Abstract
It was the general belief that DNA partitioning in prokaryotes is independent of a cytoskeletal structure, which in eukaryotic cells is indispensable for DNA segregation. Recently, however, immunofluorescence microscopy revealed highly dynamic, filamentous structures along the longitudinal axis of Escherichia coli formed by ParM, a plasmid-encoded protein required for accurate segregation of low-copy-number plasmid R1. We show here that ParM polymerizes into double helical protofilaments with a longitudinal repeat similar to filamentous actin (F-actin) and MreB filaments that maintain the cell shape of non-spherical bacteria. The crystal structure of ParM with and without ADP demonstrates that it is a member of the actin family of proteins and shows a domain movement of 25° upon nucleotide binding. Furthermore, the crystal structure of ParM reveals major differences in the protofilament interface compared with F-actin, despite the similar arrangement of the subunits within the filaments. Thus, there is now evidence for cytoskeletal structures, formed by actin-like filaments that are involved in plasmid partitioning in E.coli.
Keywords: DNA partitioning/F-actin/MreB/ParM
Introduction
The mitotic apparatus in eukaryotes is instrumental for the correct distribution of DNA among progeny cells (reviewed in Heald, 2000). It has long been thought that DNA segregation in bacteria is independent of cytoskeletal structures such as the mitotic spindle. However, new data on the rapid and direct translocation of newly replicated plasmids and origins of replication on bacterial chromosomes suggest a force-generating process (Niki and Hiraga, 1998; Jensen and Shapiro, 1999; Sharpe and Errington, 1999; Gordon and Wright, 2000). Although little is known about the molecular detail underlying bacterial DNA partitioning, recent studies on plasmid segregation have provided the first clues to a possible mechanism. Low-copy-number plasmids encode partitioning genes (par) (Austin and Abeles, 1983; Ogura and Hiraga, 1983; Gerdes et al., 1985) that are required for faithful segregation of the plasmid before cell division (for a recent review, see Gerdes et al., 2000). More recently, orthologues of the par genes were identified on bacterial chromosomes (Ogasawara and Yoshikawa, 1992; Ireton et al., 1994; Mohl and Gober, 1997). The directional movement and positioning of newly replicated plasmids and chromosomes are dependent on the par loci (for recent reviews, see Jensen and Shapiro, 1999; Sharpe and Errington, 1999; Gerdes et al., 2000; Møller-Jensen et al., 2000). All known par loci encode three components: a cis-acting centromere-like DNA sequence and two trans-acting proteins that together form a nucleoprotein complex (partitioning complex). Transcription of the par genes is autoregulated by binding of one or both trans-acting proteins to the par operon promoter region. The centromere-like region interacts with one of the trans-acting proteins. The other protein component of the partitioning complex is an ATPase that either belongs to the actin family of proteins, as determined by weak sequence homology, or is a Walker-type ATPase (Koonin, 1993). In the case of the nucleoprotein complex in Escherichia coli that actively segregates the low-copy-number plasmid R1, the ATPase is ParM (also known as StbA), the centromere-binding protein is ParR and the centromeric region is parC. Two plasmid molecules are paired at their centromeric regions through the interaction with ParR (Jensen et al., 1998). ParM interacts with the ParR–parC complex that stimulates its ATPase activity (Jensen and Gerdes, 1997; Møller-Jensen et al., 2002). A sequence database search revealed that the active site of ParM matches the consensus sequence of the actin family of proteins (Bork et al., 1992). However, this does not necessarily imply that the role of ParM is related to that of actin, as bacterial relatives of the actin superfamily of proteins have very diverse functions. Bacterial proteins that have been identified as belonging to the actin family of proteins include DnaK, FtsA, MreB and ParM. DnaK, whose ATPase domain is structurally related to actin, helps other proteins in the folding process (reviewed in Netzer and Hartl, 1998). The bacterial cell division protein FtsA is crucial for cytokinesis (Rothfield et al., 1999), but its precise role is not yet understood. Although the overall three-dimensional architecture of FtsA clearly indicates that it is a member of the actin family of proteins (van den Ent and Löwe, 2000), the second subdomain has a unique position, which probably prevents actin-like filament formation. The propensity of MreB to self-assemble into actin-like filaments, which, like F-actin, are indispensable for maintenance of cell shape has been the primary reason to regard MreB as a true bacterial actin homologue (Jones et al., 2001; van den Ent et al., 2001a).
In the past, cell division protein FtsZ was identified as the bacterial homologue of eukaryotic tubulin (Löwe and Amos, 1998). FtsZ polymerizes in an analogous fashion to tubulin and forms a ring-like structure that mediates bacterial cell division (Bi and Lutkenhaus, 1991; Addinall and Lutkenhaus, 1996; Erickson, 1998; Löwe and Amos, 1999). The existence in bacteria of these cytoskeletal structures comprising actin and tubulin homologues has eliminated an apparent fundamental difference between prokayotes and eukaryotes (van den Ent et al., 2001b).
