Abstract
Objective: This study was designed to detect the expression of bcl-2 and p53 proteins in colorectal carcinomas and to determine their association with the patient survival and stage of the diseases. Methods: Immunohistochemistry method was used to detect the expression of bcl-2 and p53 proteins in 93 cases of colorectal carcinoma. The stain results were obtained by analyzing the clinic-pathological characteristics of patients. Results: Fifty-seven percent (53/93) of the colorectal carcinomas were bcl-2 protein positive. The positive rate of bcl-2 protein in lymph node involvement cases was lower (15/37) than the cases without node involvement (38/58, P<0.01). The positive rate of p53 protein was 43% (40/93) in colon-rectum carcinomas. No significant correlation was observed between p53 protein expression and clinic-pathological manifestations (P>0.05) but the survival was significantly worse (P=0.0001) in the p53 protein positive cases. Neither bcl-2 nor p53 alone was correlated with stage of the disease. When combined bcl-2/p53 status was analyzed, a group with bcl-2(+) and p53(−) had the best prognosis. This group was significantly associated with earlier Dukes’ stages (P=0.1763). In multivariate Cox regression analysis, lymph node involvement and p53 protein expression were two independent factors correlated with survival time. Conclusion: The expression of bcl-2 and p53 represent biological characteristics of colorectal carcinomas. Assessment of both bcl-2 and p53 status may be valuable in predicting the prognosis of patients.
Keywords: Colorectal carcinoma, bcl-2, p53, Prognosis
INTRODUCTION
Colorectal cancer is the most common cancer of the Western world and accounts for approx. Eleven percent of all cancer deaths in the United States. As with other malignancies, colorectal adenocarcinoma is thought to develop through the accumulation of genetic alterations that inhibit cell growth. Two of the oncogenes that have been implicated in this process are bcl-2 and p53, the products of which are involved in apoptosis, cell proliferation and tumor development (Garewal et al., 1996).
Several studies (Flamini et al., 1996; Flohil et al., 1996; Kaklamanis et al., 1996) of colorectal adenocarcinomas have detected expression of bcl-2 protein and p53 protein, but the results among the populations studied are contradictory, and how the concomitant expression of bcl-2 and p53 affects survival in patients with colorectal cancer is unknown, so we analyzed bcl-2 and p53 protein expression in primary colorectal adenocarcinomas using immunohistochemistry, the results of which were compared with the clinical outcome of patients to determine the prognostic value of detecting one or both oncogenic protein in colorectal adenocarcinomas.
MATERIALS AND METHODS
Tissues and patient history
Formalin-fixed, paraffin-embedded tissue samples of primary colorectal adenocarcinomas resected between 1987 and 1988 were collected from the Second Affiliated Hospital of Zhejiang University. On completion of the study, surgical pathology report, demographic information and follow-up report were obtained from the hospital. Hematoxylin and eosin-stained slides of all cases were reviewed for tumor stage and differentiation. Pathological Dukes tumor staging was classified. Survival or cause of death (whether it was attributed to colorectal cancer or not) was determined from hospital charts or tumor registries. Patients were followed until date of death or date of last documented contact. Median follow-up was 60 months.
Forty-seven of the patients were male and the median age of patients was 51. The surgical pathology reports indicated that the patients with well or moderately differentiated adenocarcinoma comprised 72 cases and poorly differentiated comprised 21 cases. Metastasis to the regional lymph nodes comprised 37 cases and 56 cases had no regional lymph nodes involvement. The cases with Dukes A, B and C tumor stage were 22, 35 and 36.
