Abstract
Thermodynamic parameters of complexation of naphto-15-crown-5 with four alkaline earth ions in aqueous media was determined using titration microcalorimetry at 298.15 K. The stability of the complexes, thermal effect and entropy effect of the complexation is discussed on the basis of the guest ions structure and the solvent effect. The stability constants tendency to vary with ion radius was interpreted. Complex of naphtha-15-crown-5 with calcium ion is very stable due to the synergism of static electric interaction and size selectivity between the host and the guest.
Keywords: Microcalorimetry, Host-guest complexation, Naphtho-15-crown-5, Alkaline earth metal, Molecular recognition
INTRODUCTION
As biologic model compounds, crown ethers can interact with many guests including inorganic ions and neutral organic molecules through weak non-covalent interactions (Yi et al., 1997). Since the first series of crown ethers was synthesized by Pedersen (1967) interest in this macro-cyclic compound has been growing steadily, due to its great importance in supramolecular chemistry (Lehn, 1988), close relation to photochemistry (Krzysztof et al., 2002), catalysis and a number of new scientific areas (Jensen et al., 2002; Lu et al., 1994; Li et al., 1998). Complexes of potassium with cyclohexano-15-crown-5 and dicyclohexano-18-crown-6 formed in THF had been studied (Zbigniew et al., 2002); and the decomposition mechanism had been explored; the bis-barium complex of a butterfly crown ether azobis (benzo-18-crown-6) had been found to be phototunable supramolecular catalyst in the basic ethanolysis of anilide derivatives (Roberta et al., 2003); stability constants of complexes of Ag (I), Co (II), Cu (II), Mn (II), Ni (II), Zn (II), Pb (II), Cr (III) and Fe (III) with the macro-cyclic compounds, namely, 15-crown-5, benzo-15-crown-5, 18-crown-6, dibenzo-18-crown-6, dicyclohexano-18-crown-6, dibenzo-24-crown-8. Dicyclohexano-24-crown-8 had been determined with conductometric titrations (Vijaykumar and Ashwini, 2003); syntheses and luminescence behavior of the diaquatris (thienoyltrifluoracetonate) europium (III) with dibenzo 18-crown-6 and 18-crown-6 in the solid state had been reported (Claudia et al., 2003). Caesium complexation by tetrabenzo-24-crown-8 in 1,2-DCE had been synthesized and characterized (Levitskaia et al., 2000; 2001; Bryan et al., 2000; Talanova et al., 1999). It was found that improved solubility, strong caesium binding and weak ion-pairing tendency make 4,5′,4′′,5′′′-tetra-tert-butyltetrabenzo-24-crown-8 an attractive candidate for caesium-selective complexation and transport in biphasic liquid systems; water soluble crown-6 derivatives of calx[4] arene had been synthesized and their affinity toward Cs+, Rb+, K+ and Na+ cations in methanol and basic aqueous media had been studied (Pellet-Rostaing et al., 1998; 2001). Various aspects of the use of these new ligands in a nanofiltration-complexation process had been summarized; their use might be an efficient way to separate trace amount of Cs+ from highly salted medium. Complexation of three cyclofructants in whose molecular centers crown ether 6, 7, 8 skeletons were respectively contained. Alkaline and alkaline earth metal cations had been reported (Hamada et al., 2003), and the thermodynamic parameters of the host-guest complex processes in THF and acetone had been determined at 298 K. More fascinatingly, chiral bis-pyridino-18-crown-6(rare earth metal triflate complexes had been used as catalysts for asymmetric aldol reactions in aqueous media (Shiozuma et al., 2001).
It is well known that water is a solvent in living organisms. So studies on interactions between such hosts as crown ethers and calixarenes with inorganic ions are very helpful for gaining understanding of the important processes of ion carrying and transporting in living systems, in which some kind of peptides and natural ionophores such as monensin and valinomycin play crucial roles (Tanabe et al., 1987). It is widely accepted that interaction between crown ethers and ions must in general be governed by a subtle balance of interactions of electron donors with the acceptors, guest ion with its counter ion, and the host or guest with solvent. These weak interactions will only be identified by the study of a wide range of combinations of crown ethers with ions, which are most easily generated by using different types of crown ethers and ions. The more detailed is the thermodynamic information on host-guest interaction, the better is our understanding of its mechanism. As a reliable method, microcalorimetric measurement had been used to identify the factors and driving forces governing the interactions in such systems (Alizadeh and Shamsipur, 1996). In the present paper, we report a calorimetric study on the thermodynamics of the complexation of naphtho-15-crown-5 with alkaline earth cations, Mg2+, Ca2+, Sr2+, Ba2+, in aqueous solution at 298.15 K, and discuss the experimental results regarding crown ether molecular structure, size of the guest ion, and solvent effect. To the best of our knowledge, no thermodynamic research on complexation between naphtho-crown ether and metal ions based on data from microcalorimetry has been reported.
