Abstract
Objective: To observe the clinicopathological characteristics of gastric stump cancer (GSC) and evaluate the benefits of radical surgery of GSC. Methods: The clinicopathological characteristics and postoperative survival time of 37 GSC patients who underwent surgery were investigated retrospectively. The survival time was compared according to the type of surgical operation (radical resection vs palliative operation). Twenty-one cases that received radical resection were analyzed based on the pTMN stage. Survival curves were traced by using Kaplan-Meier methods. Results: Most GSC (32/37) was detected in patients who had received Billroth II reconstruction after partial gastrectomy for benign gastric disease. The lesser curvature side and the suture line of anastomosis were the most frequent sites where GSC occurred (27/37). Differentiated adenocarcinoma was the dominant histopathological type (24/37). The postoperative 5-year survival rate of early stage GSC patients (n=9) was significantly higher than advanced stage GSC (n=12) (55.6% vs 16.5%, xL 2=11.48, P<0.01). Five-year survival rate of 21 GSC patients with radical resection were 75% (3/4) for stage I, 60% (3/5) for stage II, 14.2% (1/7) for stage III, and 0% (0/5) for stage IV respectively. The median survival time of 21 GSC patients who underwent radical resection was longer than those undergoing palliative operation (43.0 m vs 13.0 m, xL 2=36.31, P<0.01), the median survival time of stage IV patients with radical resection was 23.8 months. Conclusions: Without remote metastasis, radical resection for GSC is possible, and is an effective way to improve the prognosis of GSC. Even in stage IV GSC, radical resection can still prolong the survival time. It is necessary for the patients with benign gastric diseases who received partial gastrectomy to carry out the endoscopy follow-up, especially in patients with Billroth II reconstruction procedure at 15–20 years.
Keywords: Gastric stump cancer, Surgery, Partial gastrectomy, Endoscopy, Prognosis
INTRODUCTION
Gastric stump cancer (GSC) is defined as carcinoma that occurred in gastric stump, which had undergone partial gastrectomy for benign gastric disease 5 years before (Matsui et al., 2001). Carcinoma developed in the gastric stump after subtotal gastrectomy for gastric cancer ten years before should also be taken into account (Tanigawa et al., 2002). Due to its lack of specific clinical manifestation in the early stage and relatively low surgical resection rate, GSC is still termed as a poor prognosis disease. With increased use of diagnostic endoscopy, GSC has been detected at earlier stages. Recent studies showed that GSC had survival rates similar to those of primary proximal gastric carcinoma (Gannon et al., 2001). Although the incidence of primary gastric cancer is decreasing, the incidence of GSC is increasing. The reasons could be related to the high frequency benign digestive ulcer partial gastrectomy performed in the previous decades. Between Aug 1993 and April 2003, 37 GSC patients underwent surgical operation in our department. We carried out a follow-up program to investigate and discuss the benefits of radical surgery in improving the prognosis of GSC.
MATERIALS AND METHODS
Patients
Thirty-seven GSC patients underwent surgery in our department from 1993 to 2003, 29 male and 8 female patients were involved in this series, where mean age was 60.0 years (range, 47–74 years). The time duration between primary gastrectomy for benign gastric disease and occurrence of GSC ranged from 7 to 40 years. The median time was 20.5 years. The interval time of 6 patients was 5–10 years, with 31 patients exceeding 10 years. Thirty-two of the 37 patients underwent Billroth II reconstruction, 3 underwent Billroth I reconstruction, and 2 received R-Y gastrojejunal anastomosis in the primary gastrectomy.
All patients had endoscopic examination and biopsy before GSC was diagnosed. In 27 patients, the tumor was located at the lesser curvature and anastomosis of the gastrojejunostomy. In 10 patients, the tumor was at the upper side of the gastric stump including that near the cardia. According to the classification of the Japanese Association of Gastric Cancer (JGCA, 1998), there were 9 early stage GSC and 28 advanced stage GSC. Among 9 early stage patients, 5 were elevated types; 1 was flat type and 3 were depressed types. Among 28 advanced stage GSC patients, 7 were type I; 15 were type III; and 6 were type IV. The predominant histopathological type was differentiated type (24/37, including papillary, well differentiated and moderately differentiated adenocarcinoma); the rest were undifferentiated types (including poorly differentiated adenocarcinoma, signet ring cell and adenosquamous carcinoma).
