Abstract
This paper presents the evaluation of an aqueous two-phase system (ATPS) for extracting elastase produced by Bacillus sp. EL31410. The elastase and cell partition behavio r in polyethylene glycol (PEG)/salt systems was investigated. The suitable system for elastase extraction was PEG/KH2PO4-K2HPO4, in which elastase is mainly partitioned into the PEG-rich phase, while the cells remained in the other phase. The influence of defined system parameters (e.g. PEG molecular mass, pH, NaCl addition) on the partitioning behavior of elastase is described. The concentration of phase forming components, PEG and KH2PO4-K2HPO4, was optimized for elastase recovery by means of response surface methodology, and it was found that they greatly influenced extraction recovery. The optimal ATPS was 23.1% (w/w) PEG 2 000 and 11.7% (w/w) KH2PO4-K2HPO4. The predicted recovery was about 89.5%, so this process is suggested to be a rapid and convenient method for elastase extraction.
Keywords: Elastase, Aqueous two-phase system (ATPS), Bioseparation, Purification, Optimization, Response surface methodology (RSM)
INTRODUCTION
Aqueous two-phase system (ATPS) is widely used in biochemistry and biotechnology for purification of proteins (Diamond and Hsu, 1992; Balasubramaniam et al., 2003), enzymes (Pan et al., 2001; Bim and Franco, 2000), amino acids (Li et al., 2002), antibiotics (Yang et al., 1994; Guan et al., 1994), nucleic acids (Ohlsson et al., 1978; Kimura, 2000), aroma compounds (Marco et al., 2000) and lactic acid (Planas et al., 1998). ATPS is mainly composed of two-incompatible polymers (e.g. Dextran/PEG) or a polymer and a salt (usually phosphate and sulfate) and has great potential for industrial applications because it can be used to obtain a concentrated and purified product in one step by addition of crude broths containing suspended matter (e.g. cells), and offers gentle nontoxic environments for labile biomolecules. Well-known advantages of ATPS are volume reduction, high capacity, rapid separations, easy scale-up and suitability for continuous large-scale operations (Yan, 2001). PEG/salt systems, especially, are widely applied for bioseparation of proteins because of their low cost and wide range of hydrophobicity differences between the two-phase systems (Li et al., 2001). The uneven partitioning of a protein in ATPS depends not only on the surface properties (such as the size, charge and hydrophobicity) of the protein but also on the physicochemical traits of the two phases. The nature of the system is influenced by changing factors such as polymers, polymer molecular mass and concentration, the type of phase forming salt and its concentration, NaCl concentration as well as the pH of the system. The partition of compounds in these systems is very complex due to several factors, including hydrogen bonds, charge, hydrophobic interactions and static effects. The mechanisms still remain unclear for this kind of uneven partitioning of materials. For a particular application, experimentation is necessary to define an optimal system.
Elastase is a protease that catalyzes the hydrolysis of elastin (Morihara, 1967), and is widely applied in biochemical medicine, as a tenderizer for meat in the food industry, in cosmetics, and in environmental protection. When compared to extraction of elastase from pancreatic tissue, production by fermentation technology is more promising due to its low cost, high production rate, and readily controlled conditions. To date, research has been concentrated on isolation and screening of microorganisms that produce elastase, and on the purification and characterization of newly found enzymes (Shibata et al., 1993; Tsai et al., 1988; Michelle et al., 2001). Bioseparation protocols reported were conventional methods such as centrifugation followed by fractional precipitation and chromatography. ATPS seems to be a good alternative to a first step purification as this allows the removal of several contaminants. However, the possibility of use of the ATPS process for the recovery of elastase is not documented.
We recently reported an elastase producing strain Bacillus sp. EL31410, with strong powerful ability for elastase production, and the cultivation parameters (Chen et al., 2002; He et al., 2004). This study aimed at investigating the factors affecting elastase partition in PEG/salt and optimizing the ATPS components using response surface methodology (RSM), and finding a more suitable system to develop the ATPS process for elastase recovery and purification.
MATERIALS AND METHODS
Materials
Polyethylene glycol (PEG) 400, 600, 1 000, 4 000, 6 000 were purchased from Shanghai Pudong Gaonan Chemical Company (Shanghai, China). PEG 2 000 was produced by China Medicine Shanghai Chemical Company (Shanghai, China). The salts and other chemicals used were of analytical grade.