The last predicted bacterial relative of the actin family of proteins in E.coli, ParM, has been investigated here. If a clear structural similarity between ParM and actin could be established, the role of bacterial cytoskeletal structures could be extended to DNA segregation. Recently, immunofluorescence microscopy showed that ParM of plasmid R1 forms highly dynamic, filamentous structures extending along the longitudinal axis of E.coli that are essential for the DNA partitioning process (Møller-Jensen et al., 2002). Also, in vitro, it has been shown that ParM self-assembles in an ATP-dependent manner (Møller-Jensen et al., 2002).
Are these filamentous structures actin-like filaments? Here, we show by electron microscope analyses that ParM self-assembles into double helical protofilaments with a longitudinal repeat similar to actin and MreB. The elucidation of the crystal structure of ParM demonstrates the structural homology to actin and MreB at the atomic level. The structure of ParM in both the unbound and the ADP-bound state was determined. A large rigid body movement between the two domains upon nucleotide binding is evident. Thus, the structural and functional properties of ParM imply a role for dynamic actin-like structures in plasmid R1 segregation.
Results and discussion
How actin like are ParM filaments?
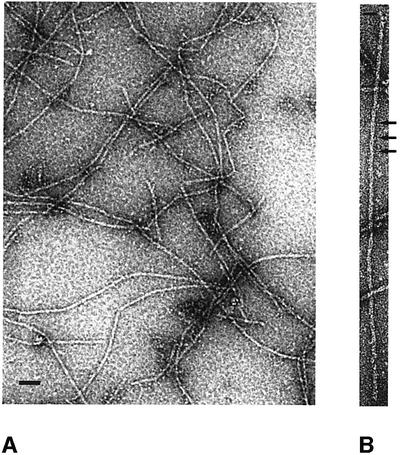
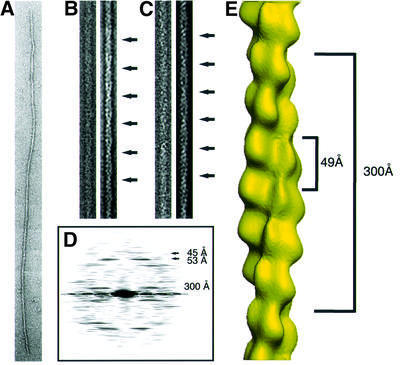
ParM self-assembles into filaments in the presence of ATP, ATPγS or AMPPNP, but not in the presence of ADP (Møller-Jensen et al., 2002). To investigate the nature of these filaments, negatively stained samples were examined under the electron microscope. As shown in Figure 1A, the majority of the protein polymerized into long, straight filaments. A closer look at those polymers revealed that they consist of two protofilaments that gently twist around each other, as shown at higher magnification (Figure 1B). This becomes even clearer in the filtered images (Figure 2B and C). The Fourier transform of the straightened polymer (Figure 2D) contains layer lines that form a pattern similar to that of eukaryotic filamentous actin (F-actin) and imply a regular helical arrangement (Table I). This is in contrast to the arrangement of the subunits of MreB (van den Ent et al., 2001a), which, in vitro, self-assemble into more or less straight polymers. The layer line at 300 Å indicates the crossover distance of the paired ParM protofilaments. The crossover distance in F-actin is variable, with an average length of 360 Å (Galkin et al., 2002). The upper group of layer lines indicate a longitudinal subunit spacing of 49 Å, which is very close to the 51 Å spacing found in MreB (van den Ent et al., 2001a) and the 55 Å spacing in F-actin (Holmes et al., 1990).
Fig. 1. Electron micrographs of negatively stained ParM filaments. (A) Typical overview of a grid containing ParM filaments. The protein (1 mg/ml) was incubated in polymerization buffer (30 mM Tris–HCl pH 7.5, 100 mM KCl, 2 mM MgCl2, 1 mM DTT) in the presence of 2 mM ATPγS for 5 min at room temperature. Scale bar, 100 nm. (B) ParM filament at higher magnification, showing the helical arrangement of the protofilaments. The crossovers are indicated by arrows. Scale bar, 100 nm.
Fig. 2. Analysis of electron micrographs of ParM. (A) A typical ParM filament before straightening. (B and C) The right panel shows the filtered image of the straightened filament in the left panel. The arrows indicate the crossovers of the two protofilaments. (D) Averaged diffraction pattern from six straightened filaments. The layer lines at 45 and 53 Å are derived from one-start left- and right-handed helices. The two-start helix produces the layer line of 300 Å. (E) Three-dimensional reconstruction. The distance of 49 Å between the subunits is the average between the layer lines from the one-start helices. The crossover distance of 300 Å is shown on the right.
