Abstract
Groundwater remediation by nanoparticles has received increasing interest in recent years. This report presents a thorough evaluation of hexavalent chromium removal in aqueous solutions using iron (Fe0) nanoparticles. Cr(VI) is a major pollutant of groundwater. Zero-valent iron, an important natural reductant of Cr(VI), is an option in the remediation of contaminated sites, transforming Cr(VI) to essentially nontoxic Cr(III). At a dose of 0.4 g/L, 100% of Cr(VI) (20 mg/L) was degraded. The Cr(VI) removal efficiency decreased significantly with increasing initial pH. Different Fe0 type was compared in the same conditions. The reactivity was in the order starch-stabilized Fe0 nanoparticles>Fe0 nanoparticles>Fe0 powder>Fe0 filings. Electrochemical analysis of the reaction process led to the conclusion that Cr(OH)3 should be the final product of Cr(VI). Iron nanoparticles are good choice for the remediation of heavy metals in groundwater.
Keywords: Hexavalent chromium, Iron nanoparticles, Starch, Stabilize, Remediation
INTRODUCTION
Chromium is a commonly identified contaminant in soils and groundwater and is widely applied in industries. In the United States, it is considered a major pollutant. Cr(VI) is toxic, carcinogenic to human and animals. In the environment Cr(VI)’s great subsurface mobility does great harm to the environment. In contrast, Cr(III) is much less toxic and immobile and can be a nutriment for human and animals. Therefore, reduction of Cr(VI) to Cr(III) is favorable for the environment and feasible method in the remediation of environmental sites.
Much research has focused on the remediation of Cr(VI) and many treatment processes have been developed. Physio-chemical adsorption has long been researched (Bowman, 2003; Hideaki et al., 2002), but the cost is high and the Cr(VI) is just transferred but not removed. Bioremediation by strains of bacteria (Chen and Hao, 1998) can effectively degrade Cr(VI) and is an economically favor but the bactericidal toxicants at many waste sites would limit their growth and effectiveness. Chemical reduction is known to remove Cr(VI) rapidly and effectively. Many reductants were employed such as H2S, Fe2+, Fe0, etc. (Hua and Deng, 2003; Buerge and Hug, 1999). Cr(VI) reduction by Fe0 (Alowitz and Scherer, 2002; Ponder et al., 2000; Powell et al., 1995; Pratt et al., 1997) appears to be one of the most promising technologies. Powell et al.(1995) suggested that the mechanisms of Cr(VI) reduction by Fe0 is a cyclic and consists of multiple reactions electrochemical corrosion. Alowitz and Scherer (2002) evaluated the effect of iron metal type, Fe0 area concentration and pH value on the rate of Cr(VI) reduction by Fe0. Ponder et al.(2000) tested nanoscale Fe0 particles for their ability to separate and immobilize Cr(VI) ions from aqueous solution. Fe0 nanoparticles, due to their extremely high effective surface area, can enhance the reduction rates markedly. However, because of the extremely high reactivity, the initially formed nanoparticles tend to either react rapidly with surrounding media [e.g., dissolved oxygen (DO) or water] or agglomerate rapidly, resulting in the formation of numerous large particles and rapid loss in reactivity. A new process must be found to prepare physically more stable Fe0 nanoparticles. Raveendran et al.(2003) showed that starch could serve as a good dispersant for preparing nanoscale Ag particles in aqueous media. He and Zhao (2005) prepared and characterized a new class of starch-stabilized bimetallic nanoparticles. Starch, a “greener” for environmental applications, could apparently prevent agglomerated nanoparticles from becoming more reactive.
