Abstract
Objective: To investigate the role of 99mTc-TRODAT-1 SPECT in diagnosis and assessing severity of idiopathic Parkinson’s disease (PD). Methods: Thirty-eight patients with primary, tentative diagnosis of PD and eighteen age-matched normal controls were studied with 99mTc-TRODAT-1 SPECT imaging. The regions of interests (ROIs) were drawn manually on cerebellum (CB), occipital cortex (OC) and three transverse plane slice-views of striatums, the semiquantitative BG (background)/[(OC+CB)/2] were then calculated. Results: A lower uptake of 99mTc-TRODAT-1 in striatums were displayed in thirty-six out of thirty-eight PD patients by visual inspection, compared to controls. In twenty-four PD cases with HYS (Hoehn and Yahr scale) stage I, a greater loss of DAT uptake was found in striatum and its subregions contralateral striatum to the affected limbs than in the same regions of the controls, although the striatal uptake was bilaterally reduced. Using Spearman correlation analysis showed that the reduction of the uptake ratios significantly correlated with the UPDRS in striatum and all its subregions in the PD group (P<0.05), a similar change was also found in the putamen by using the rating scale of Hoehn and Yahr (P<0.05). However, analysis of variance (ANOVA) did not show any relationship between the decreasing uptake of 99mTc-TRODAT-1 and increasing severity of PD patients, although the specific uptake of 99mTc-TRODAT-1 was continuously decreased in the striatum by visual inspection with the progress of PD from HYS stage I to III. Conclusion: 99mTc-TRODAT-1 SPECT imaging may serve as a useful method for improving the correct diagnosis of PD. In assessing the role of 99mTc-TRODAT-1 SPECT in disease severity of PD, UPDRS can offer a comprehensive index, although the Hoehn and Yahr assessment may be available in part.
Keywords: 99mTc-TRODAT-1, Single photon emission computed tomography (SPECT), Parkinson’s disease (PD)
INTRODUCTION
Parkinson’s disease (PD) is a progressive disorder characterized by selective degeneration of dopaminergic neurons in the substantia nigra and by a long delitescent process preceding the development of clinical symptoms such as rigidity, tremor and bradykinesia. At present, the diagnosis of PD still depends mainly on clinical criteria, the insidious onset and multifarious presentations often interfere with the accuracy of PD diagnosis, therefore, a considerable misdiagnosis in PD patients is inevitable (Gel et al., 1999; Hughes et al., 1992). In clinicopathological studies, approximately twenty-five percent of cases with clinical tentative diagnosis of idiopathic PD were not found to have characteristic changes of Lewy bodie but suffer from other diseases at postmortem examination, which include progressive supranuclear palsy (PSP), multiple system atrophy (MSA), essential tremor, vascular parkinsonism and so on (Rajput et al., 1991; Hughes et al., 1992). In fact, discrimination of these diseases is very important as each requires distinct management and has a different prognosis. However, the differential diagnosis of these disorders based on clinical symptoms alone is difficult to a certain extent, a new reliable imaging procedure that may improve the accuracy in diagnosing PD is therefore needed.
Dopamine transporters (DAT), located in the presynaptic membrane only on the terminal of the dopaminergic fibers, play a critical role in mediating the reuptake of free dopamine from the intrasynaptic cleft (Reith et al., 1997). Degeneration of the projection from the substantia nigra to the striatum results in loss of DAT (Kaufman and Madras, 1991). Several studies showed good relationship between DAT concentrations and striatum dopamine level, and the regional concentration of DAT may present the tone of dopaminergic system at that area and is thought to be a marker of dopamine terminal innervations (Mozley et al., 2000; Kung et al., 1996). In recent years, DAT imaging with positron emission tomography (PET) and single photon emission computed tomography (SPECT) has developed into an objective in vivo method to evaluate nigrostriatal neuron loss in PD (Booij et al., 1999; Kung et al., 1996; Asenbaum et al., 1997). Several results displayed a correlation between overall striatal DAT binding and global measures of the severity of PD such as the Hoehn and Yahr scale (HYS) (Hoehn and Yahr, 1967), the total score on the Unified Parkinson’s Disease Rating Scale (UPDRS). However, a number of studies found a significant inverse correlation of striatal DAT binding with UPDRS motor score.
Herein, we present our experience with thirty-eight cases suffering from primary, tentative idiopathic PD. The feasibility of using 99mTc-TRODAT-1-SPECT was tested for evaluating both the early-stage diagnosis and the relationship between the extent of DAT decline in basal ganglia and the disease severity of PD by using UPDRS and Hoehn and Yahr assessment, respectively.