Immunohistochemistry
Tissue sections (4 μm) from each case were cut continuously before immunostaining. Sections were deparaffinized in 3 changes of xylene and hydrated in 1 change each of absolute ethanol, 95% ethanol and 70% ethanol for 2 min in each solution. Sections then were soaked in Tris-buffered saline (TBS: 0.05 mol/L Tris base, 0.15 mol/L NaCl, Triton X-100 4 drops/L, pH 7.6) until the slides were placed in the racks of the automated cell-staining system. Endogenous peroxidase activity of the tissue samples was quenched with an aqueous solution of 3% hydrogen peroxide for 3 min, followed by 3 rinses with TBS. All subsequent washes were performed with TBS. Sections were blocked with casein solution to reduce non-specific background staining, followed by incubation with the monoclonal antibodies (Mabs) bcl-2 and p53. Antibodies were diluted with PBS. Each was incubated for 2 h at room temperature. BioGenex Super Sensitive Biotin-Streptavidin Horseradish Peroxidase Peroxidase Detection Kit was used as the secondary detection system. Sections were incubated with biotin-conjugated goat anti-mouse and streptavidin peroxidase for 20 min each with TBS washes between steps. For visualization of the antibody-antigen complex, sections were incubated with the diaminobenzidine tetrachloride (DAB) Super Sensitive Substrate Kit (BioGenex) for 7 min. Except for counter-staining, the remainder of the staining procedure was routinely carried out. Sections were counter-stained with methyl green stain (0.5% methyl green in 0.1 mol/L sodium acetate, pH 4.0). Breast tumor sections known to stain positively for bcl-2 and p53 were included in each run as positive controls. PBS replaced Mabs in negative controls.
Assessment of bcl-2 and p53 expression
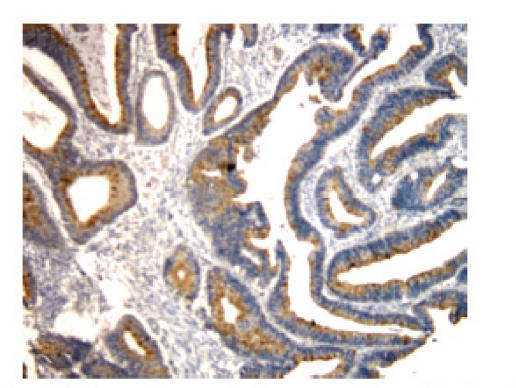
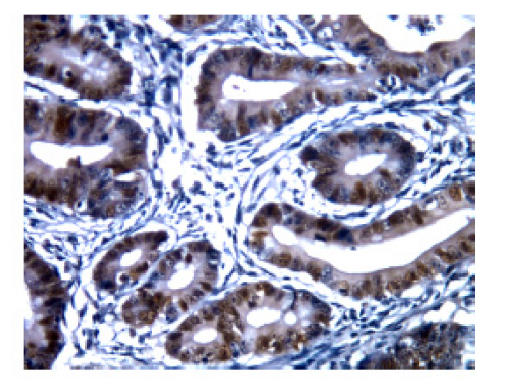
The staining pattern of bcl-2 expression in positive cells was predominantly cytoplasmic, with some staining of nuclear membranes and positive staining for p53 protein was observed in nuclei (Fig.1 and Fig.2). A semi-quantitative immunostaining score for bcl-2 was obtained as described in (Myers et al., 1995). In brief, the intensity of immunostaining of individual cells was scored on a scale from 0 (no staining) to 3 (strongest intensity). In addition, the proportion of cells stained at each intensity was estimated and scored on a scale from 0 (<10%) to 4 (>75%). The percent of cells at each intensity was multiplied by the corresponding intensity value to obtain an immunostaining score that ranged from 0 to 8. An immunostaining score of ≥2 was chosen arbitrarily to be positive for bcl-2 expression. Colorectal tumors were classified as p53-positive if positive staining cells were 5% or more of cells counted. In both cases staining intensities ranging from weak to strong were scored.
Fig. 1.
bcl-2 protein cytoplasm positive staining in patients with colorectal cancer (SP×200). The staining pattern of bcl-2 expression in positive cells was predominantly cytoplasm, with some staining of nuclear membranes
Fig. 2.
p53 protein nucleus positive staining in patients with colorectal cancer (SP×400). The staining pattern of p53 expression in positive cells was predominantly observed in nuclei
Statistical analysis
SPSS FOR WINDOWS 10.0 software was used to analyze the data. χ 2 test was used to assess the univariate association of baseline characteristics with bcl-2 and p53 expression; P values were calculated and significance was assessed at a level of 0.05. Log-rank test was used to compare Kaplan-Meier survival curves. The period from the date of resection to the date of death or last contact (if alive) was used for survival analysis. Cox proportional hazards regression analysis was conducted to compare the survival of patients with and without p53 and bcl-2 expression with adjustments for confounding variables. Clinically confounding variables of colorectal cancer used in the analysis were age, sex, Dukes stage, lymph node, recurrence and differentiation. Interaction between bcl-2 and p53 expression also was tested for significance. Univariate Kaplan-Meier survival curves were examined to assess the appropriateness of the proportional hazards assumption for bcl-2 and/or p53 status. A stepwise model-building procedure was employed to determine the significant factors in predicting colorectal cancer patient survival.
