5-HMF in the decoction, showing that the appearance of 5-HMF is not simply the decomposition of saccharide under the acid environment created by Schisandra. The difference in 5-HMF content between the decoctions of unprocessed and processed Schisandra showed that the processing method played an important role in the production of 5-HMF. Because 5-HMF is found in many herbs, the physiological and biochemical roles of 5-HMF in the herbs deserved further investigation in the future.
Acknowledgments
We thank Prof. Chen Kong-rong for his help in herb authentication. The authors are grateful to Miss Ying S.S. for her help in preparing samples and thank Prof. Sarah Radloff and Prof. Randall Hepburn for editing the article in English.
Footnotes
Project (No. 2004CCA05500) supported by the Ministry of Science and Technology, China
References
- 1.Chen W, Meng QY, Shen H, liu G, Wang XW. Experimental study on the effect of Shengmai injection in blood pressure on anesthesia dogs. Chin J Integr Tradit West Med Intensive Crit Care. 2000;7(4):229–231. (in Chinese) [Google Scholar]
- 2.Chi W, Zhang CB, Cao YH, Guo LY. Investigation of the restriction on the formation of 5-HMF. Spec J Pharm People’s Mil Surg. 1998;14(2):101–104. (in Chinese) [Google Scholar]
- 3.Cohen E, Birk Y, Mannheim CH, Saguy IS. Kinetic parameter estimation for quality change during continuous thermal processing of grapefruit juice. J Food Sci. 1994;59(1):155–158. [Google Scholar]
- 4.Dong L, Wang X. Analysis of volatile components in the extract of siraitia grosvenorii by combination of solid phase micro-extraction and gas chromatography-mass spectrometry. J Xinxiang Med College. 2004;21(1):26–28. (in Chinese) [Google Scholar]
- 5.Fang ZJ, Jiang HM, Wang LH. Therapeutic effect of Shengmai injection on respiratory function in chronic obstructive pulmonary disease. CJIM. 2000;6(2):101–103. (in Chinese) [PubMed] [Google Scholar]
- 6.Gui JL. Effect of Shengmai injection on blood pressure, heart rate and urine volume of patients with acute in ferior wall complicated with right ventricular myocardial infarction. Hebei J TCM. 2001;23(9):708–709. (in Chinese) [Google Scholar]
- 7.Guo CX, Yang XY, Lin ZF, Zhao L, Chan HW, Chen DC, Yan M. Clinical observation on effects of Shengmai injection on plasma vasoactive mediators in patients with systemic inflammatory response syndrome. Chin J TCM Crit Care. 2004;11(4):239–241. (in Chinese) [Google Scholar]
- 8.Li M, Zhao FJ, Guo JS, Li ZY. Effects of Jiawei Shengmai san on the glucocorticoid and its receptor in heat-stressed rats. Acad J Sci Mil Med Univ. 1996;17(6):542–544. (in Chinese) [Google Scholar]
- 9.Li YM, Jiang SH, Gao WY, Zhu DY. Liposoluble constituents from the roots of scrophularia ningpoensis. Acta Pharm Sinica. 1999;34(6):448–450. (in Chinese) [Google Scholar]
- 10.Li W, Zhang XM, Lu Y, Yu GQ, Lu JP, Nan QX, Chen LJ. Browning-inhibition technology for low-lactose milk. Chin Dairy Industry. 2004;32(6):20–23. (in Chinese) [Google Scholar]
- 11.Liu T, Qin CL, Zhang Y, Wang YS, Zhou JL. Comparison in anti-arrhythmia and anti-shock effects between individual decoction of Shengmaiyin’s ingredients and decoction of Shenmaiyin as a whole. Chin J Exp Tradit Med Formulae. 1998;4(5):36–38. (in Chinese) [Google Scholar]
- 12.Lo CF, Valentini C, Novelli V, Ceccon L. Liquid chromatographic determination of 2-furaldehyde and 5-hydroxymethyl-2-furaldehyde in beer. Anal Chim Acta. 1995;306(1):57–64. [Google Scholar]
- 13.Meng DL, Li X, Xiong YH, Wang JH. Study on the chemical constituents of achyranthes bidentata Bl. J Shenyang Pharm Univ. 2002;19(1):27–30. (in Chinese) [Google Scholar]