Bacterial strains, media and cultivation
Bacillus sp. EL31410 was screened and preserved by Microbiology Lab of Department of Food Science and Nutrition, Zhejiang University, China. The stock culture was maintained at 4 °C on agar slants containing (g/L) 4 beef extract, 6 peptone, 2 yeast extract, 5 NaCl and 20 agar (initial pH 7.5). The growth medium for seed culture was the same as that for the stock culture with the exception of agar. The fermentation medium consisted of (g/L) 74 glucose, 11.3 casein, 6.16 corn liquid steep, 2.06 K2HPO4·3H2O, and 0.34 MgSO4·7H2O (initial pH 7.5). The test microorganism was inoculated into 25 ml seed medium in 250 ml Erlenmeyer flasks. For the preparation of starter cultures, cultivation was for 18 h at 37 °C on a 200 r/min rotary shaker. Elastase fermentation was carried out in a 5-L bioreactor at 30 °C and 300 r/min. Inoculation size was 4%, the loading coefficient 0.6, air flux 1 vvm, initial pH value 7.5. After cultivation, the culture was harvested and centrifuged at 3000 r/min for 15 min to obtain a yellowish supernatant.
Aqueous two-phase system
Phase systems were prepared in a 25 ml beaker by adding appropriate amounts of PEG, stock salt(s) solution, and fermentation supernatant. The systems were adjusted to the required pH with buffer and distilled water was added to attain make up 15 g. Systems were thoroughly mixed by magnetic mixer. Properly proportioned ATPS (10 g) was weighed into a 10 ml graduated centrifuge tube, and then centrifuged at 2000 r/min for 10 min to expedite the phase separation. The centrifuged sample was placed into a water bath (25 °C) and allowed to stand for 30 min. After the phase volumes were measured, the top and bottom phases were separately withdrawn.
Parameters evaluated included: (1) Recovery of elastase (Y) was defined as the sum of the elastase activity in the top/initial elastase activity added into the whole system.
| Y=100×CtVt/CoVo | (1) |
where C t and C o denote the measured activity units per ml (units/ml) in the top phase and the original adding samples, respectively; V t and V o are the volumes of the top phase and the original sample, respectively; (2) The partition coefficient of cell and elastase was K cell and K elastase, respectively. The ratio of biomass in both the top phase and the bottom phase was named as K cell; the ratio of measured elastase activity in the top and that in bottom phase was defined as K elastase; (3) Purification factor (PF) was defined as SA t/SA ori, where SA t and SA ori are the specific activities of elastase in the top and in the original supernatant, respectively.
Assays
Elastolytic activity was assayed by the colorimetric method of Sachar (1955). The reaction mixture contained congo-red elastin (20 mg), 1 ml of distilled water, 2 ml of boric acid buffer (0.2 mol/L, pH 7.4) and 1 ml of suitably diluted enzyme. The reaction mixture was incubated at 37 °C and 100 r/min for 20 min. The reaction was terminated after 20 min by adding 2 ml of sodium phosphate buffer (0.7 mol/L, pH 6.0), and immediately filtered. The absorbance of the filtrate was read at 495 nm against a control (no enzyme). One unit of elastase activity was defined as the amount of enzyme required to solubilize 20 mg of elastin-congo red under the test conditions. The protein content was determined by Bradford method using bovine serum albumin (BSA) as the standard (Bradford, 1976). The cell content was evaluated at 600 nm by spectrophotometer.
Experimental design
A 22 full factorial design (FFD) yielding four sets of experiments was used to verify the most significant factors affecting elastase recovery. On the basis of the first-order model obtained by the FFD, trails were obtained in the direction of steepest ascent. In order to fit the empirical second-order polynomial model, a central composite design (CCD) with five coded levels was implemented. The quadratic model for predicting the optimum is expressed by the following equation:
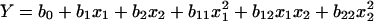 |
(2) |
, where Y is the response variable, b is the regression coefficient, and x is the coded level of the independent variable.
A full second-order polynomial model obtained by multiple regression technique for two factors using SAS (SAS Institute Inc. Cary, NC, USA) was used to describe the response surface. Response surface methodology (RSM), first documented by Box and Wilson (1951), has proved to be a very efficient tool for optimization (Box and Hunter, 1978).