Surgical treatments
All patients received surgical therapy. Twenty-one patients received radical resection under the Guideline of JGCA (1998). GSC radical resection indication was complied with as follows: (1) GSC was diagnosed with endoscopy and confirmed histopathologically; (2) no remote organs or peritoneal metastasis; (3) the patient’s physical condition indicated they could endure the surgery. On the gastric stump and at the anastomotic site of the jejunum resection, and according to the metastatic degree of the lymph node, dissection II or dissection II+ were performed. The extent of the lymph node dissection should reach beyond the positive lymph node around the neoplasm. To ensure the complete dissection of the lymph node, concomitant organ resection such as partial hepatectomy or splenectomy were done if necessary. Sixteen patients received palliative operation for remote metastasis, including five exploratory laparotomy and eleven palliative resections. There was no mortality during perioperative period (Table 1).
Table 1.
Surgical procedure characteristics of 37 patients
| EGSC (n=9) | AGSC (n=28) | |
| Type of surgical approach | ||
| Abdominal | 9 | 22 |
| Thoracoabdominal | 0 | 6 |
| Lymph node dissection | ||
| D2 | 8 | 3 |
| D2+ | 1 | 9 |
| Extent of gastric stump resection | ||
| Subtotal resection | 3 | 0 |
| Total resection | 6 | 12 |
| Concomitant resection | ||
| Partial Liver | 0 | 3 |
| Spleen | 0 | 8 |
| Middle colon | 1 | 2 |
| Partial Pancreas | 0 | 2 |
| Gallbladder | 1 | 2 |
| Alimentary reconstruction | ||
| Gastric stump-jejunum R-Y anastomosis | 3 | 0 |
| Esophagus-jejunum R-Y anastomosis | 6 | 12 |
| Palliative operation | ||
| Explored laparotomy | 0 | 5 |
| Palliative resection | 0 | 11 |
EGSC: Early stage GSC; AGSC: Advanced stage GSC
Follow-up investigation
Postoperative survival time data on 37 GSC patients were obtained through communication with patients by telephone or letter. Sixteen patients received 6 to 85 months follow-up examination in the hospital, with average follow-up time of 31.2 months. According to the type of surgical operation, 37 GSC patients were divided into radical resection and palliative operation groups to compare the postoperative survival time. In addition, postoperative survival time of 21 cases who received radical resection was analyzed based on the pTMN stage.
Statistics
Survival curves were traced by using Kaplan-Meier methods, the difference between curves was tested using the log-rank test. The significance of other data was assessed using the chi-squared test. All data were analyzed by statistical software package of SPSS 11.0; with P<0.05 being regarded as statistically significant.
RESULTS
Clinical and pathological findings
GSC showed a male preponderance, with a male: female ratio of 4:1 (29 M/8 F). Most GSC occurred in patients who had received Billroth II reconstruction (32/37, P<0.05), the lesser curvature side and the suture line of gastrojejunal anastomosis were the most frequent sites where GSC occurred (27/37). The resection rate was 100% (n=9) in early stage GSC and 42.9% (n=12) with advanced cases. Differentiated adenocarcinoma (24/37) was the dominant histopathological type in GSC.
Survival analysis
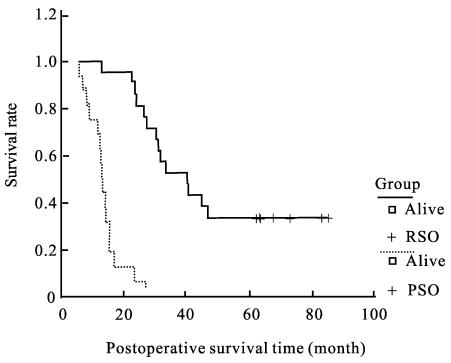
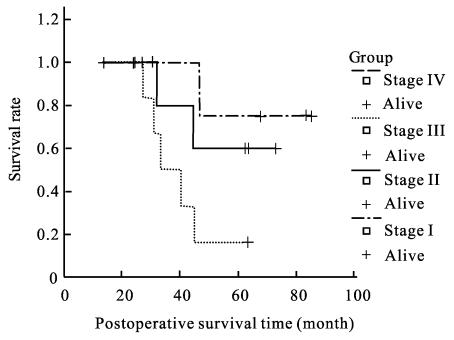
The postoperative cumulative 5-year survival rate of 9 early stage GSC patients after radical resection was significantly higher than that in advanced stage (55.6% vs 16.5%, xL 2=11.48, P<0.01). The median survival time of the GSC patients who received radical surgery was 40.3 months (Fig.1), which was significantly longer than the 13.0 months of those who underwent palliative operation (xL 2=36.31, P<0.01). According to the pTMN stage, the 5-year survival rate of 21 GSC patients with radical resection were 75% (3/4) for stage I, 60% (3/5) for stage II, 14.2% (1/7) for stage III, and 0% (0/5) for stage IV, and the median survival time of stage IV patients who underwent radical resection was about 23.8 months (Fig.2).