EXPERIMENTAL DETAILS
Materials
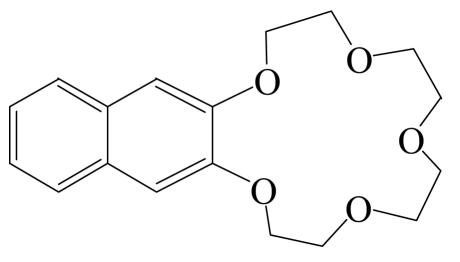
Naphtho-15-crown-5 (Naph 15C5), namely, naphtha [2,3-b]-1,4,7,10,13-pentaoxacyclopentadecin or 2,3,5,6,8,9,11,12-octahydrocyclopentadecin (Fig.1) was synthesized and purified to better than 99% in this laboratory with the method reported by Qin (1987). Magnesium chloride, calcium chloride, strontium chloride and barium chloride were analytically pure reagents purchased from the Shanghai Reagent Company (Shanghai, China). The salts were baked at 598 K for 3 h before use. Water used in the experiments was obtained by repeated twice distillation in the presence of basic potassium permanganate.
Fig. 1.
Structure of Naph 15C5
Microcalorimetric measurements
The calorimeter used was an isothermal titration microcalorimeter, Thermal Activity Monitor (TAM) Thermometric 2277 (Sweden), controlled by Digitam 4.1 software. This instrument has an electrical calibration precision of better than ±1% and the accuracy can be checked by measuring the dilution enthalpy of a concentrated sucrose solution (Bai et al., 2002). 0.100 mol/dm3 MCl2 (M=Mg, Ca, Sr, Ba) solution, was injected in small aliquots (10.00 μl) into the 4 ml stainless steel ampoule containing 2.00 ml dilute Naph 15C5 solution (titrand, 5.00×10−4 mol/dm3), using a 500 μl Hamilton syringe controlled by a 612 Lund Syringe Pump. The interval between two injections was long enough for the signal to return to the baseline. The stirrer in the ampoule rotated at a constant speed of 50 rpm. All experiments were performed at temperature (298.15±0.01) K and carried out when the baseline was stable in the presence of constant stirring so heat of stirring was deducted automatically. To deduct the dilution heats of MCl2 and the Naph 15C5 solutions, we experimented on titration of MCl2 solution into pure water and pure water into the Naph 15C5 solutions, respectively. The dilution heat of the later one was found to be negligible. The experimental data of thermal effect of complexation with volume of titrant is shown in Table 1.
Table 1.
