Abstract
The production of butyric acid by Clostridium butyricum ZJUCB at various pH values was investigated. In order to study the effect of pH on cell growth, butyric acid biosynthesis and reducing sugar consumption, different cultivation pH values ranging from 6.0 to 7.5 were evaluated in 5-L bioreactor. In controlled pH batch fermentation, the optimum pH for cell growth and butyric acid production was 6.5 with a cell yield of 3.65 g/L and butyric acid yield of 12.25 g/L. Based on these results, this study then compared batch and fed-batch fermentation of butyric acid production at pH 6.5. Maximum value (16.74 g/L) of butyric acid concentration was obtained in fed-batch fermentation compared to 12.25 g/L in batch fermentation. It was concluded that cultivation under fed-batch fermentation mode could enhance butyric acid production significantly (P<0.01) by C. butyricum ZJUCB.
Keywords: Clostridium butyricum, Batch fermentation, Fed-batch fermentation, pH, Butyric acid production, Acetic acid
INTRODUCTION
Butyric acid has several potential applications in industry. Its applications in the foodstuffs and beverage industries are widespread. It may be used as the pure acid in the dairy industry, or in the form of esters as a food additive to increase fruit fragrance. Furthermore, butyric acid could also have some important physiological functions. For example, butyric acid esters are the character-impact flavors in tropic fruits and dairy products (Centeno et al., 2002; Watson et al., 2002). Butyrate is also believed to have therapeutic effect against cancer cells (Beyer-Sehlmeyer et al., 2003; Lupton, 2004). Though butyric acid can be conventionally produced by oxidation of butyraldehyde, there has been increasing interest in the bioproduction of butyric acid via fermentation (Zigová et al., 1999). Several anaerobic bacteria can produce butyric acid as the major end product during fermentation of sugars. Among them, Clostridium species have been used preferably for butyric acid production because they have many advantages over other species due to their simple medium requirement for cell growth and relatively high product yield (Michel-Savin et al., 1990a; 1990b). Also, these strains can be readily isolated from soil, wastewater, animal digestion systems, contaminated dairy products, etc. Zigová et al.(1999) reported that the optimal cultivation conditions for butyric acid production by Clostridium butyricum were as follows: temperature of 35~37 °C and atmosphere of pure CO2 or pure N2 or N2 and CO2 in ratio of 1:9. Utilizable and/or suitable common carbon sources include glucose, as well as lactose from whey, sucrose from molasses, starch, potato wastes, wheat flour, cellulose or dextrose.
Clostridium butyricum is a typical butyric acid bacterium found in soil and intestines of healthy animals and humans (Murray et al., 1984). One strain of C. butyricum preserved in our lab was screened and identified as a probiotic bacterium (Kong et al., 2004). For butyric acid fermentation, batch, fed-batch, continuous and cell-recycle-fermentations are most frequently used. Results of such experiments offer deeper insight into strain physiology and behaviour (Zigová and Šturdik, 2000). In order to obtain a higher quantity of butyric acid, there is one important parameter which should be investigated, the pH. The most effective pH is that at which only undissociated acid is present. This value depends on the dissociation constant of the acid and for butyric acid it is 4.63. On the other hand, too low a pH is not suitable for acid production. With C. acetobutyricum the optimal pH for butyrate production (acidogenesis) is 6~6.5 and for butanol (solventogenesis) is 4.5~4.8 (Vandák et al., 1997).
This study aimed at investigating the effect of pH on cell growth and butyric acid formation by Clostridium butyricum ZJUCB. In order to find the desired pH cultivation value we first compared different pH cultivation conditions for batch fermentation in a 5-L bioreactor, and then studied the butyric acid production in fed-batch fermentation.
MATERIALS AND METHODS
Microorganism and culture media
The bacterial strain (C. butyricum ZJUCB) used in this study was preserved in the Department of Food Science and Nutrition, Zhejiang University, Hangzhou, China. The Hungate Anaerobic Culture Technique was used to isolate, purify, identify and incubate the strain of C. butyricum (Chung and Bryant, 1997; Min, 1999). The fermentation medium was composed of 20 g glucose, 3.7 g corn steep flour, 1 g (NH4)2SO4, 1.24 g NaHCO3, 0.2 g MnSO4·H2O, 0.2 g MgSO4·7H2O, 0.02 g CaCl2 per liter at pH 7.5. The medium was autoclaved at 121 °C for 20 min, and cooled to room temperature prior to use.