A range of Fe(0) and Fe(II) bearing materials promoted reduction and precipitation of Cr(VI). The net reactions of Cr(VI) reduction and coprecipitation of Cr(III) and Fe(III) are (Lee et al., 2003):
| CrO42−+Fe0+8H+→Fe3++Cr3++4H2O | (1) |
| (1−x)Fe3++(x)Cr3++2H2O→Fe(1−x)CrxOOH(S)+3H+ | (2) |
The primary objective of this work is to prepare Fe0 or starch-stabilized Fe0 nanoparticles for degration of Cr(VI). The specific objectives are to (1) characterize the Fe0 nanopraticles with environmental scanning electron microscope (ESEM); (2) quantify the effect of Fe0 nanoparticles’ dosage, Cr(VI) concentration, and initial pH on the rate of Cr(VI) reduction; (3) compare the effectiveness of Cr(VI) reduction by different type of Fe0 particles; (4) analyse the reaction process electrochemically.
MATERIALS AND METHODS
Chemicals
All chemical reagents, such as K2Cr2O4, starch, FeSO4·7H2O, sulfuric acid (H2SO4) and acetone were analytical reagent grade. The starch was purchased from Linghu foodstuff chemical plant of Huzhou, Zhejiang. The waste iron filings (30~40 mesh) were obtained from Machine factory of Zhejiang University. The commercial Fe0 powder (>98%, <200 mesh) was purchased from Jinshan Metallurgical Factory (China). Both iron particles were pretreated by wash using acetone and sulfuric acid solution (pH=2), which created fresh sites for oxidation and made the surface more active (Ruiz et al., 1995). Deionized water was used for preparing all solutions.
The Fe0 and starch-stabilized Fe0 nanoparticles were synthesized before use. The preparation of Fe0 nanoparticles and starch-stabilized Fe0 nanoparticles followed the methods described by Wei et al.(2004) and He and Zhao (2005), respectively. The iron nanoparticles were synthesized by dropwise addition of stoichiometric amounts of NaBH4 aqueous solution into a 1000 ml three-necked flask containing FeSO4·7H2O aqueous solution simultaneously with electrical stirring at ambient temperature. The ferrous iron was reduced to zero-valent iron according to the following reaction:
| Fe(H2O)62++2BH4−→Fe0↓+2B(OH)3+7H2↑ | (3) |
The Fe0 nanoparticles were then rinsed several times with deionized water.
In the preparation of starch-stabilized Fe0 nanoparticles, FeSO4·7H2O aqueous solution was added to a starch solution in a 1000 ml three-necked flask to yield a solution with desired Fe concentration and 0.2% starch concentration. The next steps were the same as those for preparation of Fe0 nanoparticles.
Batch experiments
The batch experiments for the reduction of Cr(VI) were performed in the same three-necked flask into which Fe0 nanoparticles were introduced. Then Cr2O7 2−aqueous solution was added into the flask. The reaction solution was stirred under nitrogen flow and periodically sampled by glass syringe. The sample was filtered immediately through 0.22 µm membrane filters for analysis.
The effect of various parameters on the Cr(VI) reduction was researched. Fe0 nanoparticles concentration used in this study was 0.1 to 0.4 g/L. The initial Cr(VI) concentration was 10 to 25 mg/L, and the initial pH was 3 to 10. Different size Fe0 particles at desired concentration were compared.
Characterization and analytical methods
The morphology of the metal particles was observed under an XL30-ESEM at 100 kV. Prior to scanning the fresh prepared metal particles were dried in vacuum at 25 °C for 60 min. Cr(VI) was determined spectrophotometrically with diphenylcarbazide at 540 nm using UV-VIS spectrophotometer (TU-1800PC, Beijing, China).
RESULTS AND DISCUSSION
Characterization of both Fe0 nanoparticles
Fig.1 compares the ESEM micrographs of the Fe0 nanoparticles before or after reaction. Both micrographs show that the Fe0 nanoparticles do not appear as discrete particles but form much larger dendritic flocs whose size could reach micron scale. The aggregation is attributed to the magnetic forces among the Fe particles. Similar phenomenon was observed by other researchers (Wei et al., 2004; He and Zhao, 2005). Fig.1a shows the loose structure and fine tentacles of Fe0 nanoparticles so that greater reactivity is expected. In contrast, Fig.1b shows tighter structure and thicker tentacles owing to the form of the hydroxide solid (CrxFe1−x)(OH)3 which covered the surface of the Fe0 nanoparticles.