Experimental data of thermal effect Q (mJ) of complexation with volume of titrant (V tt)
| Vtt (μl) |
Q (mJ) |
|||
| Ca2+ | Mg2+ | Sr2+ | Ba2+ | |
| 10 | −1.083 | −0.937 | −2.378 | −1.383 |
| 20 | −0.522 | −0.296 | −4.528 | −4.02 |
| 30 | −0.941 | −1.387 | −4.249 | −4.253 |
| 40 | −0.97 | −3.559 | −4.552 | −6.072 |
| 50 | −0.426 | −3.666 | −6.535 | −6.111 |
| 60 | −0.529 | −3.02 | −5.265 | −8.59 |
| 70 | −0.456 | −3.401 | −5.781 | −9.463 |
| 80 | 0.172 | −0.904 | −6.365 | −11.438 |
| 90 | 0.418 | −1.58 | −7.217 | −12.564 |
| 100 | 0.275 | −1.94 | −8.62 | −13.637 |
| 110 | 0.712 | −2.891 | −8.714 | −15.391 |
| 120 | 0.807 | −5.004 | −9.605 | −16.325 |
| 130 | 1.046 | −4.813 | −9.406 | −17.76 |
| 140 | 1.155 | −5.05 | −9.129 | −19.278 |
| 150 | 0.785 | −6.118 | −9.067 | −20.495 |
| 160 | 0.595 | −6.167 | −10.534 | −21.471 |
| 170 | 0.853 | −7.373 | −12.667 | −22.24 |
| 180 | 0.809 | −7.299 | −15.23 | −23.571 |
| 190 | 0.273 | −7.868 | −16.295 | −23.995 |
| 200 | 0.89 | −8.749 | −15.611 | −24.372 |
| 210 | 1.232 | −9.116 | −15.579 | −25.628 |
| 220 | 1.436 | −9.77 | −15.346 | −25.244 |
| 230 | 1.941 | −10.402 | −15.428 | −25.974 |
Note: Cation in titrant solution
RESULTS AND DISCUSSION
Data process
To rationalize the enthalpy of formation of complex of Naph 15C5 with the ions in aqueous solutions, apparent equilibrium constants for overall reactions have been defined as
| M+iL=MLiβi, ΔHiº | (1) |
Where i=1, or 2, or 3, M and L represent the guest (M2+) and host (Naph 15C5), respectively. The relationship between the overall equilibrium constants, βi, and the stepwise equilibrium constants, Ki, is
| βi=ΠKi | (2) |
Thermodynamic parameters, ΔHiº and βi or Ki, can be obtained by the regression method (Stödeman and Wadsö, 1995), which was performed with Ligand Binding Analysis process of Digitam 4.1 software. We obtained the optimum result by comparing the fitness of the simulated (calculated) curve with the experimental points, i.e., after calculation of three reaction models (only i=1; i=1 and 2; i=1, 2 and 3) for M2+-Naph 15C5 complex system. It was found that mainly the 1:1 stoichiometry of the host-guest complex could be formed (so other stepwise reactions could be neglected), which might be the result of the very low concentration of Naph 15C5 whose low solubility in water prevented us from getting more concentrated solution of it. All of the thermodynamic parameters corresponding to the four M2+-Naph 15C5 complex systems are listed in Table 2. The error attributable to enthalpy change is the deviation estimated by the Digitam program.
Table 2.
Thermodynamic parameters determined in this experiment and related data
Stability of the M2+-Naph 15C5 complex with the size of ions
Stability constants and the corresponding absolute value of formation Gibbs free energy of the four 1:1 complexes (Table 2) show that Ca2+-Naph 15C5 is the most stable one of the four complexes. This result accords quite well with that reported in literature (Rahman et al., 2001), indicating that interaction between Ca2+ and Naph 15C5 is stronger than that between any other baseline earth ion and the same crown ether. By comparing the values of the stability constants with the radii (Weast, 1974–1975) of the naked ions (Table 2), the size selectivity of the crown ether can be observed. The five oxygen atoms form a bowl-shaped space with negative electric charge, and only the cation with appropriate size can interact with the oxygen atoms efficiently. The stability of the complex will drop dramatically if the size of its guest ion deviates from the most appropriate radius, which indicates that Naph 15C5 possesses function of molecular recognition of alkaline earth metal. Earlier researches showed that 15-crown-5 do not have such function in aqueous media (Wu, 1992), this can be explained by the flexibility of 15-crown-5 (Sun et al., 1999).
Thermal effect and the radius of cation
All formation enthalpies (ΔH1º) of the four complexes were negative values, and the absolute value of ΔH1º obviously increased with the extension of the ion radius (r). This tendency is also related to hydration of the guest cation, because the shorter the radius is, the stronger is the hydration. According to Huang (1983), the first order hydration number of the ion changes from 14 to 1 (or 2) with the cation alternation from Mg2+ to Ba2+. A cation must be partly or completely dehydrated before its association with Naph 15C5, which is an endothermic process. Consequently, energy released by interaction between the crown ether and cation is partly consumed by the dehydration of the cation. The radius of Mg2+ is the shortest one and its hydration is the strongest, so the formation of Mg2+ (Naph 15C5) is the weakest exothermic process (ΔH1º=−0.286 kJ/mol). Conversely, the radius of Ba2+ is the largest and its hydration is the weakest, and so the formation of Ba2+ (Naph 15C5) is the strongest exothermic process (ΔH1º=−66.02 kJ/mol). If only the static electric attraction between the cation and the five oxygen atoms in Naph 15C5 molecules were considered, the formation of Ba2+ (Naph 15C5) would be the weakest exothermic process.