Fig. 1.
Comparison of postoperative survival time between radical surgical operation and palliative surgical operation PSO: palliative surgical operation; RSO: radical surgical operation
Fig. 2.
The postoperative survival time according to the pTMN stage in 21 radical resection GSC patients
DISCUSSION
The interval time of GSC development after original gastric resection is about 20 to 30 years (Lo et al., 1997), as reconfirmed by our follow-up investigation. With the prolonged life expectancy of patients with a gastric stump, the incidence of GSC will be increased. The average morbidity of GSC patients is about 2.4%~5.0%, three times higher than ordinary people (Caygill et al., 1987). GSC has no specific clinical symptom in the early stage, most patients were found to be in the end stage when they were diagnosed as suffering from GSC, which had been described as a low resectability cancer with poor prognosis, and the 5-year survival rate was still lower than what we expected.
The potential development mechanisms of GSC may be related to the lower acidic environment in the gastric stump, duodenogastric reflux and Helicobacter pylori infection (Fukuhara et al., 2003). It was shown that most GSC occurred in patients who had received Billroth II reconstruction. High level bile reflux after Billroth II gastric resection always accelerate the precancerous changes in the gastric mucosa (Lorusso et al., 2000) that may partly play a role in the etiopathogenesis of GSC after Billroth II reconstruction. A 15-year endoscopic screening program was carried out by Greene (1996), who demonstrated that early stage GSC has better prognosis than that in the advanced stage. They advocated that patients who received gastrectomy for benign gastric diseases should accept endoscopic surveillance annually. If GSC is detected at a relatively early stage, a predominant 5-year survival rate of 74% could be obtained by radical resection (Pointer et al., 1988). These findings showed that the detection of GSC in early stage can effectively improve its prognosis. Recent group studies also showed that survival of GSC patient could be benefit for endoscopy screening in early stage (Gannon et al., 2001). Nine of our early stage GSC patients underwent radical resection, whose cumulative 5-year survival rate was 55.6%, much higher than the 16.5% of advanced stage cases. There is extremely large difference between these two subgroups. According to the pTMN stage, we can see that there was a decreasing trend in our data of 5-year survival rate from stage I to stage IV. The earlier GSC is diagnosed, the longer will be the survival. So, we considered that periodic endoscopic follow-up can help detect GSC patients in relatively early stage. As the shortest interval time of GSC development after partial gastrectomy is 5 years and median time is about 20 years, it is economical for patients who had undergone partial gastrectomy for benign gastric diseases to have every 5 year follow-ups (especially after 15 years), as it was shown that the risk of gastric stump cancer increased after that period (Greene, 1996).
Surgical operation is still the only curative measure for GSC now. As well differentiated adenocarcinoma is the most common histological type (Kaneko et al., 1998), which was also reconfirmed in our datum (24/37). The curative resectability rate of GSC in this series was about 56.7%; the median survival time of GSC in patients who underwent radical resection is significantly longer than that of patients who underwent palliative operation. Despite the poor 5-year survival rate of stage IV patients who underwent radical resection, it still resulted in longer median survival time than those who underwent palliative operation. Therefore, radical resection is valuable in the management of GSC.
As a special type of primary gastric cancer in gastric stump, the lymphatic metastasis of GSC has its own rule in line with the different stages and type of reconstruction procedure in the initial operations, so the reasonable extension of the lymph node dissection in GSC should be based on the original reconstructive procedures and the extent of lymph node metastasis observed during the operation. From our experiences in management of the GSC, the lymph node compartment I metastasis around the neoplasm and the compartment II metastasis to the left of the gastric artery and the splenic hilum should be taken into account for dissection. Since a high incidence of metastasis in the node of the mesenteric root and the skip metastasis across the gastriojejunal anastomosis in GSC after Billroth II procedure, the wide resection of the jejunal mesentery (NO14) is worthy to be recommended, and might probably improve the prognosis of GSC (Thorban et al., 2000). No. 16 lympho node around the upper abdominal aorta is frequently invaded through the greater curvature and the suprapancreatic lymph nodes (Kunisaki et al., 2002), so No. 16 group lympho node should be involved in radical resection procedure theoretically. But we thought that the resection of No. 16 group lympho node should be taken on the basis of GSC stages and the physical condition of the patients. The total gastrectomy performed in advanced stage GSC was reasonable, if the lower oesophagus near the cardia and adjacent organs were invaded, concomitant resection of total gastric stump and partially related organs could yield a satisfactory result.