RESULTS
Association between bcl-2 or p53 expression and clinicopathological parameters
Of 94 colorectal adenocarcinomas, 57.0% (53/93) of tumors were classified as expressing bcl-2 (immunostaining score≥2). In addition, diffuse cytoplasmic staining of bcl-2 expression was observed in basal cells of the normal mucosa of colonic crypts adjacent to the tumor but usually not in normal colonic epithelium distant from the tumor. There was a statistically significant association between bcl-2 expression and tumor stage, metastasis to the regional lymph nodes. Lower-stage (Dukes A or B) colorectal cancer was more commonly positive for bcl-2 expression than higher-stage (Dukes C) tumors (P<0.05). Metastasis to the regional lymph nodes prevalence also was observed (P<0.01). No association was found between expression of bcl-2 and other patient variables, such as sex, age, tumor recurrence or tumor differentiation.
Positive staining for p53 protein was detected in 43% (40/93) of colorectal adenocarcinomas. When the p53 immunologic positive data were correlated with several clinicopathological parameters, there was no significant association with sex, age, metastasis to the regional lymph nodes, tumor recurrence, tumor stage or tumor differentiation (P>0.05).
Combined effect of bcl-2 and p53 expression and clinicopathological parameters
Patients were categorized into 4 groups based on the immunohistochemical staining status of bcl-2 and p53: bcl-2(+)/p53(−), bcl-2(−)/p53(−), bcl-2(+)/p53(+) and bcl-2(−)/p53(+). There were statistically significant associations between lymph nodes (involvement and non-involvement) and tumor stage with immunohistochemical staining status (P<0.05). Tumors showing bcl-2 expression but no p53 expression were more commonly observed in Dukes A or B tumor stage (41.0%, 23/56), especially in tumors without regional lymph-node involvement (41.0%, 23/56) (Table 1).
Table 1.
Correlation of bcl-2 and p53 association expression with clinicopathological factors of primary colorectal cancer
| Clinicopathological factors | Cases | bcl-2(+)/p53(−) | bcl-2(−)/p53(−) | bcl-2(+)/p53(+) | bcl-2(−)/p53(+) | P value | |
| Sex | Male | 47 | 20 | 10 | 11 | 6 | 0.156 |
| Female | 46 | 10 | 13 | 12 | 11 | 0.156 | |
| Age (year) | ≤45 | 31 | 11 | 8 | 6 | 6 | 0.863 |
| >45 | 62 | 19 | 15 | 17 | 11 | 0.863 | |
| Tumor differentiation well or moderately | 72 | 25 | 17 | 19 | 11 | 0.442 | |
| Tumor differentiation poor | 21 | 5 | 6 | 4 | 6 | 0.442 | |
| Regional lymph node involvement | 37 | 7 | 13 | 8 | 9 | 0.039 | |
| Regional lymph node non-involvement | 56 | 23 | 10 | 15 | 8 | 0.039 | |
| Dukes | A | 22 | 13 | 2 | 4 | 3 | 0.037 |
| B | 34 | 10 | 8 | 11 | 5 | 0.037 | |
| C | 37 | 7 | 13 | 8 | 9 | 0.037 | |
| Recurrence | 20 | 6 | 7 | 4 | 3 | 0.681 | |
| Non-recurrence |
73 |
24 |
16 |
19 |
14 |
0.681 |
|
| Total | 93 | 30 | 23 | 23 | 17 | ||
Survival analysis
1. bcl-2 or p53 expression and prognosis
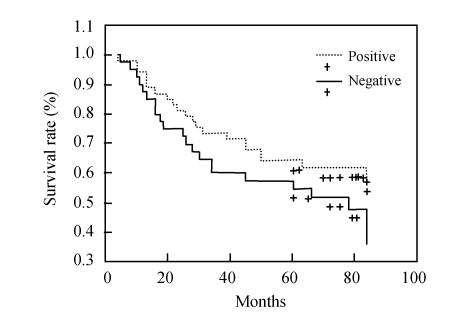
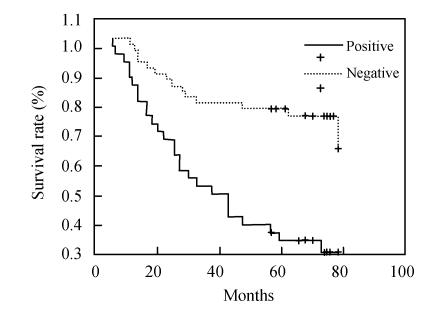
Patients with colorectal cancer whose tumors demonstrated bcl-2 expression showed no survival prevalence (5-year survival rate) than patients whose tumors were negative, using univariate Kaplan-Meier survival analysis (60.4%, 32/53 vs 47.5%, 19/40, P=0.1763) (Fig.3). A decrease in survival probability was observed in patients with p53-positive tumors compared to patients with p53-negative tumors (5-year survival rate, 32.5%, 13/40 vs 71.7%, 38/53, P=0.0001) (Fig.4).