MATERIALS AND METHODS
Subjects
The patient was no fasting. Consecutive thirty-eight cases with various severities of idiopathic PD were studied. Clinical PD was diagnosed by two neurologists according to generally accepted criteria. The criteria include at least two of the following symptoms: resting tremor, akinesia, and rigidity, with a favorable response to L-dopa therapy. The severities of PD were assessed using the HYS and the UPDRS. Twenty-four patients were in HYS stage I (13 males, 11 females, age range, 31–72 y; mean age±SD, 57.13±10.71 y), nine patients were in HYS stage II (5 males, 4 females, age range, 37–74 y; mean age±SD, 57.0±12.65 y), and five of them were in HYS stage III (2 males, 3 females, age range, 55–72 y; mean age±SD, 65.4±6.8 y) as well. Eighteen age-matched normal volunteers (10 males, 8 females, age range, 34–72 y; mean age±SD, 49.2±11.4 y) served as controls. All subjects had a low-protein diet and anti-parkinsonian medication (L-dopa) was discontinued for at least 24 h before the specific SPECT examinations were finished.
Radiopharmaceuticals
Preparation of 99mTc-TRODAT-1 was done by adding 1.11 GBq/5 ml of 99mTc-pertechnetate to an ‘all in one’ lyophilized kit from the Institute of Syncor Corporation containing free ligand, stannous chloride dihydrate, sodium glucoheptonate, sodium EDTA dihydrate, mannitol and sodium phosphate. The mixture was then autoclaved at 100 °C for 30 min. Once the kit solution had cooled down at room temperature, it could be used for the higher than 95% radiochemical purity injection. In all patients, 925 MBq (25 mCi) of 99mTc-TRODAT-1 in a 2-ml volume was injected through an indwelling needle into an antecubital vein soon after its preparation.
Image acquisition and processing
Imaging was performed 3~4 h after intravenous injection (Kao et al., 2001). The patients were placed supine, and the position of their heads was fixed with a specially designed gadget and pillows were placed under the knees, arms at body side. The head was strapped at the level of forehead and chin.
The SPECT imaging was performed with a GE Millennium VG/Discovery VH SPECT, double-head gamma cameras equipped with ultrahigh resolution fan-beam collimators and a 128×128 matrix size. The energy window was set at 140 keV with a 20% symmetric window. First, data were acquired for 50 s per projection and 180 projection angles per detector over 360°, and the SPECT images were then reconstructed using filtered back-projection with Butterworth filter with cut-off frequency of 0.5 per cm and an order of 10, and corrected for attenuation using Chang’s method. No attempt was made to correct for partial volume effects. The slice thickness and in-plane pixel size was 2.95 mm. If motion is detected the acquisition is repeated without new injection of TRODAT-1.
Image analysis
The SPECT images were shown in a 64-slice transaxial display. Three reconstructed transaxial slices were summed together, containing the image with the highest signal in the region of the basal ganglia as the central slice. The summed image was used to determine the 99mTc-TRODAT-1 activity in the caudate, putamen and occipital regions of interests (ROIs). The cerebellum and occipital area were used as non-specific sites devoid of DATs. Six ROIs were positioned on the summed slice: the left and right caudate, the left and right putamen, cerebellum (CB) and the occipital cortex (OC). All of the ROIs were drawn manually. The specific to non-specific 99mTc-TRODAT-1 binding ratios were calculated as the counts/pixel in the caudate nucleus/occipital (CN/[(CB+OC)/2]) and putamen/occipital (PT/[(CB+OC)/2]) areas.
The evaluation of TRODAT-1 SPECT studies was done visually and in a semi-quantitative manner. The images used were the transaxial cuts. Interpretation was done by comparison of DAT concentration in the striatum, caudate and putamen versus nonspecific uptake of TRODAT-1 in cerebellum and occipital cortex by consensus of two observers.
Statistical analysis
Student t test for unpaired samples was carried out for within-group comparison of the results between ipsilateral and contralateral sides and between those of PD patients and controls. Wilcoxon matched pairs test was performed for comparison of caudate nucleus and putamen between ipsilateral and contralateral sides of PD cases with different HYS stages and normal volunteers. Multiple ANOVA for multigroup from HYS stage I to III was done to investigate the role of TRODAT-1 SPECT imaging in evaluating the severity of idiopathic PD. The P-level of significance was set to 0.05 for low significant correlation or to 0.01 for highly significant correlation. Results were reported as the mean±SD. Spearman correlation and multiple regression analyses were performed to evaluate the correlation between binding ratios and disease severity, duration and age.