Entropy effect and the hydration of cation
Interaction between Naph 15C5 and a cation leads to entropy decrease, but dehydration of the cation increases entropy. The data on the entropy effect in Table 2 indicate that the formation of Ca2+ (Naph 15C5) is accompanied by the strongest dehydration while the formation of Ba2+ (Naph 15C5) is accompanied by the weakest dehydration. In fact, the first order hydration numbers of Sr2+ and Ba2+ are only 1 or 2, much less than those of Mg2+ and Ca2+. Complexation between M2+ (M=Mg, Ca, Sr, Ba) and Naph 15C5 changes from an entropy driven process to an enthalpy driven one with the radius of the cation extending from 0.066 nm to 0.134 nm.
In summary, a titration microcalorimetric study indicated that the thermodynamic stability of complex of Naph 15C5 with alkaline earth ion is obviously different with different ion radius at 298.15 K in dilute aqueous solution, and that the complex Ca2+ (Naph 15C5) is a most stable one due to the synergism of static electric interaction and the size selectivity between the host and the guest.
Footnotes
Project (No. Y2003B01) supported by the Scientific Foundation of Shandong Province, China
References
- 1.Alizadeh N, Shamsipur M. Nuclear magnetic resonance study of the legend interchange of Ba2+-18-crown-6 complex in methanol solution. J. Solution Chem. 1996;25(10):1029–1129. [Google Scholar]
- 2.Bai G, Wang Y, Yan H. Therodynamics of interaction between cationic gemini surfactants and hydrophobically bodified polymers in aqueous solutions. J. Phys. Chem. B. 2002;106(9):2153–2159. [Google Scholar]
- 3.Bryan JC, Kavallieratos K, Sachleben RA. Unusual legend coordination for cesium. Inorg. Chem. 2000;39(7):1568–1572. doi: 10.1021/ic991203v. [DOI] [PubMed] [Google Scholar]
- 4.Claudia FC, Maria FS, Tomiyama C, Brito HF, Teotonio EE, Malta OL. Synthesis and luminescent properties of supromolecules of diketonate of Eu (III) and crown ethers as ligands. J. Solid State Chemistry. 2003;171(1/2):189–194. [Google Scholar]
- 5.Hamada T, Manabe K, Ishikawa S, Nagayama S, Shiro M, Kobayashi S. Catalytic asymmetric aldol reactions in aqueous media using chiral bis-pyridino-18-crown-6-rare earth metal triflate complexes. J. Am. Chem. Soc. 2003;125(10):2989. doi: 10.1021/ja028698z. [DOI] [PubMed] [Google Scholar]
- 6.Huang ZQ. Introduction of Theory about Electrolyte Solution. Beijing, China: Science Press; 1983. pp. 49–60. (in Chinese) [Google Scholar]
- 7.Jensen MP, Dzielawa JA, Rickert P, Dietz ML. Exafs investigations of the mechanism of facilitated ion transfer into a room temperature ionic liquid. J. Am. Chem. Soc. 2002;124(36):10664–10668. doi: 10.1021/ja027476y. [DOI] [PubMed] [Google Scholar]
- 8.Krzysztof J, Juliusz S, Luboch E. Kinetics of photochromic reactions in a 10-membered dibenzoazo crown ether. Chemical Physics. 2002;285(1):47–54. [Google Scholar]
- 9.Lehn JM. Supramolecular chemistry–Scope and perspectives, molecules, supermolecules, and molecular devices (Nobel Lecture) Chem. Int. Ed. Engl. Angew. 1988;27(1):89–112. [Google Scholar]
- 10.Levitskaia TG, Bryan JC, Sachleben RA, Lamb JD, Moyer BA. A surprising host-guest relationship between 1,2-dicloroethane and the cesium complex of tetrobenzo-24-crown-ether. J. Am. Chem. Soc. 2000;122(2):554–562. [Google Scholar]
- 11.Levitskaia TG, Sachleben RA, Bryan JC, Moyer BA. Sythesis, structure, and extraction behavior of 4,5′, 4′′,5′′′-tetra-tert-butytetrabenzo-24-crown-8. J. Chem. Soc., Perkin Trans. 2001;2(5):808–814. [Google Scholar]
