Abstract
Objectives: To investigate the intestinal microflora status related to ischemia/reperfusion (I/R) liver injury and explore the possible mechanism. Methods: Specific pathogen free grade Sprague-Dawley rats were randomized into three groups: Control group (n=8), sham group (n=6) and I/R group (n=10). Rats in the control group did not receive any treatment, rats in the I/R group were subjected to 20 min of liver ischemia, and rats in the sham group were only subjected to sham operation. Twenty-two hours later, the rats were sacrificed and liver enzymes and malondialdehyde (MDA), superoxide dismutase (SOD), serum endotoxin, intestinal bacterial counts, intestinal mucosal histology, bacterial translocation to mesenteric lymph nodes, liver, spleen, and kidney were studied. Results: Ischemia/reperfusion increased liver enzymes, MDA, decreased SOD, and was associated with plasma endotoxin elevation in the I/R group campared to those in the sham group. Intestinal Bifidobacteria and Lactobacilli decreased and intestinal Enterobacterium and Enterococcus, bacterial translocation to kidney increased in the I/R group compared to the sham group. Intestinal microvilli were lost, disrupted and the interspace between cells became wider in the I/R group. Conclusion: I/R liver injury may lead to disturbance of intestinal microflora and impairment of intestinal mucosal barrier function, which contributes to endotoxemia and bacterial translocation to kidney.
Keywords: Ischemia/reperfusion (I/R), Liver injury, Microflora, Endotoxin, Bacterial translocation
INTRODUCTION
The gastrointestinal tract of an animal is colonized by complex and diverse microbial populations, and is a major reservoir of bacteria. Under normal conditions, a series of local and systemic protective mechanisms prevent passage of these potentially pathogenic bacteria beyond the intestinal lumen. The most important of these mechanisms are intestinal colonization resistance, intestinal mucosal barrier, and body reticuloendothelial system function (Wells et al., 1987). Previous studies showed that these mechanisms are severely impaired in some serious diseases, such as acute liver injury, cirrhosis, severe scalding injury and hemorrhagic shock, which could lead to excessive growth of gram negative aerobic bacteria, bacteria and endotoxin translocation, and aggravated liver injury (Li et al., 2004; Chiva et al., 2002; Kasravi et al., 1997; Wang et al., 2002). But data on the relationship between intestinal microflora status and ischemia/reperfusion (I/R) liver injury are not available. I/R liver injury is an inevitable clinical issue (Cutrín et al., 2002). Although there is a considerable study on it, the precise mechanisms leading to the damage have not been completely elucidated. From the theory of microecology, we postulated that the disturbance of intestinal microflora might occur in I/R liver injury, which might contribute to some severe clinical conditions or enhance liver injury. If the hypothesis were true, a new way, modulation of the intestinal microflora, to prevent or treat I/R liver injury would be possible. So, in the present study, we investigated the relationship between intestinal microflora status and I/R liver injury and explored the possible mechanism.
MATERIALS AND METHODS
Experimental protocol
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Review Board according to the Animal Protection Act of China. Adult male Sprague-Dawley (SD) rats (National Rodent Laboratory Animal Resources, Shanghai Branch, China) in Specific pathogen-free (SPF) grade, weighing 190–210 g were used. They had free access to standard laboratory chow and water and were exposed to a cycle of 12 h of light/darkness. According to the reference (Colak et al., 2003), 6 rats would be needed in each group to detect an expected bacterial translocation (BT) rate with an α error of 0.05 and a β error of 0.2. However, considering the possibility of death during the experiment, 28 rats were randomized into three groups with 9, 9, 10 rats in the control group, sham group, and I/R group respectively. The control group was not subjected to any surgical procedure or liver manipulation; the sham group was subjected to the surgical procedures described below without liver manipulation and maintained under anesthesia for the 20 min duration of the experiment; and the I/R group was subjected to the surgical procedures described below and underwent liver I/R.
The animals were fasted 12 h prior to the operation. Under sterile conditions, the rats were anesthetized with ketamine hydrochloride (No.1 Biochemical & Pharmaceutical Co., Shanghai, China) 50 mg/kg i.m. and inhaled ether. After shaving and skin disinfection with iodine tincture, a laparotomy was performed by a midline incision. After the organ was carefully isolated, the liver hilus was exposed. Hepatic pedicle including hepatic artery and portal vein was clamped with a vascular microclamp. Occlusion was verified visually by the change in liver color to a paler shade, and change in intestine and mesentery color to a purple shade. Twenty minutes later, liver reperfusion was established by removal of the clamp. During the procedure, the abdominal cavity was covered with gauze soaked in prewarmed physiological saline at 37 °C to minimize dehydration. The laparotomy incision was then closed in two layers with suture. Upon awakening from anesthesia, the rats had free access to sterilized water and standard rodent chow.