RESULTS AND DISCUSSION
Effects of phase-forming salt on elastase partition
The selection of salt used in the extraction system was one of the key points of this ATPS technique. Four different inorganic salts were evaluated. A film of sediment was observed at the interface of two phases. The results are summarized in Table 1. After separation and centrifugation, the cells readily entered into the bottom phase or precipitated at the interface according to the partition coefficient, especially in PEG/KH2PO4-K2HPO4; Recoveries of elastase for each system (PEG/(NH4)2SO4, PEG/K2HPO4 and PEG/KH2PO4-K2HPO4), were higher than 50%, which suggested that elastase is favorably distributed to some degree into the top phase. For PEG/KH2PO4-K2HPO4, the elastase recovery of 75.4% and K elastase recovery of 11.73 were the highest. Partitioning of the elastase was affected by the traits of the ATPSs. The interaction of a compound with each of the phases in these systems is very complex due to the several factors involved, such as hydrophobic interactions, hydrogen bonds, charge and steric effects in a mainly surface dependent process (Marcos et al., 2002). It should be pointed out, however, that the complexity of ATPSs is perhaps even greater because these factors are not absolutely mutually independent. Hydrophobicity is changed by the type of polymer and its relative molecular weight, concentration, as well as the salt concentration and pH. However, the values of purification factor observed were not high. It is not advisable to consider the recovery and purification factor in a biochemical method as a signal. In this work, high recovery was our goal, because ATPS is not the last purification step in elastase preparation. The PEG/KH2PO4-K2HPO4 system is favorably considered for subsequent study.
Table 1.
Partitioning of elastase and cells from Bacillus sp. fermentation broth in PEG-salt ATPSs
| Salts | Recovery of elastase (%) | Kelastase | Kcell | Purification factor |
| MgSO4·7H2O | 43.6±3.0c | 2.84 | 1.27 | 1.23±0.05 |
| (NH4)2SO4 | 65.0±2.9b | 6.88 | 1.90 | 1.22±0.09 |
| K2HPO4 | 64.4±2.0b | 9.34 | 0.51 | 0.74±0.03 |
| KH2PO4-K2HPO4 | 77.9±2.4a | 11.73 | 0.26 | 1.17±0.08 |
Each ATPS was composed of 20% (w/w) PEG 2000 and 13.2% (w/w) inorganic salt at pH 7.5; After elastase recovery, experiments were conducted to estimate the experimental error;
stand for the significant difference between each treatment as revealed by Duncan’s multiple range test (P=0.05)
stand for the significant difference between each treatment as revealed by Duncan’s multiple range test (P=0.05)
stand for the significant difference between each treatment as revealed by Duncan’s multiple range test (P=0.05)
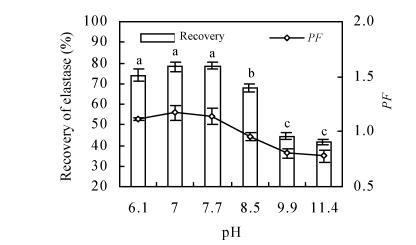
Effects of pH on elastase partition
The partition behaviors of elastase in systems with different pH values were studied in PEG/KH2PO4-K2HPO4 system containing 20% (w/w) PEG 2 000 and 13.2% (w/w) KH2PO4-K2HPO4. Fig.1 shows the partition variation over the pH range 6~11. As pH increased, the recovery of elastase decreased, especially for pH higher than 8.5. The suitable pH range was 7.0~7.7 for elastase extraction using this ATPS. The pH affected not only the targeted enzyme but also the electric traits and surface character of contaminating proteins, influenced their partition into the top and bottom phases. In addition, the ion partition proportion in the ATPS was changed, and this altered the voltage difference in-between two phases. The change of purification factor changed with the recovery, but it was still not very high.
Fig. 1.