The programs SPSS 10.0 and MS-Excel for Windows XP were used as evaluation software, on a personal computer.
RESULTS
The images were studied by both visual and semiquantitative analysis. As expected, a higher activity and bilateral symmetry of DAT binding were detected in both basal ganglias of normal controls, and the lower DAT binding were shown in global striatums of thirty-six out of thirty-eight primary and tentative idiopathic PD patients by visual inspection, as shown in Fig.1.
Fig. 1.

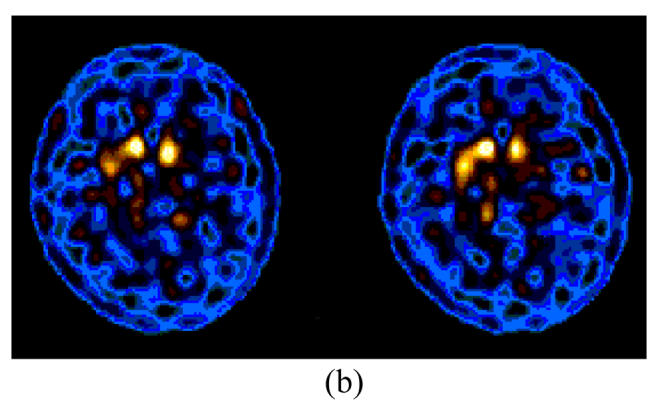


99mTc-TRODAT-1 SPECT in transverse views of normal controls and PD patients with HYS from stages I to III. A greater decline of DAT binding contralateral to more affected limbs was observed, although the striatal uptake was bilaterally reduced in PD cases with HYS stage I. A greater loss of DAT binding was noted in striatum by visual inspection in PD subjects with higher than lower HYS stage. (a) Control; (b) HYS stage I; (c) HYS stage II; (d) HYS stage III
Highly significant differences of DAT binding was found across global striatums in PD patients compared to controls, and between controls, and with stage I, stage II and stage III subjects and each other (P<0.01) (Tables 1 and 3). The decrease in radioactivity was more prominent in putamen than in caudate contralateral side to the more affected limbs in subjects with HYS stage I (P<0.01). Moreover, a significant decrease of the specific uptake was also found in putamen of cases with HYS stage II and III, compared to the ipsilateral side of twenty-four subjects with HYS stage I, respectively, although the striatal uptake was bilaterally reduced (P<0.01) (Tables 2 and 3).
Table 1.
The average specific uptake of 99mTc-TRODAT-1 from both sides of striatum in 38 subjects with PD and in controls
| Controls | Ipsilateral striatum | |
| Numbers | 18 | 38 |
| Mean±SD | 4.71±0.63 | 3.23±0.66* |
Means P<0.01
Contralateral means side opposite to symptoms or more affected limbs, ipsilateral means side of symptoms or more affected limbs
Table 3.
Specific uptake of 99mTc-TRODAT-1 in striatum and its subregions of controls and 38 subjects with PD from HYS stages I to III
| Controls | HYS stage I |
HYS stage II | HYS stage III | ||
| Contralateral | Ipsilateral | ||||
| Numbers | 18 | 24 | 24 | 9 | 5 |
| Striatum | 4.71±0.63 | 3.24±0.73* | 3.50±0.87*◊ | 2.97±0.22*△ | 3.00±0.16*△ |
| Caudate | 2.62±0.53 | 1.68±0.52* | 1.83±0.54*◊ | 1.55±0.14*△ | 1.58±0.15* |
| Putamen | 2.12±0.35 | 1.54±0.28* | 1.69±0.45*◊ | 1.42±0.12*△ | 1.42±0.05*△ |
Means P<0.01 compared with normal controls
Means P<0.01 compared with ipsilateral side
Means P<0.05 compared contralateral striatum and its subregions with those of ipsilateral side
Data of average radioactivity is from both striatums and its subregions in patients with HYS stages II and III PD
Table 2.