Batch fermentation in bioreactor
A 24-h preculture was used to inoculate the cells in a 5-L bioreactor (with 4 L working volume) at 5% level. The monitoring and/or control of various parameters, such as temperature and pH, were performed in a PID (Proportional-Integral-Derivative) control unit (B. Braun, Diessel, Germany). The bioreactor was operated at 37 °C. Anaerobiosis was ensured by flushing oxygen-free nitrogen gas through the medium. Oxygen-free nitrogen gas was also passed through the culture fluid at a rate of about 0.3~0.4 L per minute. The pH was controlled to different required pH values in the range of 6.0~7.5.
Fed-batch fermentation in bioreactor
Fermentation was initiated as batch fermentation. The pH followed the optimum pH acquired from batch fermentation. After 12 h, it was converted to fed-batch fermentation by adding concentrated glucose feed (glucose concentration=200 g/L) at a constant feed rate of 0.025 L/h. Feeding was stopped after 32 h and residual glucose in the fermentation broth was allowed to ferment by batch fermentation (Lang et al., 1997; Shoemaker and Wright, 2003).
Assays
The cell growth was measured as the dry cell weight. The culture sample (10 ml) was centrifuged at 3000 r/min for 10 min, and the cell pellet was washed twice with distilled water, dried to constant weight at 80 °C, and then weighed (Chen et al., 2004).
The glucose concentration in the medium was measured using the DNS method (Miller, 1959).
Quantitative analysis of butyric acid and acetic acid was performed by gas chromatography under the following conditions: glass column packed with Porapak Q (1.5 m×1.5 mm), N2 as the carrier gas, temperatures of the flame ionization detector (FID) and injection port set at 260 °C, and column temperature at 215 °C. The concentration of butyric acid was determined according to a standard calibration curve.
Statistical analysis
Each experiment was performed in triplicate. Data of interest were analyzed with t-test.
RESULTS AND DISCUSSION
Batch fermentation
1. Butyric acid production at different pH values
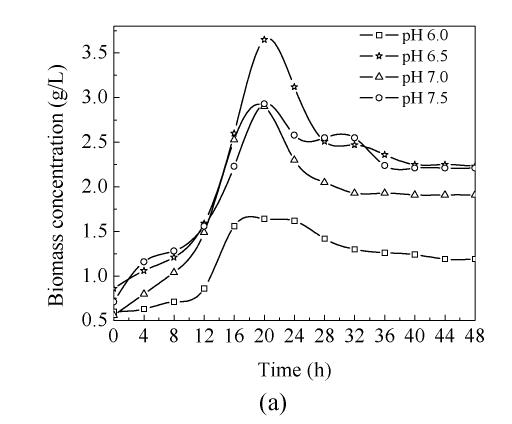
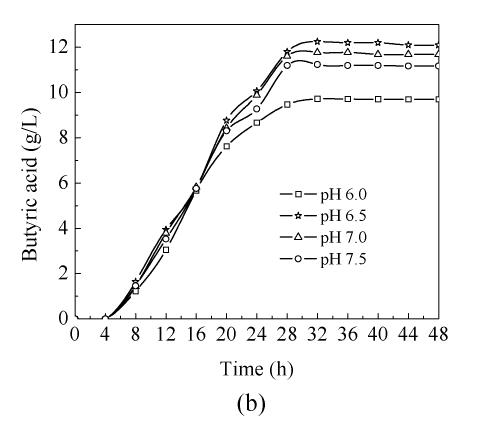
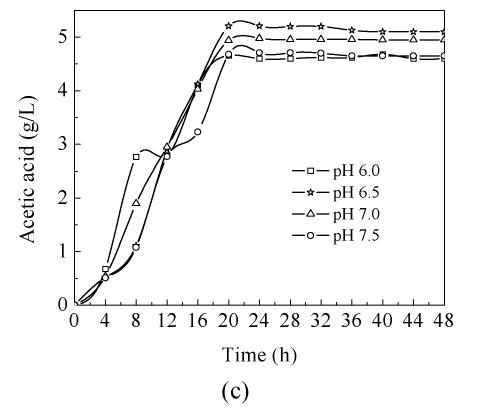
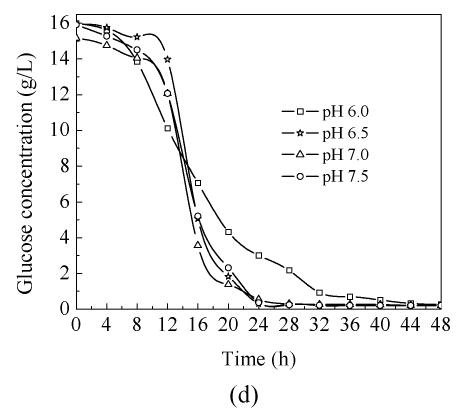