Effect of pH on the elastase recovery and purification factor (PF). Enzyme partitioning at certain pH was done in systems containing 20% (w/w) PEG 2 000 and 13.2% (w/w) KH2PO4-K2HPO4. Three replicates were made to estimate experimental error and where a, b, c over the columns stand for the significant difference (of recovery) by Duncan’s multiple range test (P=0.05)
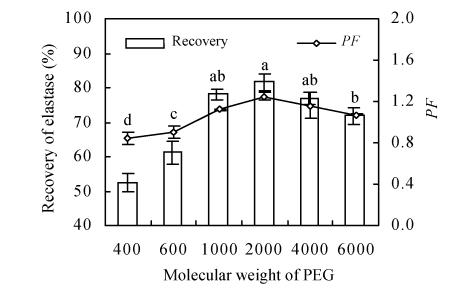
Effects of PEGs molecular weight on elastase partition
Using the above results, the 20% (w/w) PEG/13.2% (w/w) KH2PO4-K2HPO4 (pH 7.5) was chosen for further study. The effects of various PEGs with different degree of polymerization on the partitioning of elastase are shown in Fig.2. As anticipated, the very low molecular weight (MR) of PEGs MR (400, 600) and the much higher PEGs MR (6 000) were not helpful for obtaining good elastase partitioning. At the extreme PEG MRs (400, 600 or 20 000), neither the addition of NaCl nor pH modification affected the separation process (Héctor et al., 1995). Thus the Y-value at the top phase of the PEG 400 system was lower than that of PEG 600 for the hydrophobic protein. An increase in the MR of one of the phase polymers would cause the biomaterial to partition more strongly into the other phase. However, the magnitude of this effect decreases with increasing polymer chain length (Tanuja et al., 1997). As the MR increases (to 1 000 and 2 000), the hydrophobicity increases correspondingly, which resulted in more elastase entering into PEG-rich phase. But as the PEG MR increased from 2 000 to 6 000, the recovery decreased significantly from 80.7% to 71.8%. This can possibly be attributed to the increase in spatial resistance with the increase of PEG MR. The higher PEGs MRs (4 000, 6 000) hindered the movement of elastase distributing into the top phase. The PEG MR influenced the targeted protein partitioning both by altering the phase diagram (i.e. by influencing the composition of the phases) and by changing the number of polymer-enzyme interactions in general. Similar phenomena were observed by others (Tanuja et al., 1997; Baskir et al., 1989). Purification factor also changed with the recovery but was still less than 2. The best choice of PEG for the two-phase system was PEG 2 000, which exhibited the highest partition ratio and moderate purification factor.
Fig. 2.
Effect of PEG molecular weight on elastase partition and purification factor (PF). Enzyme partitioning with respected to MR was performed in systems containing 20% (w/w) PEG and 13.2% (w/w) KH2PO4-K2HPO4 (pH 7.5). Three replicates were made to estimate experimental error with a, b, c, d above the columns standing for the significant difference (of recovery) by Duncan’s multiple range test (P=0.05)
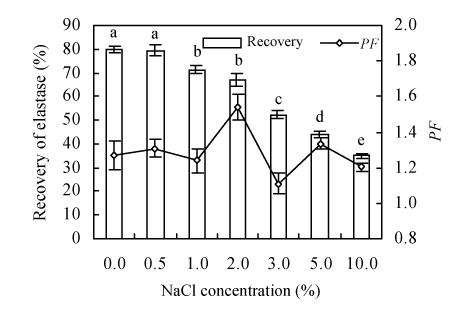
Effect of NaCl concentration on elastase partitioning
The influence of NaCl concentration on the partitioning behavior of elastase was tested in ATPS containing 20% (w/w) PEG 2 000/13.2% (w/w) KH2PO4-K2HPO4 (pH 7.5). As can be seen in Fig.3, when NaCl concentration increased from 0 to 10%, the recovery decreased form 80% to 35%. The elastase distribution pattern observed in these systems showed that increasing NaCl concentration beyond 2% could dramatically decrease the recovery. Although adding NaCl has been reported to be beneficial to targeted products (Silgia et al., 2000; Cascone et al., 1991; Marcos et al., 2002), in the current study addition of NaCl negatively influenced partitioning of elastase into the top phase. Others described similar results in other systems (Li and He, 1992; Isabel del-Val and Cristina, 2003). The purification factor (PF) value was a little higher (1.54) in the system containing 2.0% NaCl than in other trails, but it was not significantly adjusted. Based on the above, NaCl will be excluded from subsequent studies.
Fig. 3.
Effect of NaCl concentration on the recovery of elastase and on purification factor (PF). Enzyme partitioning with respected to NaCl was implemented in systems containing 20% (w/w) PEG 2 000/13.2% (w/w) KH2PO4-K2HPO4 (pH 7.5). Three replicates were implemented to estimate experimental error with a, b, c, d, e above the columns standing for the significant difference (of recovery) revealed by Duncan’s multiple range test (P=0.05)
Full factorial design (FFD) of PEG and KH2PO4-K2HPO4 concentration and analysis
The 22 factorial design and the results are presented in Table 2. Data obtained by this design were used to derive an equation describing correlation between the independent variables and the dependent variable. The model is as follows:
| Y=74.07+12.75x1−6.15x2 | (3) |
Table 2.