In conclusion, this retrospective study demonstrated it is necessary for the patients who received partial gastrectomy for benign gastric diseases to undergo endoscopy follow-up, especially for patients who underwent Billroth II reconstruction procedure at 15–20 years. Without remote metastasis, radical resection for GSC is possible, even in stage IV GSC, radical resection can still prolong the survival time.
Footnotes
Project (No. 2004C34010) supported by the Science and Technology Bureau of Zhejiang Province, China
References
- 1.Caygill CP, Hill MJ, Hall CN, Kirkham JS, Northfield TC. Increased risk of cancer at multiple site after gastric surgery for peptic ulcer. Gut. 1987;28(8):924–928. doi: 10.1136/gut.28.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuhara K, Osugi H, Takada N, Takemura M, Ohmoto Y, Kinoshita H. Quantitative determinations of duodenogastric reflux, prevalence of Helicobacter pylori infection, and concentration of interleukin-8. World J Surg. 2003;27(5):567–570. doi: 10.1007/s00268-003-6796-z. [DOI] [PubMed] [Google Scholar]
- 3.Gannon CJ, Engbrecht B, Napolitano LM, Bass BL. Gastric remnant carcinoma: reevaluation of screening endoscopy. Surg Endosc. 2001;15(12):1488–1494. doi: 10.1007/s00464-001-4175-0. [DOI] [PubMed] [Google Scholar]
- 4.Greene FL. Management of gastric remnant carcinoma based on the results of a 15-year endoscopic screening program. Ann Surg. 1996;223(6):701–708. doi: 10.1097/00000658-199606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.JGCA (Japanese Gastric Cancer Association) Japanese Classification of Gastric Carcinoma. 2nd English edition. Gastric Cancer. 1998;1(1):10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko K, Kondo H, Saito D, Shirao K, Yamaguchi H, Yokota G, Sano T, Sasako M, Yoshida S. Early gastric stump cancer following distal gastrectomy. Gut. 1998;43(3):342–344. doi: 10.1136/gut.43.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunisaki C, Shimada H, Nomura M, Hosaka N, Akiyama H, Ookubo K, Moriwaki Y, Yamaoka H. Lymph node dissection in surgical treatment for remnant stomach cancer. Hepatogastroenterology. 2002;49(44):580–584. [PubMed] [Google Scholar]
- 8.Lo SS, Wu CW, Hsieh MC, Lui WY. Is gastric remnant cancer clinically different from primary gastric cancer? Hepatogastroenterology. 1997;44(13):299–301. [PubMed] [Google Scholar]
- 9.Lorusso D, Linsalata M, Pezzolla F, Berloco P, Osella AR, Guerra V, Di LA, Demma I. Duodenogastric reflux and gastric mucosal polyamines in the non-operated stomach and in the gastric remnant after Billroth II gastric resection. A role in gastric carcinogenesis? Anticancer Res. 2000;20(3B):2197–2201. [PubMed] [Google Scholar]
- 10.Matsui N, Yao T, Akazawa K, Nawata H, Tsuneyoshi M. Different characteristics of carcinoma in the gastric remnant: histochemical and immunohistochemical studies. Oncol Rep. 2001;8(1):17–26. [PubMed] [Google Scholar]
- 11.Pointer R, Schwab G, Konigsrainer A, Bodner E, Schmid KW. Early cancer of the gastric remnant. Gut. 1988;29(3):298–301. doi: 10.1136/gut.29.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanigawa N, Nomura E, Niki M, Shinohara H, Nishiguchi K, Okuzawa M, Toyoda M, Morita S. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer. 2002;5(1):23–28. doi: 10.1007/s101200200003. [DOI] [PubMed] [Google Scholar]
- 13.Thorban S, Boattcher K, Etter M, Roder JD, Busch R, Siewert JR. Prognostic factors in gastric stump carcinoma. Ann Surg. 2000;231(2):188–194. doi: 10.1097/00000658-200002000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]