In order to study the influence of pH on C. butyricum ZJUCB cell growth and butyric acid production, four different pH (pH 6.0, pH 6.5, pH 7.0 and pH 7.5) experiments were performed in the bioreactor. The time-course of butyric acid batch fermentation at the different pH is shown in Fig.1. With rising operating pH, the maximum biomass concentration (Fig.1a) and maximum butyric acid (Fig.1b) value were higher than those at pH=6.0, and the highest biomass concentration and butyric acid value occurred at pH=6.5 with cell yield of 3.65 g/L and butyric acid yield of 12.25 g/L. During the first 20 h, faster cell growth was observed at higher pH values (pH 6.5, pH 7.0 and pH 7.5). After 20 h cultivation, the biomass declined. In general, the butyric acid biosynthesis lagged cell growth by about 8 h and then increased obviously. Acetic acid biosynthesis increased simultaneously with cell growth (Fig.1c). At the former stage, the butyric acid concentrations at different pH values were almost the same, but during mid- and later-stage the butyric acid concentrations of cells cultivated at pH 6.5, pH 7.0 and pH 7.5 were higher than those cultivated at pH 6.0. The consumption of reducing sugar at pH 7.0 was the fastest and the lowest at pH 6.0 throughout the process (Fig.1d).
Fig. 1.
Time-course of batch fermentation at pH 6.0, pH 6.5, pH 7.0 and pH 7.5 (a) Biomass concentration; (b) Butyric acid concentration; (c) Acetic acid concentration; (d) Glucose concentration
High pH was beneficial for cell growth and butyric acid biosynthesis. But sometimes cell autolyzation and enzyme inactivity are also accelerated when pH is too high, so targetted yield is negatively affected. However, the operating pH must not be too low, as biochemical reaction rate typically decreases with decreasing pH. Thus lower operating pH would decrease butyric acid production and prolong the required fermentation time as well. In addition, it is not easy to control industry-scale fermentation when the pH is too low or too high. Based on the discussion above, pH 6.5 was considered to be beneficial for C. butyricum cell growth and butyric acid production.
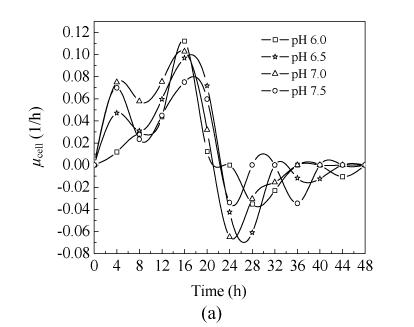
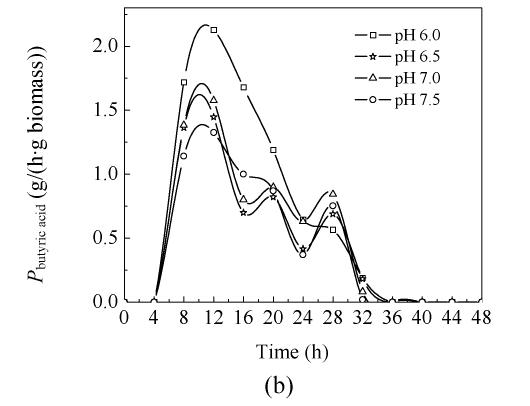
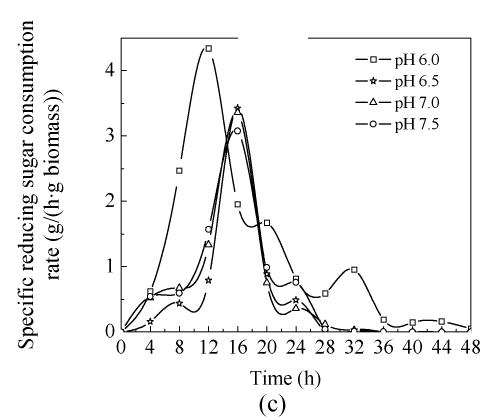
2. Effect of pH on specific growth rate
Fig.2 shows the results of μ cell, P butyric acid and specific reducing sugar consumption rate at pH 6.0, pH 6.5, pH 7.0 and pH 7.5 in 5-L bioreactor. Specific growth rate (μ cell) at the four pH values showed similar tendency before 24 h cultivation, although the highest μ cell occurred at pH 6.0; after 24 h, when μ cell decreased to minus and showed obviously different behavior. The curve fluctuated, especially during the later stage of experiment. It was thought that the microorganism autolyzed or reused the by-products in the broth and grew again during the later stage of cultivation.
