Abstract
To investigate the features of electroencephalography (EEG) power and coherence at rest and during a working memory task of patients with mild cognitive impairment (MCI). Thirty-five patients (17 males, 18 females; 52~71 years old) and 34 sex-and age-matched controls (17 males, 17 females; 51~63 years old) were recruited in the present study. Mini-Mental State Examination (MMSE) of 35 patients with MCI and 34 normal controls revealed that the scores of MCI patients did not differ significantly from those of normal controls (P>0.05). Then, EEGs at rest and during working memory task with three levels of working memory load were recorded. The EEG power was computed over 10 channels: right and left frontal (F3, F4), central (C3, C4), parietal (P3, P4), temporal (T5, T6) and occipital (O1, O2); inter-hemispheric coherences were computed from five electrode pairs of F3-F4, C3-C4, P3-P4, T5-T6 and O1-O2 for delta (1.0~3.5 Hz), theta (4.0~7.5 Hz), alpha-1 (8.0~10.0 Hz), alpha-2 (10.5~13.0 Hz), beta-1 (13.5~18.0 Hz) and beta-2 (18.5~30.0 Hz) frequency bands. All values of the EEG power of MCI patients were found to be higher than those of normal controls at rest and during working memory tasks. Furthermore, the values of EEG power in the theta, alpha-1, alpha-2 and beta-1 bands of patients with MCI were significantly high (P<0.05) in comparison with those of normal controls. Correlation analysis indicated a significant negative correlation between the EEG powers and MMSE scores. In addition, during working memory tasks, the EEG coherences in all bands were significantly higher in the MCI group in comparison with those in the control group (P<0.05). However, there was no significant difference in EEG coherences between two groups at rest. These findings comprise evidence that MCI patients have higher EEG power at rest, and higher EEG power and coherence during working conditions. It suggests that MCI may be associated with compensatory processes at rest and during working memory tasks. Moreover, failure of normal cortical connections may be exist in MCI patients.
Keywords: Mild cognitive impairment (MCI), Electroencephalography (EEG), Spectral power, Coherence, Working memory
INTRODUCTION
Mild cognitive impairment (MCI) has been defined as a boundary or transitional state between normal aging and dementia. Subjects with MCI having memory impairment beyond that expected for age and education yet are not demented. Reviews of several studies revealed that those subjects are at increased risk of developing Alzheimer’s disease (AD) ranging from 1% to 25% per year (Petersen et al., 1999). Therefore, patients with MCI are becoming of interest for treatment trials. Furthermore, those individuals are also becoming the focus of many prediction studies and early intervention trials for AD.
Working memory (WM) is an important cognitive function, involving encoding, maintenance and retrieval of temporary information and has become one of the hotspots of learning and memory study. On the other hand, electroencephalography (EEG) is easily performed and requires minimal cooperation from the patients, thus making this technique useful for clinical evaluation of demented patients. Some recent studies applied EEG for examining functional changes associated with the performance of a perceptual or cognitive task. EEG analysis is considered to be useful for assessing cerebral functioning (Kikuchi et al., 2002). Quantitative EEG and coherence provide additional sources of information on the topography of synchronous oscillatory activity and potential cortico-cortical interactions during cognitive testing (Hogan et al., 2003). Even without EEG abnormality present in MCI, quantitative EEG analysis could help to identify MCI subgroups that develop to AD (Jelic et al., 2000).
To our knowledge, there is no reported study on examining topographical differences between MCI and normal controls in either EEG spectral power or coherence during memory processing. The objective of the present study is to investigate the group differences in EEG power and coherence at rest and during working memory task for MCI patients and normal controls.
METHODS
Subjects
The patient group consisted of 35 patients (18 females and 17 males) who consulted the psychiatric outpatient clinic of the Department of Psychiatry, Second Affiliated Hospital, Zhejiang University, China. The patients satisfied DSM-IV criteria (American Psychiatric Association, 1994) for the study diagnosis of MCI and the following criteria: (1) memory complaint; (2) normal activities of daily living; (3) normal general cognitive function; (4) abnormal memory for age; (5) not demented; (6) the course of memory damage was over three months. Their mean age 62.3 years (SD=6.5), range 52 to 71 years. Each patient was evaluated by the Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR) and Activities of Daily Living Scale (ADL). The mean MMSE score was 26.6 (SD=2.0), range 25 to 30; the CDR score was 0.5; the ADL score was <22. None of the patients were receiving psychoactive medications such as antipsychotic drugs or cerebral vasodilators. Moreover, in order to rule out other organic brain disease, e.g. multiple sub-cortical infarctions with mild cognitive impairment, examination of magnetic resonance imaging (MRI) and/or computed tomography (CT) for patient group was applied.