Fig. 1.
ESEM image of Fe0 nanoparticles (a) before reaction and (b) after reaction
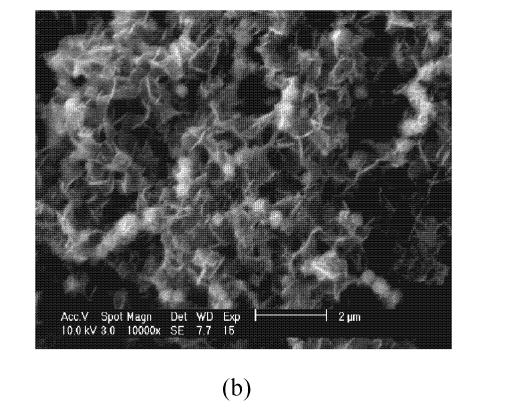
Fig.2 compares the ESEM images of the Fe0 nanoparticles without or with starch stabilizer. Fig.2a shows that Fe0 nanoparticles formed much larger dendritic flocs without starch. Fig.2b shows discrete Fe0 nanoscale particles in the presence of starch (splinter-shaped crystalloid). The presence of starch effectively prevents agglomeration of the iron particles and thus maintains high surface area and great reactivity.
Fig. 2.
ESEM image of (a) Fe nanoparticles without starch and (b) starch-stabilized Fe0 nanoparticles
Effect of Fe0 nanoparticles concentration
Four Fe0 nanoparticles concentrations were employed in this study. Fig.3 shows that the increase of Fe0 concentration greatly enhanced the removal efficiency. All Cr(VI) was removed when the Fe0 mass concentration was 0.4 g/L, but only 26% was removed when the Fe0 mass concentration was 0.1 g/L. Cr(VI) concentration decreased dramatically in the initial one minute, then slightly decreased in the later reaction. It was apparent that there was an initial sorption phase which appeared to be complete after 1 min. Thus, the overall mechanism is more complicated than just chemical reaction.
Fig. 3.
Effect of Fe0 nanoparticles concentration on the Cr(VI) removal efficiency
C Cr(VI)=20 mg/L, pH=7.0, T=25 °C, ω=600 r/min
Effect of initial Cr(VI) concentration
Fig.4 shows results of batch experiments conducted at concentrations of 10 to 25 mg/L. The Cr(VI) removal efficiency increased inversely with the concentration of initial Cr(VI), 42% at concentration of 25 mg/L and 100% at concentration of 10 mg/L. The proper mass ratio of Fe0 nanoparticles to Cr(VI) was about 20:1.
Fig. 4.
Effect of initial Cr(VI) concentration on the Cr(VI) removal efficiency
C Fe=0.2 g/L, pH=7.0, T=25 °C, ω=600 r/min
Effect of the initial pH value
As shown in Fig.5, the Cr(VI) removal efficiency increased significantly with decreasing pH, mainly because in acid condition, the accelerated corrosion of Fe0 enhanced the reaction rate. In the initial five minutes, the plots below pH 5 deceased rapidly, indicating that the Fe0 nanoparticles were still high reactivity. In contrast, the pH>8 plots decreased gently because of the form of Fe(OH)3 during high pH value. The almost horizontal segment of the plots after sixty minutes was caused by Fe0 oxidation.
Fig. 5.