Experimental design and results of factorial design
| No. | Coded level |
Real values |
Y (%) | ||
| x1 | x2 | X1 (%) | X2 (%) | ||
| 1 | −1 | −1 | 15 | 10 | 63.1 |
| 2 | 1 | −1 | 25 | 10 | 87.8 |
| 3 | −1 | 1 | 15 | 20 | 50.0 |
| 4 | 1 | 1 | 25 | 20 | 76.3 |
| 5 | 0 | 0 | 20 | 15 | 80.5 |
| 6 | 0 | 0 | 20 | 15 | 76.8 |
| 7 | 0 | 0 | 20 | 15 | 79.2 |
| 8 | 0 | 0 | 20 | 15 | 77.5 |
| 9 | 0 | 0 | 20 | 15 | 78.4 |
X 1: Concentration of PEG 2 000, % (w/w); X 2: Concentration of KH2PO4-K2HPO4, % (w/w); Y: Recovery of elastase (%)
Analysis of variance (ANOVA) in Table 3 showed that the factors PEG 2 000 and KH2PO4-K2HPO4 concentration were significant for P<0.01 and P<0.1, respectively. This verified that both two variables were important factors influencing the distribution of elastase. Increasing the concentration of PEG 2000 and decreasing that of KH2PO4-K2HPO4, according to the signs of their main effects, should have a positive impact on elastase recovery. The coefficient of determination, R 2 (determinant coefficient)=0.827, and F=14.34 (>F 2, 6, 0.01=10.9) verified that the model was valid at probability level of 99%. The results of t-test for variance between the observed average of two-level experiment and the center point showed that the difference was not significant. This result indicated that optimum point was not in the scope of our experiment. Experimentation on the steepest ascent path was necessary to reach optimum domain.
Table 3.
Results of factorial design regression analysis for recovery of elastase (Y)
| Term | Parameter estimate | Standard error | T for H0: Parameter=0 | Prob>|T| | |||
| Intercept | 74.07 | 1.76 | 42.029 | 0.0001 | |||
| x1 | 12.75 | 2.64 | 4.823 | 0.0029 | |||
|
x2 |
−6.15 |
2.64 |
−2.327 |
0.0589 |
|||
| Root MSE | 5.29 | R-square | 0.827 | ||||
| Dep mean | 74.07 | Adj R-square | 0.769 | ||||
| C.V. | 7.138 |
Steepest ascent experiment on PEG and KH2PO4-K2HPO4 concentration and analysis
Results shown in Table 2 and Table 3 indicated obviously that the optimal region was outside the current design space. Under this situation, a directional search method, like the steepest ascent, can be carried out to determine the next set of experiments. Based on the first-order model equation (Eq.(3)), the path of steepest ascent was aimed at increasing the concentrations of PEG 2 000 and decreasing that of KH2PO4-K2HPO4 in order to improve elastase recovery. The values of elastase recovery and purification factor in these experiments are listed in Table 4. The data clearly suggested that the recovery of elastase increased when the PEG concentration (X 1) increased and KH2PO4-K2HPO4 concentration (X 2) decreased both by 0.8% (Trails No. 10 to No. 14). However, after the fourth step on the path, further experimentation could not increase the recovery. It obviously appeared that the recovery plateau occurred in the fourth step. These data showed that the results were approaching the neighborhood of optimum recovery. This composition of ATPS was chosen for the subsequent experiments.
Table 4.
Experimental design and results of path of steepest ascent
| No. | X1 (%) | X2 (%) | Y (%) |
| 10 | 20.8 | 14.2 | 77.6 |
| 11 | 21.6 | 13.4 | 80.9 |
| 12 | 22.4 | 12.6 | 82.3 |
| 13 | 23.2 | 11.8 | 85.7 |
| 14 | 24.0 | 11.0 | 81.8 |
| 15 | 24.8 | 10.2 | 78.0 |
X 1: Concentration of PEG 2 000, % (w/w); X 2: Concentration of KH2PO4-K2HPO4, % (w/w); Y: Recovery of elastase (%)
Central composite design and response surface analysis for optimization of PEG and KH2PO4-K2HPO4 concentration
A response surface design was appropriate when the optimal region for running the course has been identified. Further optimization of elastase recovery was carried out by using a Box-Wilson central composite design with four star points and five replicates at center point for each of two factors (Box and Wilson, 1951). Table 5 presents the design of this experiment and the results. Regression analysis was conducted to fit the response function with the experimental data. In order to check the statistical significance of the second-order model equation, F-test (ANOVA) was done and data shown in Table 6. The value of R 2 of the polynomial model (R 2=0.9455) showed that 95% of the variability in the response could be explained by the model (Eq.(4)). So the Eq.(4) was a suitable model of elastase in ATPS as measured by the recovery. The highest elastase recovery possible was determined by the confirmation of the maximum of the model. Also, Eq.(4) showed that the signs of b 11, b 12 and b 22 were all negative and that the parabolas open downward and have a maximum point. The ANOVA results proved that this model was appropriate. Additional, ANOVA analysis suggested that the recovery of elastase was primarily determined by the quadratic terms PEG 2 000 and KH2PO4-K2HPO4 of the model, and also indicated that no significant interaction existed between the two factors.