The control group consisted of 34 healthy volunteers (17 females and 17 males) without personal or family history of psychiatric or neurological abnormality. Their mean age was 57.4 years (SD=4.0), range 51 to 63 years. Their mean MMSE score was 29.1 (SD=1.3), range 27 to 30. The normal controls were sought from the community population in Hangzhou City, China. They were functioning normally in the community and have no cognitive impairment. Patients were not significantly different from controls in age, gender and education. All subjects were right-handed and agreed to participate in the study with full knowledge of the experimental nature of the research.
EEG recording and analysis
During EEG recording the subjects were in a resting state with eyes closed, sitting in a semi-darkened, electrically shielded, sound attenuated room. According to the international 10~20 system, original EEG signals were recorded from scalp electrodes and separate ear electrodes A1 and A2, with electrodes referenced to linked ear lobes. Impedance of electrode/skin was kept below 5 000 Ω. The signals were amplified and filtered by a 16-channel electroencephalograph (EEG-NATION918, Shanghai, China) with an upper frequency cut-off of 60 Hz and 0.1 s time constant. EEGs were recorded at 16 electrode sites: Fp1, Fp2, F3, F4, F7, F8, C3, C4, T3, T4, T5, T6, P3, P4, O1 and O2 electrodes for 10 min for each subject with eyes closed. Names of electrode sites are defined by the rule of international 10~20 system. Original EEG records were digitally transformed into a common montage of 10 signals from F3, F4, C3, C4, T5, T6, P3, P4, O1 and O2 electrodes. Selection of segments recorded when eyes were closed but awaked was based on visual inspection of EEG and electro-oculographic (EOG) recordings. Segments containing eye movements, blinks, or muscle activity were excluded from the analysis.
EEG coherence was calculated by the Fast Fourier Transform (FFT) method. One epoch consisted of 2 s, and 20 artifact-free epochs per subject were processed with a spectral resolution of 0.5 Hz. Coherence between two waveforms x and y was calculated spectrally as
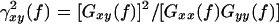 |
, where Gxy(f) is the mean cross-power density and Gxx(f) and Gyy(f) are the respective mean auto-power spectral densities. The details of the method for calculating the coherence are published in Jiang (2004; 2005). In this study, inter-hemispheric EEG coherence was measured between the following 5 homologous electrode pairs: left-right frontal (F3-F4), left-right centrals (C3-C4), left-right parietals (P3-P4), left-right temporals (T5-T6) and left-right occipitals (O1-O2). The coherence coefficients were calculated and banded into delta band (1.0~3.5 Hz), theta band (4.0~7.5 Hz), alpha-1 band (8.0~10.0 Hz), alpha-2 band (10.5~13.0 Hz), beta-1 band (13.5~18.0 Hz) and beta-2 band (18.5~30.0 Hz).
Working memory task
After routine EEG examination, a working memory task was performed by each subject. The sums (arithmetic) are designed with three levels of working memory load, recitation of three-digit numbers and mental calculation based on Salthouse and Babcock (1991). First level of working memory is two simple unit numerals added one time; the second level of working memory is two simple unit numeral add two times; the third level of working memory is two simple unit numeral add three times. They were asked to remember the answer during ever level of working memory task. Along with the increased working memory demands, EEG was recorded during the process of each question being given until the question was answered.
Statistics
In this study, a logarithmic transformation of absolute power and Fisher’s Z transformation of coherence values of each band in each derivation were implemented to normalize the distribution of power and coherence values, respectively. Differences between the MCI patients and the normal controls were analyzed on each frequency band by using two-way analyses of variance (ANOVA) with a grouping factor (patients vs controls) and a within-subject factor (electrode position). As the EEG recording method for analysis of EEG power within subject factor, i.e., electrode position involved ten levels; for analysis of coherence within subject factor, i.e., electrode pair involved five levels. Separate ANOVAs were conducted for different frequency bands in order to test the EEG power and coherence, respectively. The testing conditions, such as resting and working memory state, were used as condition variables. Then, two-tailed student’s t-test was conducted to compare the values of EEG power and coherence between the two groups. In addition, Pearson correlations were computed to examine the relationships of EEG band power and coherences with the clinical severity assessed by the MMSE score. Statistical significance was defined as P<0.05.