- 12.Li HP, Wang PF, Wu SK. A new flavonecrown ether and its complexation with alkaline and alkaline earth metal cations. Chemical Journal of Chinese Universities. 1998;19(9):1431–1435. (in Chinese) [Google Scholar]
- 13.Lu XR, Zhang LF, Zhou XC. Polysioxane with pendant benzo crown ether via a spacer of undecyloxymethyl as stationary of capillary chromatography. Chemical Research in Chinese Universities. 1994;10(2):163–166. (in Chinese) [Google Scholar]
- 14.Pedersen CJ. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967;89(26):7017–7036. [Google Scholar]
- 15.Pellet-Rostaing S, Chitry F, Lemaire M, Barnier H, Federici V. Synthesis of water soluble molecular receptor from calyx[4] arene-bis-crown-6. Tetrahedron Lett. 1998;39(51):9443–9446. [Google Scholar]
- 16.Pellet-Rostaing S, Chitry F, Nicod L, Lemaire M. Synthesis and complexation properties of 1,3-alternate calyx[4] arene-bis (crown-6) derivatives. J. Chem. Soc., Perkin Trans. 2001;2(8):1426–1432. [Google Scholar]
- 17.Qin SY. Synthesis of dinaphtho-18-crown-6 and naphtha-15C5. Chemical Reagents (Huaxue Shiji) 1987;9(4):203–204. (in Chinese) [Google Scholar]
- 18.Rahman MM, Doe H, Sakurada N, Arakawa R. Facilitated ion-transfer of alkaline-earth metal cations by naphthao-15-crown-5 across the water. Electrochimica Acta. 2001;47(4):623–631. [Google Scholar]
- 19.Roberta C, Stefano DS, Luigi M. The bis-barium complex of a butterfly crown ether as a phototunable supramolecular catalyst. J. Am. Chem. Soc. 2003;125(8):2224–2227. doi: 10.1021/ja029331x. [DOI] [PubMed] [Google Scholar]
- 20.Shiozuma M, Takai Y, Kawamujra M, Takeda T, Sawada M. Complexation characteristics of permethylated cycloinulohexaose, cycloinuloheptaose, and cycloinuloocttaose with metal cations. J. Chem. Soc., Perkin Trans. 2001;2(8):1306–1314. [Google Scholar]
- 21.Stödeman M, Wadsö I. Scope of microcalorimetry in the area of microcyclic chemistry. Pure & Appl. Chem. 1995;67(7):1059–1068. [Google Scholar]
- 22.Sun DZ, Chen J, Lu WM, Zheng XM. Solution thermodynamics of some organic solutes in liquid film of dibenzo-18-crown-6. Indian J. Chem. 1999;38A(11):1195–1199. [Google Scholar]
- 23.Talanova GG, Elkarim NSA, Talanov VS, Hanes REJr, Hwang HS, Bartsch RA, Rogers RD. The “picrate effect” on extraction selectivities of aromatic group-containing crown ethers for alkali metal cations. J. Am. Chem. Soc. 1999;121(49):11281–11290. [Google Scholar]
- 24.Tanabe T, Takeshima M, Mikami A. Primary structure of the receptor for calcium channal blockers from skeletal muscle. Nature. 1987;328(1):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- 25.Vijaykumar SI, Ashwini KS. Complexation of macrocyclic compounds with mono-, di- and tri-valent transition and heavy metal ions in 90% (v/v) DMSO+water medium. Polyhedron. 2003;22(4):569–574. [Google Scholar]
- 26.Weast RC. CRC Handbook of Chemistry and Physics. 55th Edition. Ohio: CRC Press; 1974–1975. pp. 1–198. [Google Scholar]
- 27.Wu CT. Chemistry of Crown Ethers. Beijing, China: Science Press; 1992. pp. 114–156. (in Chinese) [Google Scholar]
- 28.Yi P, Yu Q, Lin R, Zong H. Coordination reactions of 18-crown-6 with the alkali ions in alcohol solvents. Acta Phys. Chim. 1997;13(6):569–572. [Google Scholar]
- 29.Zbigniew G, Andrzej S, Barbara MB. Preparation and decomposition of potassium alkalid-lipophilic crown ether complexes in tetrahydrofuran. J. Org. Chem. 2002;67(22):7807–7812. doi: 10.1021/jo026086r. [DOI] [PubMed] [Google Scholar]