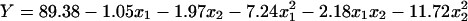 |
(4) |
Table 5.
Experimental design and results of the central composite design (CCD) for the recovery of elastase (Y)
| No. | Coded level |
Real values |
Y (%) | ||
| x1 | x2 | X1 | X2 | ||
| 16 | −1 | −1 | 21.2 | 9.8 | 71.2 |
| 17 | 1 | −1 | 25.2 | 9.8 | 76.8 |
| 18 | −1 | 1 | 21.2 | 13.8 | 72.9 |
| 19 | 1 | 1 | 25.2 | 13.8 | 69.8 |
| 20 | −1.41421 | 0 | 20.4 | 11.8 | 76.5 |
| 21 | 1.41421 | 0 | 26.0 | 11.8 | 68.8 |
| 22 | 0 | −1.41421 | 23.2 | 9.0 | 67.4 |
| 23 | 0 | 1.41421 | 23.2 | 14.6 | 60.0 |
| 24 | 0 | 0 | 23.2 | 11.8 | 89.8 |
| 25 | 0 | 0 | 23.2 | 11.8 | 87.6 |
| 26 | 0 | 0 | 23.2 | 11.8 | 90.6 |
| 27 | 0 | 0 | 23.2 | 11.8 | 88.9 |
| 28 | 0 | 0 | 23.2 | 11.8 | 90.0 |
X 1: Concentration % (w/w) of PEG 2 000; X 2: Concentration % (w/w) of KH2PO4-K2HPO4; Y: Recovery of elastase (%); x 1=(X 1−23.2)/2.0; x 2=(X 2−11.8)/2.0. The experiments were conducted in triplicate, with Y being the average value
Table 6.
ANOVA results for elastase recovery (Y) obtained by CCD
| Regression | DF | Type I sum of squares | R-square | F-ratio | Prob>F |
| Linear | 2 | 39.9 | 0.0303 | 1.95 | 0.2128 |
| Quadratic | 2 | 1185.6 | 0.9009 | 57.88 | 0.0000 |
| Cross product | 1 | 18.9 | 0.0144 | 1.85 | 0.2162 |
| Total regress | 5 | 1244.4 | 0.9455 | 24.30 | 0.0003 |
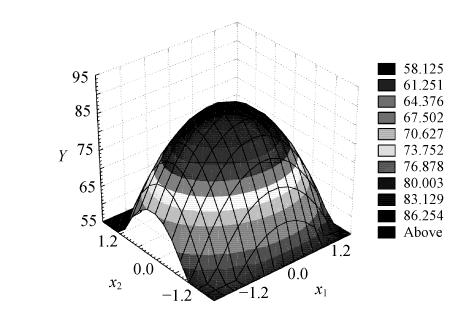
The three-dimensional graph obtained from the calculated response surface is shown in Fig.4. The three-dimensional response surface profile of PEG 2-000 and KH2PO4-K2HPO4 concentrations against recovery of elastase can further explain clearly the results of the statistical and mathematical analyses. It was evident from the plot that recovery of elastase reached its maximum at a combination of coded level −0.043 (x 1) and −0.055 (x 2). This reconfirms that the fitted surface has a maximum point that was 23.1% for PEG 2 000 and 11.7% for KH2PO4-K2HPO4. The model predicted a maximum response of 89.5% for this peak. In order to validate these results, experimental validations were done using a system involving this maximum point, and an average recovery value of 89.9% (N=4) was obtained. The good correlation between these two results confirmed the validity of the response model and the model was proven to be adequate. However, purification factor (average value 1.7) still needs to be improved. The corresponding experimentations are being researched further.
Fig. 4.