- 14.Qasim AK, Farrukh AS, Hadi SM. Mutagenicity of furfural in plasmid DNA. Cancer Letters. 1995;89:95–99. doi: 10.1016/0304-3835(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 15.Qian XQ, Chen H, Jin ZQ. Effects of Shengmai-Siwu decoction on insulin-like growth factor-1 in the patients with hypertophic cardiomyopathy. Chin J Integr Med on Cardio/Cerebrovascular Disease. 2004;2(8):440–441. (in Chinese) [Google Scholar]
- 16.Reinald P, Bellmunt MJ, Manel P, David R, Joan P. Chromatographic evidence for amadori product formation in rat liver aminophospholipids. Life Sci. 1995;57(9):873–879. doi: 10.1016/0024-3205(95)02020-j. [DOI] [PubMed] [Google Scholar]
- 17.Wang JS, Niu FY, Sun P, Li T. Investigation on the toxicity of furfural. J Health Toxicology. 1994;8(3):21–23. [Google Scholar]
- 18.Wang YF, Mu TH, Chen JJ, Luo SD. Studies on chemical constituents from the root of polygonatum kingianum. Chin J Chin Marterial Med. 2003;28(6):524–527. (in Chinese) [PubMed] [Google Scholar]
- 19.Wang L, Gong MX, Chen X. Protective effects of Shengmai injection on oxidative injury in cultured PC12 cells. Chin J the Practical Chin with Modern Med. 2004;4(17):1792–1794. (in Chinese) [Google Scholar]
- 20.Wang ZQ, Yu CF, Tian ZHL. Prevention of premature initial aprea with Shengmai injection. J Beijing Univ of TCM (Clinical Med) 2004;11(2):1–3. (in Chinese) [Google Scholar]
- 21.Wu CR, Zhao WN, Liu HB, He ST. Immune-modulation effect of Shengmaisan with astraglus membranceus on cell-mediated immunity of mouse with wound stress. J Hunan College TCM. 1997;17(2):48–50. (in Chinese) [Google Scholar]
- 22.Xia Y, Li ZM, Zhu DN, Yan YQ. A research on chemical dynamic changes and drug efficacy of SMS compound prescription chemical researches on SMS prescription (i) Chin J Chin Material Med. 1998;23(4):230–231. (in Chinese) [PubMed] [Google Scholar]
- 23.Xu CY, He W, Fang B, Pan YG, Yuan H. Regulatory effect of Shengmai Chenggu recipe on collagen type I in necrotic femoral head. Modern J Integr Tradit Chin West Med. 2003;12(6):574–576. (in Chinese) [Google Scholar]
- 24.Xu BD, Chen K, Lin WJ, Zhang HL. Comparative study on chemical components of supercitical extracts from herba ephedrae and honey-prepared herba ephedrae. J Guangzhou Univ TCM. 2004;21(3):211–212. (in Chinese) [Google Scholar]
- 25.Yi YX, Yang Y, Qu XB, Liu YL. Clinical effect and mechanism of Shengmai injection in treating senile cerebral infarction. CJIM. 2003;9(1):60–62. [Google Scholar]
- 26.Yuan JZ, Wu LJ, Chen YJ, Li W, Xiao CYN, Er JTB. Isolation, identification of the chemical constituents from smilax glabra roxb. Chin J Med Chem. 2004;14(5):291–295. (in Chinese) [Google Scholar]
- 27.Zdzienicka M, Tudek B, Zielenska M, Szymczyk T. Mutagenic activity of furfural in salmonella typhimurjum TA100. Mutat Res. 1978;58:205–209. doi: 10.1016/0165-1218(78)90010-1. [DOI] [PubMed] [Google Scholar]
- 28.Zeng XH, Zeng XJ. Injection Shengmai therapy for chronic heart failure and ginsenoside re plasma concentration monitoring by HPLC. J Chengdu Univ (Natural Science) 2004;23(1):1–8. (in Chinese) [Google Scholar]
- 29.Zeng ZL, Ye ZX, Wan DM, Xiong T, Peng SH. The effect on the content of HMF of honey by heating process. J Nanchang Univ (Natural Sci) 2002;26(1):67–70. (in Chinese) [Google Scholar]
- 30.Zhang QB, Qiao F, Chen XX, Chen J, Zhang YH. Determination of 5-HMF in radix rehmanniac preparata by HPLC. Chin Drug Standards. 2001;2(4):33–34. (in Chinese) [Google Scholar]
- 31.Zhu DN, Yan YQ, Li ZM. A research on chemical dynamic changes and drug efficacy of SMS compound prescription chemical researches on SMS prescription (iii) Chin J Chin Material Med. 1998;23(8):483–485. (in Chinese) [PubMed] [Google Scholar]
- 32.Zhu DN, Li ZM, Yan YQ, Zhu JG. A research on chemical dynamic changes and drug efficacy of SMS compound prescription chemical researches on SMS prescription (ii) Chin J Chin Material Med. 1998;23(5):291–293. (in Chinese) [PubMed] [Google Scholar]