Effect of initial pH value on the Cr(VI) removal efficiency
C Fe=0.2 g/L, C Cr(VI)=20 mg/L, T=25 °C, ω=600 r/min
Comparison of different Fe0 type
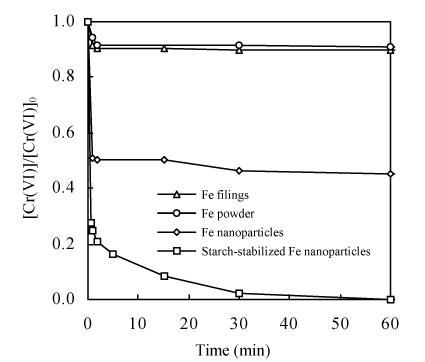
Fig.6 shows that the removal rates, especially for Fe0 nanoparticles and starch-stabilized Fe0 nanoparticles, were rapid in the first minute. There was an initial sorption phase combined with Cr(VI) reduction, which were enhanced with the increasing surface area of Fe0. After the first minute, the Cr(VI) removal rates were significantly slower. The removal efficiency was around 10% for Fe0 filings and powder in 60 min, but was 55% and 98% for Fe0 nanoparticles and starch-stabilized Fe0 nanoparticles, respectively. The reactivity was in the order starch-stabilized Fe0 nanoparticles>Fe0 nanoparticles>Fe0 powder>Fe0 filings. The comparison shows that Fe0 nanoparticles could reach significantly higher efficiency than Fe0 filings or powder, but could not maintain the activity for long because of the agglomeration. While starch-stabilized Fe0 nanoparticles were apparently prevented to agglomerated to become more reactive and keep longer activity.
Fig. 6.
Comparison of different Fe0 type on the Cr(VI) removal efficiency
C Fe=0.2 g/L, C Cr(VI)=20 mg/L, T=25 °C, pH=7.0, ω=600 r/min
Electrochemical analysis of the reaction process
The standard reduction potentials of E Fe(II)/Fe(0) Θ and E Cr(VI)/Cr(III) Θ are −0.44 V and 1.33 V, respectively. When mixed with Cr2O7 2−, Fe0 would be corroded and dissolved, causing the reduction of Cr(VI) to Cr(III). The standard reduction potential of E Cr(III)/Cr(0) Θ is −0.74 V, which is lower than that of Fe0. It means that the Cr(III) or Cr(III) hydroxide should be the steady product in the reaction. In fact, several investigators have reported that the removal mechanism involves reduction of highly soluble Cr2O7 2− to weakly soluble Cr(III) compounds at the surface of the iron. Spectroscopic data showed that Cr(OH)3 and mixed Cr(III)/Fe(III) hydroxides are precipitated on the iron surface (Ponder et al., 2000; Powell et al., 1995; Pratt et al., 1997). The net reactions of Cr(VI) reduction with Fe(0) and coprecipitation of Cr(III) and Fe(III) could be expressed by Eq.(1) and Eq.(2). No dissolved Cr(III) was detected during the reaction, which indicated that all the Cr(III) was coprecipitated with Fe(III). This observation is consistent with former observation (Pratt et al., 1997).
Insight into the process of Cr(VI) removal can also be obtained by detecting the reduction potential E and pH values in solution. The E and pH values of the Cr2O7 2− solution (10−4 mol/L) were 0.239 V and 7.00, respectively. In Fig.7, the reduction potential E decreased with increasing pH initially in the reaction, which indicates that Cr(VI) trended to form Cr(OH)3. After achieving minimum, E increased with the decreasing pH due to the form of Cr(III)/Fe(III) hydroxides which covered the surface of Fe0 nanoparticles to cut down the reaction activity. The detected point during the reaction was always in the area where Cr(OH)3 was dominant. This observation led to the conclusion that Cr(OH)3 should be the final and dominant product of Cr(VI).
Fig. 7.