Fig. 3.
Survival curve of bcl-2 positive and negative expression groups in patients with colorectal neoplasm. Patients with colorectal cancer whose tumors showed bcl-2 expression indicated no survival prevalence than patients whose tumors were negative, using univariate Kaplan-Meier survival analysis (P=0.1763)
Fig. 4.
Survival curve of p53 positive and negative expression groups in patients with colorectal neoplasm. A decrease in survival probability was observed in patients with p53-positive tumors compared to patients with p53-negative tumors (P=0.0001)
2. Combined effect of bcl-2 expression and p53 expression on prognosis
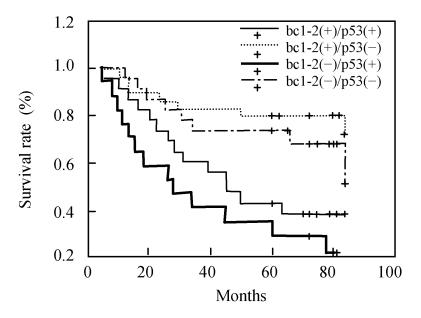
As above-mentioned, patients were categorized into 4 groups based on the immunohistochemical staining status of bcl-2 and p53. Four possible combinations of bcl-2 expression and p53 expression were considered for assessment of patient survival, and all four Kaplan-Meier survival curves of these combinations were significantly different from each other (log rank, P=0.0003). Furthermore, statistically significant differences in clinical outcome (5-year survival rate) were observed when the following survival curves were compared: bcl-2-positive, p53-negative (76.7%, 23/30) vs bcl-2-negative, p53-positive (23.5%, 4/17) and bcl-2-negative, p53-negative (65.2%, 15/23) vs bcl-2-negative, p53-positive (23.5%, 4/17). Although not statistically significant (P>0.05), an inverse trend between the expression of p53 and bcl-2 was observed in the colorectal tumors, that is, patients with tumors expressing p53 but without bcl-2 expression showed the lowest overall survival probability (Fig.5). The best overall survival probability was observed in patients with tumors showing bcl-2 expression and no p53 expression.
Fig. 5.
Survival curve of different combinations of bcl-2 expression and p53 expression in patients with colorectal neoplasm. All four Kaplan-Meier survival curves were significantly different from each other (log rank, P=0.0003). Statistically significant differences in clinical outcome were observed when the following survival curves were compared: bcl-2-positive, p53-negative (76.7%, 23/30) vs bcl-2-negative, p53-positive (23.5%, 4/17) and bcl-2-negative, p53-negative (65.2%, 15/23) vs bcl-2-negative, p53-positive (23.5%, 4/17)
Multivariate survival analysis
Cox regression analysis was used to evaluate the independent prognostic significance of bcl-2 expression and/or p53 expression. Variables included in the proportional hazards model were bcl-2 expression, p53 expression, combined effect of bcl-2 and p53 expression, sex, age, tumor differentiation, recurrence, metastasis to lymph node and tumor stage. Multivariate analysis showed that p53 expression and lymph node status of the tumor are the significant independent prognostic factors of colorectal adenocarcinoma (P=0.001) and no association was found between other patient variables, such as expression of bcl-2, combined effect of bcl-2 and p53 expression, sex, age, tumor recurrence, tumor differentiation and the prognosis of colorectal cancer.