Twenty-two hours after the reperfusion, all animals underwent relaparotomy through the previous incision. The abdominal aorta was punctured and blood samples were collected for measurement of plasma endotoxin levels, liver enzymes and serum total bilirubin (TBil). The mesenteric lymph nodes (MLN) from the ileo-cecal area, tissue samples from the left lobe of the liver, spleen, kidney, and ileal contents were taken for microbiological study. Additional liver tissue samples from the left lobe were collected, washed with cold physiologic saline solution and immediately frozen in −80 °C refrigerator until malondialdehyde (MDA) levels and superoxide dismutase (SOD) determination. Ileal mucosal samples were biopsied from the places about 2 cm to the ileocecal valve, fixed in 2.5% glutaraldehyde for later histological study by electronic-microscopy.
Liver function and plasma endotoxin tests
The blood sample was centrifuged at 3000 g for 10 min at room temperature to separate serum for analysis. The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and TBil were measured with a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
The blood sample collected in the pyrogen-free heparin-containing tube was centrifuged at 3000 g for 15 min at 4 °C. The plasma endotoxin levels were determined using a quantitative, chromogenic Limulus Amebocyte Lysate (LAL) assay following the instructions of the supplier (Eihua Medical Co., Shanghai, China). The detection limit of endotoxin for the assay was 0.20 EU. Endotoxemia was expressed as EU/ml.
Lipid peroxide assay
The frozen liver tissues (−80 °C) were thawed and homogenized in appropriate cold physiologic saline (1:10) for each analysis by ultrasonication (Sonics, Vibra-Cell, Sonics and Materials Inc., USA) in iced bath. The homogenates were then centrifuged at 3000 g for 10 min at 4 °C and the supernatant was collected for use in the following assays; the protein in homogenates was detected with Coomassie BBG250 (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Liver malondialdehyde (MDA) levels, which are indications of hepatic lipid peroxidation damage, were determined by thiobarbituric acid reaction. The final results were expressed in nmol per milligram of protein (nmol/mg protein). Liver SOD activity, which reflects anti-oxidation ability of tissues, was measured by hydroxylamine assay-developed from xanthine oxidase assay. The final results were expressed in U per milligram of protein (U/mg protein).
Microbiologic analysis
Intestinal microflora was analyzed using seven selected media according to the method of Mitsuoka et al., with slight modification (Li and Qiu, 1998). Samples from distal small intestines contents (ileal feces) were collected in sterile tubes, weighed, transferred into other sterile tubes containing appropriate anaerobic buffer (phosphate buffered saline with 0.5 g cysteine, HCl, 0.5 ml tween 80, and 0.5 g agar per liter) to make sure that the samples were diluted 10-fold. They were then serially diluted in 10 ml of the same medium seven times. Within 30 min of the samples collection, bacterial cultures were done by placing 50 μl of the dilutions on 7 selective agar media respectively: Neomycin sulfate-brilliant green-taurocholate-blood (NBGT) (Li and Qiu, 1998) agar prepared with EG agar (Nissui Pharmcy Co., Japan) according to the instructions for Bacteriodaceae; MRS with vancomycin and bromocresol green (LAMVAB) medium (Hartemink et al., 1997) for Lactobacillus spp.; Bifidobacterium selective agar (BS, prepared according to the instructions) (Li and Qiu, 1998) for Bifidobacterium spp.; TSN (bioMérieux, France) for Clostridium perfringens (C. perfringens); Eosin methylene blue agar (EMB, Hangzhou microbiological Co., China) for Enterobacteriaceae; Enterococcus agar (EC, prepared according to the instructions) (Li and Qiu, 1998) for Enterococcus; and Sabouraud agar media (bioMérieux, France) for Yeasts. The plates for anaerobical bacteria were incubated anaerobically in Anaerobic system (Forma Scientific, USA) 48 h at 37 °C, and plates for aerobic bacteria were incubated aerobically for 48 h at 37 °C. At the mentioned time, colony forming units (CFU) on each plate were counted and calculated to the original weight of the samples. The results expressed as log10CFU/g of ileal feces.