Specific uptake of 99mTc-TRODAT-1 in 24 PD patients with HYS stage I contralateral and ipsilateral to symptoms, compared to that of controls
| Controls | Contralateral striatum | Ipsilateral striatum | |
| Numbers | 18 | 24 | 24 |
| Mean±SD | 4.71±0.63 | 3.24±0.73* | 3.50±0.87* |
Means P<0.01
Spearman correlation analysis showed that the reduction of the uptake ratios significantly correlated with the UPDRS in striatum and all its subregions in the PD group (P<0.05), a similar change was found in putamen with the HYS assessment (P<0.05) (Fig.2). In analysis of variance (ANOVA), however, the decline of DAT binding across all striatal subregions did not show significant correlation with the disease severity of the PD assessed by using the HYS, as shown in Tables 1 to 4.
Fig. 2.
The correlation of 99mTc-TRODAT-1 uptake with UPDRS (a) and HYS stage (b), respectively. (a) The binding ratios of 99mTc-TRODAT-1 displayed significant negative correlation with UPDRS in striatum, caudate and putamen, respectively; (b) The binding ratios were significantly correlated with HYS stage only in putamen
Table 4.
Analysis of variance of specific uptake of 99mTc-TRODAT-1 in striatum and its subregions of 38 subjects with PD among HYS stages I, II and III
| HYS stage I | HYS stage II | HYS stage III | ANOVA | P value | |
| Numbers | 24 | 9 | 5 | ||
| Striatum | 3.24±0.73 | 2.97±0.22 | 3.00±0.16 | 0.88 | 0.425 |
| Caudate | 1.68±0.52 | 1.55±0.14 | 1.58±0.15 | 0.35 | 0.706 |
| Putamen | 1.54±0.28 | 1.42±0.12 | 1.42±0.05 | 1.2 | 0.313 |
Note: Specific uptake of 99mTc-TRODAT-1 in striatum and its subregions of patients with HYS stage I is from contralateral side to symptoms; Data on average radioactivity is from both striatums and its subregions in patients with HYS stages II and III PD
DISCUSSION
This study revealed steady reduction of TRODAT-1 uptake in thirty-six out of thirty-eight PD cases with minimal to severe disability by visual inspection of images, compared to normal controls. Semiquantitative analysis of DAT binding showed that the striatal uptake values were bilaterally reduced, compared with controls (P<0.01). In the hemiparkinsonian patients the reduction was greater on the side contralateral than ispilateral to the initial symptoms, and the specific uptake were more obviously deficit in the putamen than in the caudate. These results indicated that the greater loss in putamen than in caudate neucleus and the asymmetry of DAT binding on both sides of basal ganglias may serve as important parameters in improving sensitivity and accuracy of 99mTc-TRODAT-1 SPECT imaging for early diagnosis of PD. Compared with normal controls, a significant decrease of specific uptake was also found in striatum, and especially in putamen ipsilateral to the symptomatic side. Similar results were reported and suggested that 99mTc-TRODAT-1 SPECT imaging may be able to detect dopaminergic changes due to neurodegenerative disorders before the onset of clinical symptoms (Mozley et al., 2000; Huang et al., 2003). As expected, this unilateral change was eventually worsened to a similar level of specific uptake in striatum on the side contralateral to the initially affected limbs with the progression of PD (Huang et al., 2001).
DAT is responsible for removing dopamine and taking it up into the presynaptic deposit vesicles (Reith et al., 1997). DAT concentration obviously diminished during SPECT imaging when quantities of dopaminergic neurodegeneration was over 50% in the striatum. Therefore, both neurodegeneration and lower retake ability of DAT play a critical role in the severity of PD. It was reported that DAT level in basal ganglias could be 30%~60% down in early PD patients, which may explain why the concentration of DAT in striatal, especially in putamen is a high sensitivity parameter for detecting the early stage of PD (Nutt et al., 2004). Several studies also showed correlation between a progressive decline of overall striatal DAT binding and increasing disability of PD by using the HYS and the total score on the UPDRS (Marek et al., 2001; Pirker, 2003). In the present study, Spearman analysis also showed negative correlation of TRODAT-1 uptake in overall striatum with the UPDRS in assessing disease severity of PD. However, it was interesting to note that a similar change was just found in putamen of PD subjects by using Hoehn and Yahr assessment. In addition, we particularly pay our attention to this assessment in evaluating the relationship between the reduction of uptake ratios and the progress of PD, because the majority of PD cases with unilateral symptoms (HYS stage I) were involved in the present study. ANOVA is therefore necessary as the results did not show significant correlation of disease severity with the decline of DAT binding in overall striatum and its subregions, although a greater loss of 99mTc-TRODAT-1 SPECT imaging was observed by visual inspection in those areas with the increasing severity of PD graded according to the HYS stage. These findings suggested that the UPDRS is more fit than the Hoehn and Yahr method for studying disease severity of PD by using 99mTc-TRODAT-1 SPECT imaging.