DISCUSSION
Colorectal cancer is a major cause of cancer death and, in most cases, develops from a multistep process characterised by accumulation of molecular genetic alterations causing disorders in cell growth, differentiation and apoptosis. It is generally believed that the balance between the rates of cell growth and apoptosis maintains intestinal epithelial cell homeostasis, and that during cancer development this balance gets progressively disturbed. Two of the oncogenes that have been implicated in this process are bcl-2 and p53. The bcl-2 oncogene is a known inhibitor of apoptosis that may allow the accumulation and propagation of cells containing genetic alterations. Usually expression of bcl-2 in colorectal cancers has been shown as being a favorable prognostic factor (Manne et al., 1997). In contrast, Bosari et al.(1995) investigated by immunocytochemistry bcl-2 expression in normal colonic mucosa, hyperplastic polyps, adenomas, and adenocarcinomas of the large bowel in a large US population. In colorectal carcinomas bcl-2 expression (76.9%) was higher than that in adenomas (59.0%), but bcl-2 expression in colorectal cancer and adenomas was not correlated with relevant clinicopathologic parameters, including dysplastic change, differentiation, and had no prognostic significance by univariate or multivariate analysis. These results suggest that bcl-2 oncogenic protein may play a role in colorectal tumorigenesis; probably in the early phases of the adenoma-carcinoma sequence. bcl-2 expression in established tumors has no prognostic significance.
As another known inhibitor of apoptosis, the wild-type p53 tumor suppressor gene prevents the accumulation and propagation of cells containing genetic alterations (Mosnier et al., 1996). Mutations in the p53 gene have been implicated in the development of colorectal adenocarcinomas as approximately half of all colorectal cancers show p53 (TP53) gene mutations, with higher frequencies observed in distal colon and rectal tumors and lower frequencies in proximal tumors. But the relationship between p53 abnormalities and patient survival is a subject of controversy in patients with colorectal adenocarcinomas. Some studies found that p53 in colorectal tumors, as assessed by immunohistochemistry, is of limited value in predicting clinical outcome (Garewal et al., 1995). In contrast, Iacopetta (2003) think the evaluation of p53 overexpression, using a standardized immunohistochemical (IHC) procedure, could be a clinically useful marker, while other studies indicated that expression of p53 in colorectal tumors is an indicator of shortened patient survival (Belluco et al., 1996). In contrast, Dix et al.(1994) showed a significant association between over-expression of p53 and better prognosis in an Australian population of patients with colorectal cancer. Gonzalez-Aguilera et al.(2004) reported simultaneous mutations in K-ras and TP53 are indicative of a worse prognosis in sporadic colorectal cancer while Westra et al.(2005) showed both mutant TP53 andmicrosatellite instability-high (MSI-H) seem to be prognostic indicators for disease-free survival, but only TP53 retains statistical significance after adjusting for clinical heterogeneity. Thus, in adjuvantly treated patients with stage III colon cancer, presence or absence of a TP53 mutation should be considered as a better predictor for disease-free survival (DFS) than MSI status.
The identification of molecular markers that provide insight into the potential behavior or aggressiveness of tumors is a necessary step for the improvement of cancer treatment; however, few individual biomarkers that provide prognostic information have been identified. The limited value of a single molecular marker is not surprising because tumors frequently express multiple protooncogenes, suppressor genes and onco-fetal antigens, each of which may contribute to tumor progression and metastasis. Thus, better understanding of tumor behavior or aggressiveness may be gained by examining the co-expression of multiple markers.