RESULTS
EEG power and coherence in resting state
Table 1 shows the mean log-transformed absolute power of the resting EEG in MCI and control groups. The two-way ANOVA revealed significant group differences in EEG power for the theta [F(1, 9)=8.460, P=0.005], alpha-1 [F(1, 9)=11.672, P=0.001], alpha-2 [F(1, 9)=8.512, P=0.005] and beta-1 [F(1, 9)=4.235, P=0.043] bands. Post-hoc analysis by t-test indicated that the MCI patients had significantly higher EEG power at F3, F4, C3, C4, P3 and P4 for the theta band, at all electrodes for the alpha-1 band, at F3, F4, C3, C4, P3, P4, T5 and T6 for the alpha-2 band, as well as at F4, P3, T6 and O1 for the beta-1 band (P<0.05). No significant group difference was found, however, in the delta [F(1, 9)=0.994, P=0.322] and beta-2 [F(1, 9)=0.674, P=0.422] bands.
Table 1.
EEG power (μV2, mean±SD) at rest and analysis results by ANOVA
| Bands | F3 | F4 | C3 | C4 | P3 | P4 | T5 | T6 | O1 | O2 | ANOVA |
||||
| df | F | P | |||||||||||||
| δ | MCI | 0.73±0.21 | 0.70±0.22 | 0.54±0.26 | 0.54±0.26 | 0.55±0.27 | 0.62±0.22 | 0.50±0.26 | 0.49±0.25 | 0.33±0.26 | 0.39±0.26 | 1 | 0.994 | 0.322 | |
| Con | 0.64±0.19 | 0.68±0.17 | 0.53±0.19 | 0.50±0.12 | 0.54±0.24 | 0.57±0.12 | 0.43±0.26 | 0.43±0.20 | 0.33±0.29 | 0.32±0.16 | |||||
| θ | MCI | 0.94±0.19* | 0.97±0.21* | 0.74±0.22* | 0.80±0.22* | 0.82±0.23* | 0.94±0.22* | 0.76±0.28 | 0.84±0.29 | 0.52±0.25 | 0.70±0.29* | 1 | 8.460 | 0.005* | |
| Con | 0.76±0.23 | 0.73±0.26 | 0.60±0.31 | 0.58±0.29 | 0.66±0.33 | 0.70±0.30 | 0.61±0.38 | 0.64±0.39 | 0.41±0.37 | 0.43±0.35 | |||||
| α1 | MCI | 0.82±0.40* | 0.86±0.40* | 0.66±0.41* | 0.76±0.39* | 0.95±0.52* | 1.14±0.53* | 0.98±0.68* | 1.13±0.61* | 0.94±0.69* | 0.91±0.58* | 1 | 11.672 | 0.001* | |
| Con | 0.53±0.40 | 0.51±0.45 | 0.36±0.45 | 0.47±0.37 | 0.54±0.45 | 0.58±0.53 | 0.61±0.54 | 0.64±0.55 | 0.58±0.61 | 0.59±0.32 | |||||
| α2 | MCI | 0.63±0.27* | 0.66±0.27* | 0.55±0.29* | 0.64±0.26* | 0.92±0.43* | 1.03±0.47* | 0.84±0.35* | 1.00±0.54* | 0.53±0.48 | 0.75±0.48 | 1 | 8.512 | 0.005* | |
| Con | 0.45±0.40 | 0.42±0.24 | 0.37±0.34 | 0.36±0.24 | 0.55±0.35 | 0.60±0.50 | 0.57±0.35 | 0.57±0.47 | 0.48±0.28 | 0.56±0.51 | |||||
| β1 | MCI | 0.51±0.26 | 0.52±0.26* | 0.44±0.26 | 0.50±0.24 | 0.64±0.28* | 0.69±0.29 | 0.55±0.34 | 0.61±0.35* | 0.34±0.34* | 0.43±0.30 | 1 | 4.235 | 0.043* | |
| Con | 0.37±0.27 | 0.35±0.24 | 0.34±0.29 | 0.36±0.31 | 0.45±0.24 | 0.52±0.42 | 0.38±0.32 | 0.40±0.36 | 0.18±0.39 | 0.35±0.22 | |||||
| β2 | MCI | 0.44±0.30 | 0.45±0.27 | 0.38±0.28 | 0.37±0.26 | 0.46±0.29 | 0.49±0.27 | 0.38±0.28 | 0.41±0.29 | 0.37±0.17 | 0.33±0.21 | 1 | 0.674 | 0.422 | |