Response surface plot of the effect of PEG concentration (x 1) and KH2PO4-K2HPO4 concentration (x 2) on elastase recovery
CONCLUSION
A simple process for extraction and purification of elastase from Bacillus sp. EL31410 fermentation culture was developed. Extraction of elastase in PEG/salt ATPS with high recovery was successfully proved to be a rapid and convenient method that integrated elastase extraction with cell removal. This procedure of extracting elastase using PEG/salt ATPS is an economic approach to large-scale elastase recovery. Statistical experimental designs proved to be useful tools in optimizing elastase extraction in PEG/salt ATPS. The system compositions, PEG 2 000 and KH2PO4-K2HPO4, strongly affected elastase recovery. The optimal ATPS for elastase extraction from the culture was 23.1% (w/w) PEG 2 000 and 11.7% (w/w) KH2PO4-K2HPO4 at pH 7.5. In addition to establishing optimal extraction parameters for process operation, the present methodology also makes it possible to predict the elastase recovery when the system is disturbed in some way.
Footnotes
Project (No. 20276064) supported by the National Natural Science Foundation of China
References
- 1.Balasubramaniam D, Wilkinson C, Cott KV, Zhang CM. Tobacco protein separation by aqueous two-phase extraction. J Chromatogr A. 2003;989:119–129. doi: 10.1016/S0021-9673(02)01900-3. [DOI] [PubMed] [Google Scholar]
- 2.Baskir NJ, Hatton TA, Suter UW. Protein partitioning in two-phase aqueous polymer systems. Biotechnol Bioeng. 1989;34:541–558. doi: 10.1002/bit.260340414. [DOI] [PubMed] [Google Scholar]
- 3.Bim MA, Franco TT. Extraction in aqueous two-phase systems of alkaline xylanase produced by Bacillus pumilus and its application in kraft pulp bleaching. J Chromatogr B. 2000;743:349–356. doi: 10.1016/s0378-4347(00)00223-1. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Box GEP, Wilson KB. On the experimental attainment of optimum conditions. J Roy Stat Soc B. 1951;13:145. [Google Scholar]
- 6.Box GEP, Hunter WG. Statistics for Experimenters. NY: John Wiley and Sons; 1978. [Google Scholar]
- 7.Cascone O, Andrews BA, Asenjo JA. Partitioning and purification of thaumatin in aqueous two-phase systems. Enzyme Microb Technol. 1991;13:629–635. doi: 10.1016/0141-0229(91)90076-M. [DOI] [Google Scholar]
- 8.Chen QH, He GQ, Mokhtar AMA. Optimization of medium composition for the production of elastase by Bacillus sp. EL31410 with response surface methodology. Enzyme Microb Technol. 2002;30:667–672. doi: 10.1016/S0141-0229(02)00028-5. [DOI] [Google Scholar]
- 9.Diamond AD, Hsu JT. Aqueous two-phase systems for biomolecule separation. Adv Biochem Eng Biotechnol. 1992;47:89–135. doi: 10.1007/BFb0046198. [DOI] [PubMed] [Google Scholar]
- 10.Guan YX, Mei LH, Zhu ZQ. Recovery of antibiotics by aqueous two-phase partition-partitioning behavior of pure acetylspiramycin solution in polyethylene glycol/potassium phosphate aqueous two-phase systems. Biotechnol Techniques. 1994;8:491–496. [Google Scholar]
- 11.He GQ, Xu Y, Chen QH, Ruan H, Li JJ. Effect of temperature on batch elastase production by Bacillus sp. EL31410. J Zhejiang Univ Sci. 2004;5:1583–1589. doi: 10.1631/jzus.2004.1583. [DOI] [PubMed] [Google Scholar]
- 12.Héctor M, Fernóndez L, María VM, Elda R, Fraile MJ, Biscoglio JB, Osvaldo C. Partition behavior and purification of a Mucor Bacilliformis acid protease in aqueous two-phase systems. Process Biochem. 1995;30:615–621. doi: 10.1016/0032-9592(95)95727-Z. [DOI] [Google Scholar]
- 13.Isabel del-Val M, Cristina O. Biphasic aqueous media containing polyethylene glycol for the enzymatic synthesis of oligosaccharides from lactose. Enzyme Microb Technol. 2003;33:118–126. doi: 10.1016/S0141-0229(03)00098-X. [DOI] [Google Scholar]