Concomitant expression of bcl-2 and p53 has been correlated with poor survival in patients with b-cell lymphomas and urinary tract transitional-cell carcinomas. In contrast, Baretton et al.(1996) reported a poor clinical outcome of patients with non-metastatic colorectal tumors that did not express bcl-2 but showed nuclear accumulation of p53. Watson et al.(2005) reported patient stratification by combined p53/bcl-2 phenotype provides stage-independent prognostic information in colorectal cancer. Specifically, that up to a quarter of patients showed a good prognosis p53(−)/bcl-2(+) phenotype. This may indicate a more clinically indolent phenotype and a subset of patients for whom less aggressive adjuvant treatment is appropriate. In our study, there was a statistically significant difference between lymph nodes and tumor stage and the immunohistochemical staining status of bcl-2 and p53 (P<0.05). Tumors showing bcl-2 expression but no p53 expression were more commonly observed in Dukes A or B tumor stage (41.0%, 23/56), especially in tumors without regional lymph-node involvement (41.0%, 23/56). Although not statistically significant (P>0.05), an inverse trend between the expression of p53 and bcl-2 was observed in colorectal tumors, that is, patients with tumors expressing p53 but not bcl-2 showed the lowest overall survival probability. The best overall survival probability was observed in patients with tumors that showed bcl-2 expression but not p53 expression. These findings suggest that bcl-2 expressing tumors are less aggressive and progress more slowly than tumors in which bcl-2 expression could not be detected. This concept is supported further by the statistically significant correlation between bcl-2 expression and clinical outcome.
These findings seem paradoxical in view of the role of bcl-2 as an inhibitor of apoptosis. The biological role or function of bcl-2 in the development and progression of the malignant cell is not understood completely, and the bcl-2 protein may be involved in cellular processes other than apoptosis. In vitro studies in normal human breast epithelial cells suggested that bcl-2 expression may regulate an early commitment step of the cell with respect either to proliferation or to apoptosis. In addition, studies on breast adenocarcinomas showed an association between increased bcl-2 expression and decreased proliferative potential. By inhibiting cellular proliferation, bcl-2 expression may define a sub-group of colorectal tumors with less potential for progression (Upadhyay et al., 1995).
As we know, expression of bcl-2 and p53 is related to the multidrug resistance of chemotheraputic agents. So maybe it is useful to evaluate the sensitivity of agents and the prognosis of colorecatal cancer by examining the co-expression of bcl-2 and p53.
Lymph node status is the common indicator of prognosis in clinic. In our study, multivariate analysis showed that p53 expression and lymph node status of the tumor are the significant independent prognostic factors of colorectal adenocarcinoma (P=0.001) and no association was found between other patient variables, even as expression of bcl-2. These findings supported further the role of lymph node in indicating the prognosis.
References
- 1.Baretton GB, Diebold J, Christoforis G, Vogt M, Muller C, Dopfer K, Schneiderbanger K, Schmidt M, Lohrs U. Apoptosis and immunohistochemical bcl-2 expression in colorectal adenomas and carcinomas. Cancer. 1996;77:255–264. doi: 10.1002/(SICI)1097-0142(19960115)77:2<255::AID-CNCR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Belluco C, Guillem JG, Kemeny N, Huang Y, Klimstra D, Berger MF, Cohen AM. p53 nuclear protein overexpression in colorectal cancer. A dominant predictor of survival in patients with advanced hepatic metastases. J Clin Oncol. 1996;14:2696–2701. doi: 10.1200/JCO.1996.14.10.2696. [DOI] [PubMed] [Google Scholar]