| Con | 0.41±0.29 | 0.40±0.29 | 0.35±0.21 | 0.35±0.23 | 0.38±0.23 | 0.39±0.31 | 0.37±0.16 | 0.36±0.20 | 0.31±0.24 | 0.31±0.24 | |||||
EEG power by log-transformed; Compared between two group (two-tailed t-test; MCI: Patients with MCI; Con: The normal controls
P<0.05
EEG coherence values at rest for the controls and MCI are shown in Table 2 showing that no significant group differences were found, in the delta [F(1, 4)=2.862, P=0.095], theta [F(1, 4)=2.436, P=0.123], alpha-1 [F(1, 4)=3.150, P=0.080], alpha-2 [F(1, 4)=2.565, P=0.114], beta-1 [F(1, 4)=2.514, P=0.117] and beta-2 [F(1, 4)=2.801, P=0.099] bands.
Table 2.
EEG coherence values (mean±SD) at rest and analysis results by ANOVA
| Bands | F3-F4 | C3-C4 | P3-P4 | T5-T6 | O1-O2 | ANOVA |
|||
| df | F | P | |||||||
| δ | MCI | 1.26±0.10 | 1.29±0.11 | 1.27±0.08 | 1.27±0.09 | 1.28±0.08 | 1 | 2.862 | 0.095 |
| Con | 1.22±0.09 | 1.24±0.12 | 1.24±0.11 | 1.22±0.11 | 1.26±0.10 | ||||
| θ | MCI | 1.21±0.09 | 1.24±0.08 | 1.22±0.08 | 1.23±0.08 | 1.24±0.09 | 1 | 2.436 | 0.123 |
| Con | 1.18±0.09 | 1.20±0.12 | 1.19±0.10 | 1.18±0.11 | 1.22±0.07 | ||||
| α1 | MCI | 1.33±0.10 | 1.36±0.10 | 1.34±0.09 | 1.35±0.09 | 1.35±0.08 | 1 | 3.150 | 0.080 |
| Con | 1.29±0.10 | 1.31±0.13 | 1.30±0.11 | 1.29±0.11 | 1.32±0.11 | ||||
| α2 | MCI | 1.20±0.09 | 1.23±0.09 | 1.21±0.07 | 1.21±0.08 | 1.23±0.08 | 1 | 2.565 | 0.114 |
| Con | 1.16±0.09 | 1.18±0.12 | 1.18±0.10 | 1.16±0.10 | 1.20±0.10 | ||||
| β1 | MCI | 1.18±0.10 | 1.22±0.11 | 1.20±0.09 | 1.20±0.10 | 1.21±0.10 | 1 | 2.514 | 0.117 |
| Con | 1.16±0.10 | 1.18±0.12 | 1.18±0.10 | 1.16±0.10 | 1.20±0.11 | ||||
| β2 | MCI | 1.18±0.10 | 1.21±0.10 | 1.19±0.10 | 1.19±0.10 | 1.20±0.09 | 1 | 2.801 | 0.099 |
| Con | 1.16±0.10 | 1.17±0.12 | 1.17±0.11 | 1.15±0.10 | 1.19±0.11 | ||||
Coherence values transformed to Fisher’s Z scores; Compared between two groups (two-tailed t-test); MCI: Patients with MCI; Con: The normal controls
EEG power and coherence during working memory
Table 3 shows EEG power during working memory for the controls and MCI. The analysis during working memory data by two-way ANOVA revealed that there are significant group differences in EEG power for theta [F(1, 9)=4.950, P=0.029], alpha-1 [F(1, 9)=10.137, P=0.002], alpha-2 [F(1, 9)=9.264, P=0.003] and beta-1 [F(1, 9)=4.712, P=0.034] bands. As shown in Table 3, a subsequent t-test showed that the MCI patients had significantly higher power at P4, T6 and O1 for theta band, at F4, C4, P3, P4, T5, T6 and O2 for alpha-1 band, at F4, C3, C4, P3, P4, T6 and O2 for alpha-2 band, and at P3 and T6 for beta-1 band (P<0.05). However, no significant group difference was found in the delta [F(1, 9)=0.205, P=0.652] and beta-2 [F(1, 9)=0.670, P=0.416] bands.