Samples from the liver, spleen, kidney and mesenteric lymph nodes were weighed and placed in sterile glass homogenizer containing 9-fold amount of anaerobic buffer (as described above). They were homogenized and 50 μl of 10% homogenate was placed on Blood agar base (bioMérieux, France) and Anaerobic Blood Agar (CDC) base (bioMérieux, France) within 30 min of the samples collection, incubated for 48 h at 37 °C in anaerobic atmosphere and in aerobic atmosphere respectively. The sterile swabs were passed over the parietal peritoneal surface and then cultured on the same medium as an index of aseptic technique. The number of CFU was counted at the end time of culture and expressed as CFU/g of tissues. BT was defined as the positivity of the tissue cultures, which suggested the existence of translocated bacteria in these organs.
Investigation of intestinal mucosal ultrastructure
Ileal mucosal specimens collected from various groups underwent standard technical procedures for electron microscopy. Briefly, they were fixed in 2.5% glutaraldehyde (4 °C, pH 7.4), postfixed in 1% osmium tetroxide, and embedded in an epon-araldite mixture. Ultrathin sections, cut and placed onto mesh copper grids and stained with uranyl acetate and lead citrate, were observed with a Philips Tecnai 10 electron microscope. Particular attention was paid to the ultrastructure of microvilli, tight junctions and paracellular regions.
Statistical analysis
Values were presented as means±standard deviation (SD) when appropriate. Differences between groups were evaluated by using one-way ANOVA, followed by a least-significant difference (LSD). The incidence of BT was statistically evaluated using non-parametric Kruskal-Wallis and Mann-Whitney U. All analyses were performed using the statistical software SPSS 11.0 (SPSS Inc., USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Three rats in the sham group and one rat in the control group died before operation (after anesthetization). Consequently, 8 rats in the control group, 6 rats in the sham group and 10 rats in the I/R group were assessed for each outcome.
Liver function and plasma endotoxin
To assess the influence of hepatic I/R on liver function, we measured serum ALT, AST, and TBil. Serum ALT levels in the sham group (40.50±9.670 U/L) were similar to those in the control group (34.10±8.741 U/L, P>0.05, Table 1), but increased greatly in the I/R group (122.80±56.983 U/L, P<0.01) compared to the sham group or the control group. AST levels were increased in the sham group (159.83±46.028 U/L) compared to the control group (79.90±18.977 U/L, P<0.05), and increased even more in the I/R group (295.90±216.923 U/L) compared to the sham group (P<0.01). Levels of TBil were not statistically difference between the three groups.
Table 1.
Liver function and plasma endotoxin levels in different experimental groups (mean±SD)
| Group | N | ALT (U/L) | AST (U/L) | TBil (μmol/L) | Endotoxin (EU/ml) |
| Control | 8 | 34.10±8.741 | 79.90±18.977 | 0.63±0.744 | 0.34±0.178 |
| Sham | 6 | 40.50±9.670 | 159.83±46.028* | 0.83±0.753 | 0.52±0.289 |
| I/R | 10 | 122.80±56.938**†† | 295.90±216.923**† | 0.90±0.876 | 0.80±0.262** |
Control: normal rats not subjected to any surgical procedure; Sham: rats subjected to surgical procedures except for hepatic ischemia/reperfusion (I/R); I/R: rats subjected to hepatic I/R surgical operation.
P<0.05 as compared to the control group
P<0.01 as compared to the control group
P<0.05 as compared to the sham group
P<0.01 as compared to the sham group
Plasma endotoxin levels in rats from the sham group (0.52±0.289 EU/ml) were similar to those of the control group (0.34±0.178 EU/ml, Table 1), but significantly increased in rats from the I/R group (0.80±0.262 EU/ml) compared to the control group or the sham group (P<0.01). These suggested that hepatic I/R accompanied by liver injury and the elevation of plasma endotonxin.
MDA levels and SOD activity in liver
To evaluate whether lipid peroxidation reaction participated in liver injury induced by hepatic I/R and the anti-oxidation ability of liver, liver MDA levels and SOD activity were measured. As shown in Table 2, no significant difference of MDA levels in liver was noted between the sham group (0.49±0.217 nmol/mg protein) and the control group (0.48±0.142 nmol/mg protein, P>0.05). MDA levels in the I/R group (0.72±0.124 nmol/mg protein) were much higher than those in the control group (P<0.01) or the sham group (P<0.05). Liver SOD activity in the sham group (314.41±88.525 U/L) was similar to that in the control group (336.99±67.633, P>0.05). The significant low liver SOD activity was observed in rats from the I/R group (240.76±63.674) compared to the control group (P<0.01). These indicated lipid peroxidation reaction participated in the liver injury and that the anti-oxidation ability of liver was impaired in rats subjected to hepatic I/R.