- 14.Kimura K. Simultaneous accumulation of low-molecular-mass RNA at the interface along with accumulation of high-molecular-mass RNA on aqueous two-phase system partitioning. J Chromatogr B. 2000;743:421–429. doi: 10.1016/s0378-4347(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 15.Li QM, He BL. Study on the partition of aminoacylase from Aspergillus oryzae in aqueous polyethylene glycol-salt two-phase system. Ion Exchange Absorp. 1992;8:417–423. [Google Scholar]
- 16.Li C, Bai JH, Li W, Cai ZL, Ouyang F. Optimization of conditions for bacteriocin extraction in PEG/salt aqueous two-phase systems using statistical experimental designs. Biotechnol Progr. 2001;17:366–368. doi: 10.1021/bp000167w. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Zhu ZQ, Rodrigues AE. Process integration of separation of amino acids by a temperature-induced aqueous two-phase system. Industrial Eng Chem Res. 2002;41:251–256. doi: 10.1021/ie010060t. [DOI] [Google Scholar]
- 18.Marco RP, Alejandro N, Enrique G, Leobardo SC. Aroma compounds recovery from mycelial cultures in aqueous two-phase processes. J Chromatogr B. 2000;743:403–408. doi: 10.1016/s0378-4347(00)00073-6. [DOI] [PubMed] [Google Scholar]
- 19.Marcos JC, Fonseca LP, Ramalho MT, Cabral JMS. Application of surface response analysis to the optimization of penicillin acylase purification in aqueous two-phase systems. Enzyme Microb Technol. 2002;31:1006–1013. doi: 10.1016/S0141-0229(02)00230-2. [DOI] [Google Scholar]
- 20.Michelle MZ, Chad AZ, Shana RP, Lynn R. Expression and partial characterization of an elastase from Chromobacterium violaceum . Vet Microb. 2001;80:63–74. doi: 10.1016/S0378-1135(00)00370-9. [DOI] [PubMed] [Google Scholar]
- 21.Morihara K. Elastolytic properties of various proteases from microbial origin. Arch Biochem Biophys. 1967;120:68–78. doi: 10.1016/0003-9861(67)90599-1. [DOI] [PubMed] [Google Scholar]
- 22.Ohlsson R, Hentschel CC, Williams JG. A rapid method for the isolation of circular DNA using an aqueous two-phase partition system. Nucleic Acids Res. 1978;5:583–590. doi: 10.1093/nar/5.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan IH, Yao HJ, Li YK. Effective extraction and purification of β-xylosidase from Trichoderma koningii fermentation culture by aqueous two-phase partitioning. Enzyme Microb Technol. 2001;28:196–201. doi: 10.1016/S0141-0229(00)00291-X. [DOI] [PubMed] [Google Scholar]
- 24.Planas J, Varelas V, Tjerneld F, Hahn-Hägerdal B. Amine-based aqueous polymers for the simultaneous titration and extraction of lactic acid in aqueous two-phase systems. J Chromatogr B. 1998;711:265–275. doi: 10.1016/s0378-4347(97)00663-4. [DOI] [PubMed] [Google Scholar]
- 25.Sachar LA. Photometry method for estimation of elastase activity. Proc Soc Expeti Biol Med. 1955;90:323–325. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- 26.Shibata Y, Fujimura S, Nakamura T. Purification and partial characterization of an elastolytic serine protease of Prevotella intermedia. . Appl Environm Microbial. 1993;59:2107–2111. doi: 10.1128/aem.59.7.2107-2111.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silgia AC, Adslberto PJ, Inês CR. Partitioning of xylanolitic complex from Penicillium janthinellum by an aqueous two-phase systems. J Chromatogr B. 2000;743:339–348. doi: 10.1016/s0378-4347(00)00212-7. [DOI] [PubMed] [Google Scholar]
- 28.Tanuja S, Srinivas ND, Raghava KR, Gowthaman MK. Aqueous two-phase extraction for downstream processing of amyloglucosidase. Process Biochem. 1997;32:635–641. doi: 10.1016/S0032-9592(97)00009-5. [DOI] [Google Scholar]
- 29.Tsai YC, Jung RY, Lin SF. Production and further characterization of an alkaline elastase production by alkalophilic Bacillus strain YaB. Appl Environm Microbial. 1988;54:3156–3161. doi: 10.1128/aem.54.12.3156-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan XK. Bioseperation Engineering. Beijing: Chemistry Industry Press; 2001. pp. 169–187. (in Chinese) [Google Scholar]
- 31.Yang WY, Lin CD, Chu IM, Lee CJ. Extraction of Cephalosporin C from whole broth and separation of desacetyl Cephalosporin C by aqueous two-phase partition. Biotechnol Bioeng. 1994;35:439–445. doi: 10.1002/bit.260430602. [DOI] [PubMed] [Google Scholar]