Table 3.
EEG power (μV2, mean±SD) during working memory and analysis results by ANOVA
| Bands | F3 | F4 | C3 | C4 | P3 | P4 | T5 | T6 | O1 | O2 | ANOVA |
|||
| df | F | P | ||||||||||||
| δ | MCI | 1.37±0.34 | 1.38±0.30 | 1.17±0.31 | 1.13±0.37 | 1.07±0.39 | 1.18±0.28 | 0.93±0.41 | 1.00±0.32 | 0.95±0.38 | 1.13±0.38 | 1 | 0.205 | 0.652 |
| Con | 1.26±0.31 | 1.32±0.38 | 1.07±0.35 | 1.12±0.40 | 1.01±0.29 | 1.03±0.41 | 0.91±0.32 | 0.87±0.45 | 0.91±0.36 | 0.81±0.42 | ||||
| θ | MCI | 1.03±0.28 | 1.05±0.16 | 0.81±0.20 | 0.84±0.19 | 0.84±0.25 | 0.93±0.20* | 0.76±0.29 | 0.79±0.28* | 0.63±0.23 | 0.76±0.29* | 1 | 4.950 | 0.029* |
| Con | 0.98±0.24 | 0.95±0.25 | 0.77±0.31 | 0.74±0.32 | 0.73±0.27 | 0.69±0.30 | 0.67±0.29 | 0.64±0.34 | 0.54±0.33 | 0.50±0.31 | ||||
| α1 | MCI | 0.52±0.32 | 0.55±0.26* | 0.35±0.26 | 0.40±0.26* | 0.50±0.30* | 0.61±0.32* | 0.48±0.39* | 0.57±0.44* | 0.32±0.20 | 0.40±0.34* | 1 | 10.137 | 0.002* |
| Con | 0.40±0.21 | 0.38±0.19 | 0.29±0.23 | 0.27±0.21 | 0.30±0.27 | 0.29±0.26 | 0.37±0.27 | 0.40±0.28 | 0.30±0.18 | 0.28±0.16 | ||||
| α2 | MCI | 0.41±0.34 | 0.44±0.33* | 0.31±0.21* | 0.38±0.28* | 0.56±0.36* | 0.69±0.40* | 0.54±0.41 | 0.71±0.48* | 0.34±0.23 | 0.47±0.43* | 1 | 9.264 | 0.003* |
| Con | 0.28±0.24 | 0.29±0.25 | 0.14±0.12 | 0.19±0.12 | 0.29±0.27 | 0.36±0.35 | 0.43±0.34 | 0.39±0.33 | 0.33±0.19 | 0.18±0.15 | ||||
| β1 | MCI | 0.34±0.25 | 0.33±0.26 | 0.26±0.24 | 0.31±0.22 | 0.48±0.29* | 0.53±0.26 | 0.45±0.37 | 0.56±0.35* | 0.35±0.25 | 0.35±0.29 | 1 | 4.712 | 0.034* |
| Con | 0.24±0.20 | 0.25±0.23 | 0.24±0.23 | 0.29±0.21 | 0.31±0.28 | 0.40±0.33 | 0.41±0.37 | 0.37±0.36 | 0.33±0.13 | 0.26±0.23 | ||||
| β2 | MCI | 0.32±0.22 | 0.25±0.20 | 0.22±0.19 | 0.24±0.18 | 0.32±0.24 | 0.32±0.24 | 0.30±0.26 | 0.28±0.23 | 0.25±0.12 | 0.27±0.18 | 1 | 0.670 | 0.416 |
| Con | 0.24±0.23 | 0.24±0.23 | 0.20±0.18 | 0.23±0.16 | 0.28±0.15 | 0.28±0.16 | 0.21±0.23 | 0.22±0.21 | 0.20±0.15 | 0.23±0.11 | ||||
EEG power by log-transformed; Compared between two groups (two-tailed t-test); MCI: Patients with MCI; Con: The normal controls;
P<0.05
On the other hand, as seen in Table 4, significant group differences were found in EEG coherence during working memory in the delta [F(1, 4)=18.435, P=0.000], theta [F(1, 4)=18.010, P=0.000], alpha-1 [F(1, 4)=19.283, P=0.000], alpha-2 [F(1, 4)=19.316, P=0.000], beta-1 [F(1, 4)=18.186, P=0.000] and beta-2 [F(1, 4)=15.231, P=0.005] bands.