The E-pH diagram of chromium (C total Cr=10−4 mol/L)
Δ: Actually detected in the reaction, C Fe=0.2 g/L, C Cr(VI)=10−4 mol/L, T=25 °C, ω=600 r/min
CONCLUSION
Reduction of Cr(VI) by Fe0 nanoparticles as reductant was studied using potassium dichromate solution as the model contaminant. The concentration of Fe0 nanoparticles had significant effect on the reduction of Cr(VI). When the mass ratio of Fe0 to Cr(VI) was 20:1, 100% removal efficiency was achieved. The reaction occurred in broad pH value scale and the reaction efficiency increased significantly with decreasing initial pH. The starch-stabilized Fe0 nanoparticles exhibited higher removal efficiency because starch as a good dispersant could prevent Fe0 nanoparticles agglomeration. Electrochemical analysis of the reaction process showed that Cr(OH)3 should be the final and dominant product of Cr(VI). The remediation of Cr(VI) was widely researched before. This study indicated that Fe0 nanoparticles, especially those which were starch-stabilized, can yield a high removal efficiency in the remediation of Cr(VI)-contaminated soils and groundwater.
Footnotes
Project (No. 20407015) supported by the National Natural Science Foundation of China
References
- 1.Alowitz MJ, Scherer MM. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ Sci Technol. 2002;36:299–306. doi: 10.1021/es011000h. [DOI] [PubMed] [Google Scholar]
- 2.Bowman RS. Applications of surfactant-modified zeolites to environmental remediation. Microporous and Mesoporous Materials. 2003;61:43–56. [Google Scholar]
- 3.Buerge IJ, Hug SJ. Influence of mineral surfaces on chromium(VI) reduction by iron(II) Environ Sci Technol. 1999;33:4285–4291. [Google Scholar]
- 4.Chen JM, Hao OJ. Microbial chromium(VI) reduction. Critical Rev Environ Sci Technol. 1998;28:219–251. [Google Scholar]
- 5.He F, Zhao D. Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environ Sci Technol. 2005;39:3314–3320. doi: 10.1021/es048743y. [DOI] [PubMed] [Google Scholar]
- 6.Hideaki Y, Toshiiyuki Y, Takashi T. Adsorption of chromate and arsenate by amino-functionalized MCM-41 and SBA-1. Chem Mater. 2002;14:4603–4610. [Google Scholar]
- 7.Hua B, Deng B. Influences of water vapor on Cr(VI) reduction by gaseous hydrogen sulfide. Environ Sci Technol. 2003;37:4771–4777. doi: 10.1021/es0342446. [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Lim H, Lee Y, Park J. Use of waste iron metal for removal of Cr(VI) from water. Chemosphere. 2003;53:479–485. doi: 10.1016/S0045-6535(03)00548-4. [DOI] [PubMed] [Google Scholar]
- 9.Ponder SM, Darab JG, Mallouk TE. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ Sci Technol. 2000;34:2564–2569. [Google Scholar]
- 10.Powell RM, Puls RW, Hightower SK, Sabantini DA. Coupled iron corrosion and chromate reduction: mechanisms for subsurface remediation. Environ Sci Technol. 1995;29:1913–1922. doi: 10.1021/es00008a008. [DOI] [PubMed] [Google Scholar]
- 11.Pratt AR, Blowes DW, Ptacek CJ. Products of chromate reduction on proposed subsurface remediation material. Environ Sci Technol. 1997;31:2492–2498. [Google Scholar]
- 12.Raveendran P, Fu J, Wallen SL. Complete “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125:13940–13941. doi: 10.1021/ja029267j. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz N, Seal S, Reinhart D. Surface chemical reactivity in selected zero-valent iron samples used in groundwater remediation. Journal of Hazardous Materials. 1995;B80:107–117. doi: 10.1016/s0304-3894(00)00281-8. [DOI] [PubMed] [Google Scholar]
- 14.Wei JJ, Xu XH, Liu Y. Kinetics and mechanism of dechlorination of o-chlorophenol by nanoscale Pd/Fe. Chem Res Chinese U. 2004;20:73–76. [Google Scholar]