- 3.Bosari S, Moneghini L, Graziani D, Lee AK, Murray JJ, Coggi G, Viale G. bcl-2 oncoprotein in colorectal hyperplastic polyps, adenomas, and adenocarcinomas. Hum Pathol. 1995;26(5):534–540. doi: 10.1016/0046-8177(95)90250-3. [DOI] [PubMed] [Google Scholar]
- 4.Dix BR, Robbins P, Soong R. The common molecular genetic alterations in Dukes’ B and C colorectal carcinomas are not short-term prognostic indicators of survival. Int J Cancer. 1994;59(6):747–751. doi: 10.1002/ijc.2910590606. [DOI] [PubMed] [Google Scholar]
- 5.Flamini G, Curiglian G, Ratto C, Astone A, Ferretti G, Nucera P, Sofo L, Sgambato A, Boninsegna A, Crucitti F, et al. Prognostic significance of cytoplasmic p53 overexpression in colorectal cancer. An Immunohistochemical Analysis Europ J Cancer. 1996;32A:802–806. doi: 10.1016/0959-8049(95)00625-7. [DOI] [PubMed] [Google Scholar]
- 6.Flohil CC, Janssen PA, Bosman FT. Expression of bcl-2 protein in hyperplastic polyps, adenomas, and carcinomas of the colon. J Pathol. 1996;178:393–397. doi: 10.1002/(SICI)1096-9896(199604)178:4<393::AID-PATH488>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Garewal H, Guillem JG, Klimstra DS. p53 nuclear overexpression may not be an independent prognostic marker in early colorectal cancer. Dis Colon Rectum. 1995;38:1176–1181. doi: 10.1007/BF02048333. [DOI] [PubMed] [Google Scholar]
- 8.Garewal H, Bernstein N, Bernstein C, Sampliner R, Payne C. Reduced bile acid-induced apoptosis in “normal” colorectal mucosa: a potential biological marker for cancer risk. Cancer Res. 1996;56(12):1480–1483. [PubMed] [Google Scholar]
- 9.Gonzalez-Aguilera JJ, Oliart S, Azcoita MM, Fernandez-Peralta AM. Simultaneous mutations in K-ras and TP53 are indicative of poor prognosis in sporadic colorectal cancer. Am J Clin Oncol. 2004;27(1):39–45. doi: 10.1097/01.coc.0000045920.49210.7A. [DOI] [PubMed] [Google Scholar]
- 10.Iacopetta B. TP53 mutation in colorectal cancer. Hum Mutat. 2003;21(3):271–276. doi: 10.1002/humu.10175. [DOI] [PubMed] [Google Scholar]
- 11.Kaklamanis L, Savage A, Mortensen N, Tsiotos P, Doussis-Anagnostopoulou I, Biddolph S, Whitehouse R, Harris AL, Gatter KC. Early expression of bcl-2 protein in the adenoma–carcinoma sequence of colorectal neoplasia. J Pathol. 1996;179:10–14. doi: 10.1002/(SICI)1096-9896(199605)179:1<10::AID-PATH540>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Manne U, Myers RB, Moran C, Poczatek RB, Dillard S, Weiss H, Brown D, Srivastava S, Grizzle WE. Prognostic significance of bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer. 1997;74:346–358. doi: 10.1002/(sici)1097-0215(19970620)74:3<346::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Mosnier JF, Perret AG, Vindimian M, Dumollard JM, Balique JG, Perpoint B, Boucheron S. An immunohistochemical study of the simultaneous expression of bcl-2 and p53 oncoproteins in epithelial tumor of the colon and rectum. Arch Pathol Lab Med. 1996;120(7):654. [PubMed] [Google Scholar]
- 14.Myers RB, Lampejo O, Herrera GA. TGF-alpha expression is a relatively late event in the progression of prostatic adenocarcinoma. J Urol Pathol. 1995;3:195–204. [Google Scholar]
- 15.Upadhyay S, Li G, Liu H. bcl-2 suppresses expression of p21WAF/CIP1 in breast epithelial cells. Cancer Res. 1995;55:4520–4524. [PubMed] [Google Scholar]
- 16.Watson NF, Madjd Z, Scrimegour D, Spendlove I, Ellis IO, Scholefield JH, Durrant LG. Evidence that the p53 negative/bcl-2 positive phenotype is an independent indicator of good prognosis in colorectal cancer: a tissue microarray study of 460 patients. World J Surg Oncol. 2005;3:47. doi: 10.1186/1477-7819-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westra JL, Schaapveld M, Hollema H, de Boer JP, Kraak MM, de Jong D, ter Elst A, Mulder NH, Buys CH, Hofstra RM, et al. Determination of TP53 mutation is more relevant than microsatellite instability status for the prediction of disease-free survival in adjuvant-treated stage III colon cancer patients. J Clin Oncol. 2005;23(24):5635–5643. doi: 10.1200/JCO.2005.04.096. [DOI] [PubMed] [Google Scholar]