CONCLUSION
The present results support previous findings that suggested 99mTc-TRODAT-1 SPECT imaging techniques can be used as a new convenient method for evaluating degeneration of dopaminergic projections in striatum and enhancing accuracy of diagnosis in patients with PD. Both the significant decrease and the asymmetry of specific DAT binding in striatums and its subregions provide an objective means for diagnosis of early-stage PD. The UPDRS but not the Hoehn and Yahr assessment can offer a comprehensive index to assess the role of 99mTc-TRODAT-1 SPECT in disease severity of PD. Moreover, the level of specific DAT uptake in striatums and their subregions ipsilateral to the affected limbs may present a clue for following the progression of PD, because this level may present the vestigial functional integrity of dopaminergic fibers in striatum of patients with early-stage PD.
References
- 1.Asenbaum S, Brücke T, Pirker W, Podreka I, Angelberger P, Wenger S, Wober C, Muller C, Deecke L. Imaging of dopamine transporters with iodine-123-beta-CIT and SPECT in Parkinson’s disease. J Nucl Med. 1997;38(1):1–6. [PubMed] [Google Scholar]
- 2.Booij J, Tissingh G, Winogrodzka A, van Poyen EZ. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. Eur J Nucl Med. 1999;26(2):171–182. doi: 10.1007/s002590050374. [DOI] [PubMed] [Google Scholar]
- 3.Gel DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Hoehn MW, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology (Minneap) 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 5.Huang WS, Lin SZ, Lin JC, Wey SP, Ting G, Liu RS. Evaluation of early-stage Parkinson’s disease with 99mTc-TRODAT-1 imaging. J Nucl Med. 2001;42(9):1303–1308. [PubMed] [Google Scholar]
- 6.Huang WS, Chiang YH, Lin JC, Chou YH, Cheng CY, Liu RS. Crossover study of (99m)Tc-TRODAT-1 SPECT and (18)F-FDOPA PET in Parkinson’s disease patients. J Nucl Med. 2003;44(7):999–1005. [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kao PF, Tzen KY, Yen TC, Lu CS, Weng YH, Wey SP, Ting G. The optimal imaging time for [99mTc]TRODAT-1/SPET in normal subjects and patients with Parkinson’s disease. Nucl Med Commun. 2001;22(2):151–154. doi: 10.1097/00006231-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman MJ, Madras BK. Severe depletion of cocaine recognition sites associated withthe dopamine transporter in Parkinson’s diseased striatum. Synapse. 1991;9(1):43–49. doi: 10.1002/syn.890090107. [DOI] [PubMed] [Google Scholar]
- 10.Kung HF, Kim HJ, Kung MP, Meegalla SK, Plossl K, Lee HK. Imaging of dopamine transporter in humans with technetium-99mTRODAT-1. Eur J Nucl Med. 1996;23(11):1527–1530. doi: 10.1007/BF01254479. [DOI] [PubMed] [Google Scholar]
- 11.Marek K, Innis R, van Dyck C, Fussell B, Early M, Eberly S, Oakes D, Seibyl J. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology. 2001;57(11):2089–2094. doi: 10.1212/wnl.57.11.2089. [DOI] [PubMed] [Google Scholar]
- 12.Mozley PD, Schneider JS, Acton PD, Plossl K, Stern MB, Siderowf A, Leopold NA, Li PY, Alavi A, Kung HF. Binding of [99mTc]TRODAT-1 to dopamine transporters in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med. 2000;41(4):584–589. [PubMed] [Google Scholar]
- 13.Nutt JG, Carter JH, Sexton GJ. The dopamine transporter: Importance in Parkinson’s disease. Ann Neurol. 2004;55(6):766–773. doi: 10.1002/ana.20089. [DOI] [PubMed] [Google Scholar]
- 14.Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18((Suppl 7)):S43–S51. doi: 10.1002/mds.10579. [DOI] [PubMed] [Google Scholar]
- 15.Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in Parkinsonism–a prospective study. Can J Neurol Sci. 1991;18(3):275–278. doi: 10.1017/s0317167100031814. [DOI] [PubMed] [Google Scholar]
- 16.Reith ME, Xu C, Chen NH. Pharmacology and regulation of the neuronal dopamine transporter. Eur J Pharmacol. 1997;324(1):1–10. doi: 10.1016/s0014-2999(97)00065-4. [DOI] [PubMed] [Google Scholar]