Table 4.
EEG coherence values (mean±SD) during working memory and analysis results by ANOVA
| Bands | F3-F4 | C3-C4 | P3-P4 | T5-T6 | O1-O2 | ANOVA |
|||
| df | F | P | |||||||
| δ | MCI | 1.11±0.11* | 1.10±0.09* | 1.12±0.07* | 1.11±0.08* | 1.08±0.08 | 1 | 18.435 | 0.000* |
| Con | 1.05±0.05 | 1.04±0.08 | 1.06±0.08 | 1.04±0.07 | 1.05±0.06 | ||||
| θ | MCI | 1.08±0.10* | 1.08±0.09* | 1.09±0.06* | 1.10±0.08* | 1.05±0.08 | 1 | 18.010 | 0.000* |
| Con | 1.02±0.04 | 1.01±0.08 | 1.03±0.08 | 1.08±0.06 | 1.02±0.06 | ||||
| α1 | MCI | 1.15±0.11* | 1.15±0.10* | 1.18±0.07* | 1.16±0.09* | 1.13±0.09 | 1 | 19.283 | 0.000* |
| Con | 1.08±0.05 | 1.10±0.08 | 1.11±0.08 | 1.06±0.07 | 1.10±0.07 | ||||
| α2 | MCI | 1.07±0.10* | 1.06±0.09* | 1.08±0.06* | 1.07±0.09* | 1.05±0.08 | 1 | 19.316 | 0.000* |
| Con | 1.01±0.05 | 1.00±0.08 | 1.02±0.08 | 0.99±0.06 | 1.01±0.06 | ||||
| β1 | MCI | 1.06±0.10* | 1.06±0.09* | 1.08±0.06* | 1.07±0.09* | 1.04±0.08 | 1 | 18.186 | 0.000* |
| Con | 1.01±0.04 | 1.00±0.08 | 1.02±0.08 | 0.99±0.06 | 1.01±0.06 | ||||
| β2 | MCI | 1.06±0.10* | 1.06±0.09* | 1.07±0.06* | 1.06±0.08* | 1.04±0.08 | 1 | 15.231 | 0.005* |
| Con | 1.01±0.04 | 1.00±0.08 | 1.01±0.08 | 0.98±0.06 | 1.01±0.06 | ||||
Coherence values transformed to Fisher’s Z scores; Compared between two groups (two-tailed t-test); MCI: Patients with MCI; Con: The normal controls;
P<0.05
During working memory, a subsequent t-test showed that MCI patients had significantly higher EEG coherence at all electrode pairs (P<0.05) in all bands, except at O1-O2 electrode pair.
Correlations
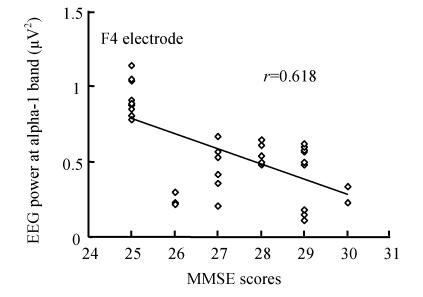
We have analyzed the correlations between the severity of cognitive impairment estimated by the MMSE scores and EEG power during working memory. Correlation analysis showed that the alpha-1 band powers of F3 (r=−0.437, P=0.009), F4 (r=−0.618, P=0.000), C3 (r=−0.532, P=0.001) and C4 (r=−0.355, P=0.036), as well as the alpha-2 band powers of F3 (r=−0.343, P=0.043), F4 (r=−0.524, P=0.001), C3 (r=−0.456, P=0.006), P4 (r=−0.379, P=0.025) and O2 (r=−0.382, P=0.024) were negatively correlated to scores of MMSE during working memory. However, the analysis did not show the correlation between the MMSE score and EEG power at rest. Fig.1 is the scatter plot of correlation between alpha-1 band EEG power of F4 electrode and MMSE scores of MCI patients.