Table 2.
Liver MDA concentrations and SOD activity in the different experimental groups (mean±SD)
| Group | N | Liver SOD (U/mg protein) | Liver MDA (nmol/mg protein) |
| Control | 8 | 336.99±67.633 | 0.48±0.142 |
| Sham | 6 | 314.41±88.525 | 0.49±0.217 |
| I/R | 10 | 240.76±63.674** | 0.72±0.124**†† |
Control: normal rats not subjected to any surgical procedure; Sham: rats subjected to surgical procedures except for hepatic ischemia/reperfusion (I/R); I/R: rats subjected to hepatic I/R surgical operation.
P<0.01 as compared to the control group
P<0.01 as compared to the sham group
Intestinal microflora and BT
In order to determine whether intestinal microflora altered in rats suffering from hepatic I/R, the microbiological study of the ileal content was conducted with the results being summarized in Table 3. No significant difference in the counts of various bacteria in ileal feces was observed between the sham group and the control group. The counts of Enterococci and Enterobacteria were higher (P<0.05) and the counts of Lactobacilli and Bifidobacteria were lower (P<0.01) in the I/R group compared to the control group. There were no differences of the counts of C. perfringens and Yeasts in ileal feces in different groups.
Table 3.
Ileal microflora in the different experimental groups (log10CFU/g, mean±SD)
| Group | N | Bifidobacterium | Lactobacillus | Enterococcus | Enterobacter | Bacteroides |
| Control | 8 | 8.62±0.467 | 9.77±0.396 | 6.66±0.459 | 7.42±1.027 | 7.41±0.557 |
| Sham | 6 | 8.48±0.994 | 9.98±0.540 | 7.16±0.630 | 8.01±1.044 | 7.38±1.174 |
| I/R | 10 | 7.54±0.849*† | 8.54±1.143**†† | 8.04±0.692** | 9.22±0.897**† | 6.84±0.959 |
Control: normal rats not subjected to any surgical procedure; Sham: rats subjected to surgical procedures except for hepatic ischemia/reperfusion (I/R); I/R: rats subjected to hepatic I/R surgical operation; CFU: colony-forming units.
P<0.05 as compared to the control group
P<0.01 as compared to the control group
P<0.05 as compared to the sham group
P<0.01 as compared to the sham group
BT to MLN, liver, spleen and kidney was also tested (results shown in Table 4). The CFU of aerobic and anaerobic bacteria in each instance are given in parentheses. There was no bacterial growth in the visceral swab cultures, which indicates that the visceral surface was not contaminated. The incidence of BT to MLN, liver, spleen and kidney was higher in the I/R group than in the other two groups, and BT to kidney in the I/R group significantly increased (5/10, P<0.05) compared to the other two groups.
Table 4.
Incidence of bacterial translocation in the different experimental groups (average CFU counts/g tissue, aerobic/anaerobic in parentheses)
| Group | Liver | Spleen | Kidney | MLN |
| Control | 1/8 (0/100) | 0/8 | 0/8 | 2/8 (150/75) |
| Sham | 1/6 (33/25) | 1/6 (100/133) | 0/6 | 2/6 (400/166) |
| I/R | 2/10 (1000/240) | 2/10 (220/100) | 5/10 (220/200)*† | 6/10 (2900/2060) |
Control: normal rats not subjected to any surgical procedure; Sham: rats subjected to surgical procedures except for hepatic ischemia/reperfusion (I/R); I/R: rats subjected to hepatic I/R surgical operation; MLN: mesenteric lymph node.
P<0.05 as compared to the control group
P<0.05 as compared to the sham group
These results demonstrated that I/R liver injury led to disturbance of intestinal microflroa and induced BT to extraintestinal sites.