Fig. 2.
Batch fermentation at pH 6.0, pH 6.5, pH 7.0 and pH 7.5 carried out in 5-L bioreactor (a) μ cell, (b) P butyric acid, (c) specific reducing sugar consumption rate
3. Effect of pH on the specific butyric acid production rate
Fig.2b shows that during the period of 0 h to 24 h, specific butyric acid formation rate (P butyric acid) at pH 6.0 was the fastest because the biomass concentration was the lowest at pH 6.0. After cultivation for 12 h, the maximum value of P butyric acid [2.128 g/(h·g biomass)] occurred at pH 6.0.
4. Effect of pH on specific reducing sugar consumption rate
The consumption rate of reducing sugar at pH 6.0 was faster than that at other pH values during the period of time=0 to 12 h. At pH 6.0 more substrate was contributed to cell growth and the production of other metabolites, so butyric acid concentration (9.717 g/L) was not high. This finding implied that the substrate metabolites may have a role in contributing to butyric acid production.
Fed-batch fermentation
According to effect of pH on butyric acid production shown above, we found the optimal cultivation pH for cell growth and butyric acid production is 6.5. In order to enhance the production of butyric acid, then we adopted the fed-batch fermentation.
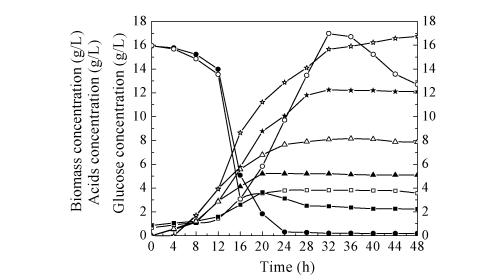
Comparative profiles of butyric acid fermentation under batch and fed-batch conditions are shown in Fig.3. Before 20 h, the biomass of C. butyricum was almost constant, and during the mid- and later-stages, the biomass in the fed-batch fermentation did not decrease. While for batch fermentation, after the biomass concentration peak, biomass rapidly declined to lower than that of the fed-batch fermentation. For fed-batch fermentation, the cell growth still remained at suitable rate after 20 h. As expected, the fed-batch fermentation biomass was higher than that of batch fermentation. Maximum biomass (about 3.81 g/L) was achieved in fed-batch fermentation. These results indicated that fed-batch fermentation strategy favors cell growth.
Fig. 3.
Comparison of batch and fed-batch fermentation at pH 6.5
■ Biomass of batch; □ Biomass of fed-batch; ● Glucose of batch; ○ Glucose of fed-batch; ▲ Acetate of batch; ∆ Acetate of fed-batch; ★ Butyrate of batch; ⋆ Butyrate of fed-batch
Similar time-course profiles of butyric acid fermentation were observed among the two cultivation protocols as shown in Fig.3. The highest value (12.25 g/L) was obtained after 32 h cultivation in batch fermentation, while the maximum value (16.74 g/L) of butyric acid concentration in fed-batch fermentation was much higher. The differences of maximum butyric acid concentrations under the two cultivation conditions were significant (P<0.01). These results suggested that fed-batch fermentation cultivation significantly improved butyric acid production as expected. Fed-batch fermentation cultivation mode could also significantly improve acetic acid production (P<0.01).
During 12 h to 24 h of cultivation, the reducing sugar was consumed at a high rate under batch fermentation strategy. While under fed-batch fermentation strategy, the reducing sugar was consumed at a high rate during 12 h to 32 h of cultivation (from 12 h to 32 h, constant addition of glucose at a rate of 5 g/h).
CONCLUSION
This study indicated that the influence of pH on butyric acid fermentation was significant. Maximum butyric acid concentration (12.25 g/L) was achieved when the cells were cultivated at pH 6.5 in batch fermentation. Comparison of the batch and fed-batch fermentation showed that fed-batch fermentation mode could significantly enhance butyric acid production (P<0.01). Maximum butyric acid concentration in fed-batch fermentation could reach 16.74 g/L at pH 6.5, representing a 36.65% increase in productivity over the batch process. These results indicate that a fed-batch process at pH 6.5 yielded higher levels of cell growth and butyric acid concentration than batch process for butyric acid production from C. butycium ZJUCB. Results of this study provide useful information to apply and industrial microbiologists, and process engineers for designing scale-up strategies for process optimization.