Fig. 1.
Correlation between alpha-1 band EEG power of F4 electrode and MMSE scores of MCI patients during working memory task (r=0.618)
On the other hand, as seen in Tables 3 and 4, only during the working task, was the result of EEG coherence found to have robust differences. Thus, one might suggest that EEG coherence is associated with the severity of cognitive impairment, as shown by the MMSE scores. However, correlation analysis did not show correlation between EEG coherence during working task and MMSE scores (r<0.205, P>0.05).
DISCUSSION
It is well known that working memory is related to the information encoding, maintenance and decoding process. Moreover, working memory tasks are also known to change the cerebral function connectivity (Hogan et al., 2003). Thus, working memory tasks would result in changes of EEG power in different electrodes and the value of coherence. Dierks et al.(1991)’s study of the correlation between EEG power in alpha and beta bands at rest and the degree of dementia in AD patients, indicated that topographical EEG power changes may reflect early signs of cortical atrophy and/or compensatory cortical reorganization early during the course of the disease. The present work’s experimental results shown in Tables 1 and 2, indicated that the EEG power in theta, alpha-1, alpha-2 and beta-1 bands of MCI patients were significantly higher than those of the controls under experimental conditions of at rest and during working memory task. This finding suggests that MCI patients may be associated with early signs of cortical atrophy and/or compensatory cortical reorganization. Furthermore, the change of the EEG sign for cortical functional connectivity for MCI patients is more distinctly apparent during the performance of cognitive task than that at rest. In contract, it seemed that there was no such compensatory process in the control group during working memory task. Pijnenburg et al.(2004) reported that MCI patients had significantly higher EEG synchronization likelihood in alpha and beta bands, and suggested that there is a compensational mechanism in MCI during cognitive performance, while the AD patients could not compensate anymore. The present work’s experimental result for EEG power accorded with Pijnenburg et al.(2004)’s suggestion of compensational mechanism existing in MCI during cognitive performance.
As seen in Table 3, MCI group had significantly higher values of EEG power in bilateral parietal (P3, P4) and temporal (T5, T6) lobes in alpha-1 and alpha-2 bands during working memory compared to normal control. The temporal lobe, hippocampus and other related cortexes play an important role in cholinergic activity in the central nerve centre; parietal lobe is responsible for collating all information into an entire perception. Both lobes are physical bases in cognition function. Recent neuroimage studies have also provided experimental evidences that the parietal lobes are more sensitive during cognitive performance (Xie et al., 2003). Consequently, when studying retarded memory, semantic memory and information encoding or decoding, damage related to the temporal lobe, hippocampus, parietal lobe and other cortexes would occur and result in a change of EEG power. The experimental results in Table 3 reveal that characteristic neuropsychological and neurophysiological changes would happen during the early stage of MCI.
EEG power and coherence estimates reflect different aspects of physiological processing that are mathematically independent. EEG coherence is a non-invasive technique for studying functional relationships between brain regions. The coherence of two EEGs recorded from separate scalp electrodes is assessed in terms of the similarity of waveform components generated by the mass action of neurons in underlying cortical regions. Previous studies showed that patterns of high coherence between EEG signals recorded at different scalp sites have functional significance and can be correlated with different kinds of cognitive information processing, such as memory, language, concept retrieval and music processing (Hogan et al., 2003). In relation to memory processes, studies on healthy humans have generally reported an increase of synchronization between two different brain regions involved in the respective task. It is known that cognitive performance is supported by a network composed by brain cortical regions (Hogan et al., 2003), therefore, MCI may suffer localized damage and connectional disturbance of cortical regions. The present study shows that compared to the controls, MCI patient had significantly higher EEG coherence values during working memory, although at rest, there were no significant differences between the two groups, which suggests that MCI patients and normal controls had differences in cortical connection, and MCI had cortical connection disturbance. Moreover, MCI patients had higher compensatory connection during active cognition than that at rest.
This study did not find any difference of MMSE scores between MCI patients and the controls. This experimental result may reflect that MCI patients could possibly maintain high levels in mental test. Although discriminating between normal older adults and MCI patients is difficult by using neuropsychological test, characteristic neurophysiological changes could be measured. Based on the compensatory mechanism, since the MMSE score was not different in the two groups, it is suggested that changes of the EEG power and coherence would appear even in the early stage of MCI.