Ultrastructure of intestinal mucosa
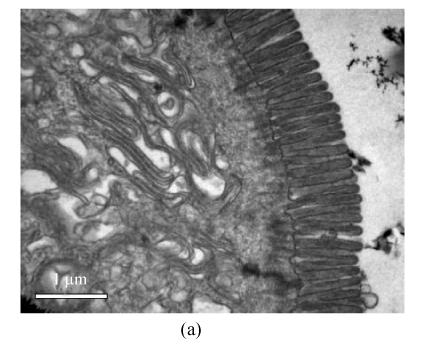
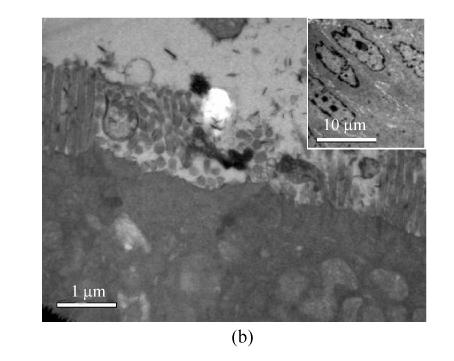
In order to determine intestinal mucosal integrity in rats with I/R liver injury, we observed the ultrastructure of ileal mucosa with electronic microscopy. Under the transmission electron microscope, the intestinal epithelial cells in the sham group appeared well and had many homogenously distributed microvilli. Lateral junction complexes and desmosomes were observed (Fig.1a). The intestinal epithelial cells in the I/R group were damaged. Microvilli disruption and loss, and wider lateral spaces between the neighbouring cells were noted (Fig.1b). These results indicated that I/R liver injury is associated with intestinal mucosal lesion.
Fig. 1.
Transmission electron micrograph of ileal mucosa structure in the two groups
(a) Sham group (×15000, bar=1 μm). The apical junction complexes in epithelial cells with many homogenously distributed microvilli; (b) I/R group (×12500 with bar=1 μm, right upper corner ×1250 with bar=10 μm). Microvilli in epithelial cells were lost and disrupted with large intercellular spaces noticed
DISCUSSION
I/R liver injury is a complex, multifactorial pathophysiologic process. It is generally believed that the process of hepatic I/R includes three different events (Armeni et al., 2000): Hepatic ischemia, portal venous congestion, and subsequent hepatic reperfusion with arterial blood and congested portal blood. Previous study mainly focused on the process of hepatic ischemia and subsequent reperfusion with blood. It has been proved that the action of oxygen derived free radicals (ODFR), the vasoconstriction of the hepatovasculature after warm I/R (Kukan et al., 1996), the activation of Kupffer cells (Caban et al., 2002) and pneutrophils releasing endothelin and some other cytokines, participated in the processes. This study showed that hepatic I/R caused significant increase in the serum levels of ALT and AST, which reflected the degree of liver injury. Moreover, the increased serum ALT and AST were accompanied by elevation of MDA concentration and reduced SOD activity in liver tissues. These results strongly indicated that I/R liver injury was mediated by reactive oxygen species (Serracino-Inglott et al., 2001; Serrano et al., 2000). In this study, AST also increased in the sham group. The increased serum AST in the sham group could have resulted from muscle injury during operation, because AST is a nonspecific marker for hepatic injury, which is also found in many body tissues including the heart, muscle, kidney, brain, and lung. TBil did not change greatly in the three groups. This was partially due to non-occlusion of the choledochus during the surgical operation. It also indicated that the I/R rat model just suffered from slight liver injury.
Portal venous congestion is also an important event in hepatic I/R. Portal venous congestion could result in extensive mesenteric venous congestion, which would greatly slow down the blood flow in the intestinal wall and cause stagnant anoxia in the tissues, intestinal mucosa lesion, and abnormalities in the coordinated motor function of the small bowel that delays intestinal transit and favor intestinal overgrowth of Enterobacteria (Albillos and De la Hera, 2002; Chan et al., 1998). Previous reports revealed that small intestine transplantation or small intestine I/R could cause intestinal microflora changes and intestine mucosal barrier dysfunction associated with BT (Colak et al., 2003; Amarri et al., 2002). In this experiment, we found that the counts of anaerobic bacteria population including Bifidobacteria and Lactobacillium were low, whereas the counts of aerobic bacteria including Enterobacteriaceae and Enterococci were high in the rats subjected to hepatic I/R. Interestingly, such alteration in both anaerobic and aerobic bacterial population was associated with the damage of microvilli in the epithelial apical surface, the disruption of epithelial tight junctions in ileal, the elevation of plasma endotoxin, and a high rate of BT to kidney. Microflora content and mucosal integrity are two important pillars supporting the intestinal barrier (Albillos and De la Hera, 2002). Normal intestinal microflora could prevent the adherence of potential pathogenic bacteria and suppress bacterial overgrowth by occupying the space closest to intestinal epithelial cells (Tuohy et al., 2003). Combined injury to the two intestinal barrier pillars results in greater intestinal permeability and susceptibility to bacterial and endotoxin translocation. Besides, the elevation of Enterobacteriaceae, a major resource of blood endotoxin in severe liver disease, may be another important causative for endotoxemia in I/R liver injury.