Acknowledgments
We deeply thank Dr. Y. Xu for her useful comments during the preparation of this manuscript.
References
- 1.Beyer-Sehlmeyer G, Glei M, Hartmann E, Hughes R, Persin C, Böhm V, Rowland I, Schubert R, Jahreis G, Pool-Zobel BL. Butyrate is only one of several growth inhibitors produced during gut flora-mediated fermentation of dietary fibre sources. Br J Nutr. 2003;90:1057–1070. doi: 10.1079/BJN20031003. [DOI] [PubMed] [Google Scholar]
- 2.Centeno JA, Tomillo FJ, Fenández-García E, Gaya P, Nuňez M. Effect of wild strains of Lactococcus lactis on the volatile profile and the sensory characteristics of Ewes’ raw milk cheese. J Dairy Sci. 2002;85:3164–3172. doi: 10.3168/jds.S0022-0302(02)74404-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen QH, He GQ, Schwarz P. Studies on cultivation kinetics for elastase production by Bacillus sp. EL31410. J Agric Food Chem. 2004;52(11):3356–3359. doi: 10.1021/jf0303161. [DOI] [PubMed] [Google Scholar]
- 4.Chung KT, Bryant MP. Robert E. Hungate: Pioneer of anaerobic microbial ecology. Anaerobe. 1997;3:213–217. doi: 10.1006/anae.1997.0109. [DOI] [PubMed] [Google Scholar]
- 5.Kong Q, He GQ, Chen QH, Chen F. Optimization of medium composition for cultivating Clostridium butyricum with response surface methodology. J Food Sci. 2004;69(7):163–168. [Google Scholar]
- 6.Lang C, Gollnitz C, Popovic M, Stahl U. Optimization of fungal polygalacturonase synthesis by Saccharomyces cerevisiae in fed-batch culture. Chem Eng J. 1997;65:219–226. doi: 10.1016/S1385-8947(97)00029-6. [DOI] [Google Scholar]
- 7.Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr. 2004;134:479–482. doi: 10.1093/jn/134.2.479. [DOI] [PubMed] [Google Scholar]
- 8.Michel-Savin D, Marchal R, Vandecasteele JP. Control of the selectivity of butyric acid production of Clostridium tyrobutyricum . Appl Microbiol Biotechnol. 1990;32:387–392. doi: 10.1007/BF00903770. [DOI] [Google Scholar]
- 9.Michel-Savin D, Marchal R, Vandecasteele JP. Butyrate production in continuous culture of Clostridium tyrobutyricum: effect of end-product inhibition. Appl Microbiol Biotechnol. 1990;33:127–131. doi: 10.1007/BF00176512. [DOI] [Google Scholar]
- 10.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–427. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 11.Min H. Microbial Research Techniques. Beijing: Science Press; 1999. [Google Scholar]
- 12.Murray RGE, Brenner DJ, Bryant MP, et al. Bergey’s Manual of Systematic Bacteriology. Baltimore: Williams & Wilkins; 1984. pp. 1160–1161. [Google Scholar]
- 13.Shoemaker SP, Wright LL. Feedstock production, genetic modification, and processing. Appl Biochem Biotechnol. 2003;105:3–4. doi: 10.1385/ABAB:105:1-3:3. [DOI] [Google Scholar]
- 14.Vandák D, Zigová J, Šturdik E, Schlosser S. Evaluation of solvent and pH for extractive fermentation of butyric acid. Process Biochem. 1997;32(3):245–251. doi: 10.1016/S0032-9592(96)00084-2. [DOI] [Google Scholar]
- 15.Watson R, Wright CJ, McBurney T, Taylor AJ, Linforth RST. Influence of harvest data and light integral on the development of strawberry flavor compounds. J Exp Bot. 2002;53(377):2121–2129. doi: 10.1093/jxb/erf088. [DOI] [PubMed] [Google Scholar]
- 16.Zigová J, Šturdik E. Advances in biotechnological production of butyric acid. J Ind Microbiol Biotechnol. 2000;24:153–160. doi: 10.1038/sj.jim.2900795. [DOI] [Google Scholar]
- 17.Zigová J, Šturdik E, Vandák D, Schlosser S. Butyric acid production by Clostridium butyricum with integrated extraction and pertraction. Process Biochem. 1999;34:835–843. doi: 10.1016/S0032-9592(99)00007-2. [DOI] [Google Scholar]