Some EEG studies have provided evidences that the alpha band is involved in information processing in the brain. Moreover, some studies (Basar et al., 1997; Schurmann and Basar, 2001) suggested that when external sensory stimulation is applied to the brain, alpha rhythms may have functional correlation with primary sensory processing and preparatory processes. Klimesch (1996) reported that the normal control had reduced values of EEG power in the alpha band during memory performance.
In this study, there are lower values of EEG power in the alpha band during working memory task for both MCI patients and the controls were more than that during rest. In addition, we have examined and confirmed that the values of EEG power in alpha band during working task were negatively correlated to MMSE scores of MCI patients. It is expected that the EEG power is increased when the MMSE scores are decreased. Considering that MCI patients may have compensatory mechanism, it was confirmed that alpha rhythms were directly related to cognitive action.
CONCLUSION
MCI patients had significantly higher EEG power at rest and significantly higher EEG power and coherence during working memory task, which suggests that MCI may be associated with compensatory processes. Furthermore, failure of normal cortical connections probably exists in MCI patients.
Acknowledgments
The author sincerely thanks the members of Clinic EEG Laboratory, Second Affiliated Hospital, School of Medicine, Zhejiang University, for their help in this study.
Footnotes
Project (No. 2004C30020) supported by the Science and Technology Project of Zhejiang Province, China
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- 2.Basar E, Yordanova J, Kolev V, Basar-Eroglu C. Is the alpha rhythm a control parameter for brain responses? Boil Cybern. 1997;76:471–480. doi: 10.1007/s004220050360. [DOI] [PubMed] [Google Scholar]
- 3.Dierks T, Perisic I, Frolich L, Ihl R, Maurer K. Topography of the quantitative electroencephalogram in dementia of the Alzheimer type: relation to severity of dementia. Psychiatry Res. 1991;40:181–194. doi: 10.1016/0165-1781(91)90157-K. [DOI] [PubMed] [Google Scholar]
- 4.Hogan MJ, Swanwick GRJ, Kaiser J, Rowan M, Lawlor B. Memory-related EEG power and coherence reduction in mild Alzheimer’s disease. Int J Psychophysiol. 2003;49:147–163. doi: 10.1016/S0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 5.Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Winblad B, Wahlund LO. Quantitative electroencephalography in mild cognitive impairment: longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol Aging. 2000;21:533–540. doi: 10.1016/S0197-4580(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 6.Jiang ZY. Research of diagnosis of Alzheimer’s disease based on coherence analysis of EEG signal. Chinese J Sensor Actuator. 2004;17(3):363–366. (in Chinese) [Google Scholar]
- 7.Jiang ZY. Abnormal cortical functional connections in Alzheimer’s disease: analysis of inter-and intra-hemispheric EEG coherence. J Zhejiang Univ SCI. 2005;6B(4):259–264. doi: 10.1631/jzus.2005.B0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi M, Wada Y, Takeda T, Oe H, Hashimoto T, Koshino Y. EEG harmonic responses to phonic stimulation in normal aging and Alzheimer’s disease: differences in interhemispheric coherence. Clin Neurophysiol. 2002;113:1045–1051. doi: 10.1016/S1388-2457(02)00129-3. [DOI] [PubMed] [Google Scholar]
- 9.Klimesch W. Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol. 1996;24:61–100. doi: 10.1016/S0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 11.Pijnenburg YAL, vd Made Y, van Cappellen van Walsum AM, Knol DL, Scheltens P, Stem CJ. EEG synchronization likelihood in mild cognitive impairment and Alzheimer’s disease during a working memory task. Clin Neurophysiol. 2004;115:1332–1339. doi: 10.1016/j.clinph.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Salthouse TA, Babcock RL. Decomposing adult age difference in working memory. Developmental Psychology. 1991;27:763–776. doi: 10.1037/0012-1649.27.5.763. [DOI] [Google Scholar]
- 13.Schurmann M, Basar E. Functional aspects of alpha oscillations in the EEG. Int J Psychophysiol. 2001;39:151–158. doi: 10.1016/S0167-8760(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 14.Xie S, Xiao JX, Jiang XX. The fMRI study of the calculation tasks in normal aged volunteers. J Peking Univ (Health Sci) 2003;35:311–313. (in Chinese) [PubMed] [Google Scholar]