As for the BT, it is known that kidney is an organ far from the intestine, compared to the spleen, liver, and MLN anatomically. It may occur that bacteria transfer to adjacent organs such as mesenteric lymph node (MLN), liver and spleen in rats with minor intestinal mucosal lesion or even normal intestinal mucosa (Albillos and De la Hera, 2002). However, BT to a remote organ such as kidney may be associated with severe intestinal mucosal impairment and significant increase in intestinal permeability. Besides, the immune defense, such as the bacteriostatic and opsonic capacity of serum, phagocytosis by neutrophils, and the effector function of immune cells circulating in blood, is also an important pillar that prevents BT to a remote organ. The significantly increased BT to kidney in I/R rats might indicate that the immune defense was defective in them. Verification by further experiments is needed.
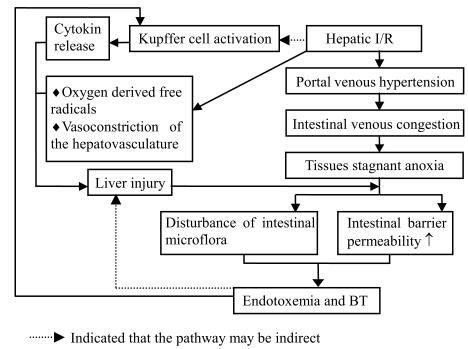
These results strongly suggested that I/R liver injury could alter the intestinal microflora and damage intestinal mucosal barrier function, which lead to the elevation of plasma endotoxin and BT to multiple organs. The potential mechanism is depicted in Fig.2. It had been shown that endotoxin was a crucial medium that could aggravate liver injury via altering hepatic energy metabolism (Ren et al., 2001) and activation of Kupffer cell, which could release vasoactive agents and highly active mediators (Serracino-Inglott et al., 2001; Vajdova et al., 2000). BT might be the source of endogenous infection. So modulating intestinal microbes and improving intestinal mucosal barrier function will be beneficial for inhibiting endotoxemia and BT and preventing I/R liver injury.
Fig. 2.
The potential mechanism of hepatic I/R inducing BT
In conclusion, the present study showed that I/R liver injury could result in disturbance of intestinal microflora and impairment of the function of intestinal mucosal barrier, which could contribute to endotoxemia and BT.
Acknowledgments
We wish to thank Lei Shui-ying for linguistic assistance with the manuscript and Dr. Xu Xiao for critically revising the manuscript. We also thank Dr. Chen Yu, Dr. Wang Jian-guo, Dr. Chen Chun-lei, Yan Dong, Fu Su-zhen, and Dai Fang-wei, Dr. Du Wei-bo, Cao Hong-cui for their help in the experiment.
Footnotes
Project supported by the National Basic Research Program (973) of China (No. 2003CB515506), Postdoctoral Fund of China (No. 20040350233) and Research Grant awarded by the First Affiliated Hospital, School of Medicine, Zhejiang University, China
References
- 1.Albillos A, De la Hera A. Multifactorial gut barrier failure in cirrhosis and bacterial translocation: working out the role of probiotics and antioxidants. J Hepatol. 2002;37:523–526. doi: 10.1016/s0168-8278(02)00265-9. [DOI] [PubMed] [Google Scholar]
- 2.Amarri S, Masetti M, Benatti F, Callegari ML, Morelli L, Villa E, Balli F, Jovine E, Pinna AD. Small intestine microflora after intestinal/multivisceral transplantation: preliminary results. Transplantat Proc. 2002;34:953–954. doi: 10.1016/s0041-1345(02)02713-6. [DOI] [PubMed] [Google Scholar]
- 3.Armeni T, Ghiselli R, Balercia G, Goffi L, Jassem W, Saba V, Principato G. Glutathione and ultrastructural changes in inflow occlusion of rat liver. J Surg Research. 2000;88:207–221. doi: 10.1006/jsre.1999.5781. [DOI] [PubMed] [Google Scholar]
- 4.Caban A, Oczkowicz G, Abdel-Samad O, Cierpka L. Influence of Kupffer cells on the early phase of liver reperfusion. Transplant Proc. 2002;34(2):694–697. doi: 10.1016/s0041-1345(01)02891-3. [DOI] [PubMed] [Google Scholar]
- 5.Chan CS, Chen GH, Lien HC, Yeh HZ. Small intestine dismotility and bacterial overgrowth in cirrhotic patients with spontane bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 6.Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, Mirelis B, Schiffrin EJ, Guarner C, Balanzo J. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol. 2002;37:456–462. doi: 10.1016/s0168-8278(02)00142-3. [DOI] [PubMed] [Google Scholar]
- 7.Colak T, Ozturk C, Polat A, Bagdatoglu O, Kanik A, Turkmenoglu O, Aydin S. Effects of trapidil on intestinal mucosal barrier function and bacterial translocation after intestinal ischemia and reperfusion in an experimental rat model. Current Therapeutic Research. 2003;64(6):355–366. doi: 10.1016/S0011-393X(03)00091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutrín JC, Perrelli MG, Cavalieri B, Peralta C, Catafau JR, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radical Biology & Medicine. 2002;33(9):1200–1208. doi: 10.1016/s0891-5849(02)01017-1. [DOI] [PubMed] [Google Scholar]
- 9.Hartemink R, Domenech VR, Rombouts FML. AMVAB–a new selective medium for the isolation of laxtobacilli from faeces. J Microbio Methods. 1997;29:77–84. [Google Scholar]
- 10.Kasravi FB, Adawi D, Molin G, Bengmark S, Jeppsson B. Effect of oral supplementation of lactobacilli on bacterial translocation in acute liver injury induced by d-galactosamine. J Hepatol. 1997;26:417–424. doi: 10.1016/s0168-8278(97)80060-8. [DOI] [PubMed] [Google Scholar]
- 11.Kukan M, Bezek S, Trnovec T. Role of hepatovasculature in warm ischaemia-reperfusion injury of rat liver. Physiol Res. 1996;45(5):427–430. [PubMed] [Google Scholar]
- 12.Li XT, Qiu H. Lecture on Mitsuoka’s method for analysing intestinal microorganism and metabolite. Chin J Microecology. 1998;10:61–63. (in Chinese) [Google Scholar]
- 13.Li LJ, Wu ZW, Xiao DS, Sheng JF. Changes of gut flora and endotoxin in rats with D-galactosamine-induced acute liver failure. World J Gastroenterol. 2004;10(14):2087–2090. doi: 10.3748/wjg.v10.i14.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren D, Han D, Zhao Y. Effect of intestinal endotoxemia on hepatic energy metabolism in acute liver failure. Chin J Pathophy. 2001;17(9):890–892. (in Chinese) [Google Scholar]
- 15.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 16.Serrano E, Diaz J, Acosta F, Palenciano CG, Parrilla P, Carbonell LF. Oxidative stress during ischemia-reperfusion in liver transplantation. Transplant Proc. 2000;32:2651–2651. doi: 10.1016/s0041-1345(00)01824-8. [DOI] [PubMed] [Google Scholar]
- 17.Tuohy KM, Probert HM, Smejkal CW, Gibson GR. Using probiotics and prebiotics to improve gut health. DDT. 2003;8(15):692–700. doi: 10.1016/s1359-6446(03)02746-6. [DOI] [PubMed] [Google Scholar]
- 18.Vajdova K, Smrekova R, Kukan M, Jakubovsky J, Roojen N, Horecky J, Lutterova M, Wsolova L. Endotoxin-induced aggravation of preservation-reperfusion injury of rat liver and its modulation. J Hepatol. 2000;32(1):112–120. doi: 10.1016/s0168-8278(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Xiao G, Yao Y, Wang H, Sheng Z, Cai B, Xiao J. The relationship between intestinal bifidobacteria and bacteria/endotoxin translocation in scalded rats. Chin J Burns. 2002;18(6):365–368. (in Chinese) [PubMed] [Google Scholar]
- 20.Wells CL, Maddaus MA, Reynolds CM, Jechorek RP, Simmons RL. Role of anaerobic flora in the translocation of aerobic and facultatively anaerobic intestinal bacteria. Infect Immun. 1987;55:2689–2694. doi: 10.1128/iai.55.11.2689-2694.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]