Table I. Electron microscopy data on filament-forming actin-like proteins.
| F-actina | ParMb | MreBc | |
|---|---|---|---|
| Protofilament repeat (Å) | 55 | 49 | 51 |
| Crossover repeat (Å) | ∼360 | ∼300 | |
| Subunits/repeat | 13 | 12.5 | |
| Rotation (°) | 166.2 | 165.6 |
aThe values for F-actin are the average for uncomplexed muscle and yeast actin (Galkin et al., 2002).
bAs described in this paper.
cSee van den Ent et al. (2001a). Since the observed filaments of MreB are straight, only the longitudinal distance between the subunits is given.
Our electron microscope analyses show that ParM filaments can be regarded as a two-start helix with a twist slightly tighter than that of F-actin and a slightly shorter longitudinal spacing of 49 Å (Figure 2E). A helical rather than a straight arrangement of the subunits in ParM filaments should increase the strength of the filaments and provides an intrinsic limitation for lateral growth.
How actin like is ParM?
Despite the homology of ParM protofilaments to F-actin, evidence that the proteins are structurally similar at the atomic level has still to be obtained. To determine the three-dimensional structure of ParM, orthorhombic and tetragonal crystals were grown (see Materials and methods). The structure of selenomethionine (SeMet)-substituted ParM was solved by multiple anomalous dispersion (MAD; Table II). The structure of the native crystal (space group P21212) was determined to 2.3 Å resolution using the model of SeMet-substituted ParM. The tetragonal ADP-bound crystals (space group P41) diffracted to 2.0 Å. The crystals were perfectly twinned, which was facilitated by identical a- and b-axes, and hence appeared to have point group 422. The structure of the ADP-bound form was solved by molecular replacement and Patterson correlation refinement to overcome the large conformational shift between the two states of ParM. The model was refined using CNS1.1 (Brünger et al., 1998), assuming a twinning fraction of 50%. Refinement statistics are summarized in Table III.
Table II. Crystallographic data.
| P21212:a = 117.68 Åb = 72.47 Åc = 87.56 Å | P41: a = b = 64.71 Å c = 199.71 Å twinning fraction 50% | |||||
| Crystal |
λ (Å) |
SG |
Resolution (Å) |
I/σIa |
Rmb |
Multiplicityc |
| PEAK | 0.9792 | P21212 | 2.6 | 22.6 (9.6) | 0.057 | 6.3 |
| INFL | 0.9798 | P21212 | 2.6 | 21.8 (8.4) | 0.060 | 6.3 |
| HREM | 0.9393 | P21212 | 2.6 | 19.1 (7.2) | 0.082 | 6.3 |
| APO | 0.9393 | P21212 | 2.3 | 14.8 (3.4) | 0.064 | 3.1 |
| ADP | 0.9600 | P41 | 2.0 | 15.4 (4.6) | 0.064 | 4.1 |
aSignal-to-noise ratio of intensities, highest resolution bin in parentheses.
bRm = ΣhΣi|I(h,i) – I(h)|/ΣhΣiI(h,i), where I(h,i) are symmetry-related intensities and I(h) is the mean intensity of the reflection with unique index h.
cMultiplicity for unique reflections; for MAD datasets I(+) and I(–) are kept separate. Correlation coefficients of anomalous differences at different wavelengths for MAD experiment: PEAK versus INFL: 0.48; PEAK versus HREM: 0.59; INFL versus HREM: 0.33.
Table III. Refinement statistics.
| |
APO |
ADP |
| Residues | 1: 1–320; | A: 1–63; 68–320 |
| 2: 1–211, 217–319 | B: 1–63; 68–320 | |
| Water, cofactor | 282 | 370, Mg-ADP |
| Resolution (Å) | 2.3 | 2.0 |
| Twinning fractiona | 0.50 | |
| R-factor, Rfreeb | 0.217, 0.266 | 0.221, 0.252 |
| B averagec (Å2) | 42.7 | 37.6 |
| Geometry bonds (Å)/angles (°)d | 0.006, 1.193 | 0.008, 1.381 |
| Ramachandrane (%) | 88.5/0.0 | 90.8/0.0 |
| PDB IDf | 1MWK | 1MWM |
aTwinning fraction as used in refinement, operator h, –k, –l.
bFive per cent of reflections were randomly selected for determination of the free R-factor (taking twinning into account where appropriate), prior to any refinement.
cTemperature factors averaged for all atoms.
dR.m.s.ds from ideal geometry for bond lengths and restraint angles (Engh and Huber, 1991).
ePercentage of residues in the ‘most favoured region’ of the Ramachandran plot and percentage of outliers (PROCHECK; Laskowski et al., 1993).
fPDB identifiers for coordinates and structure factors, respectively.
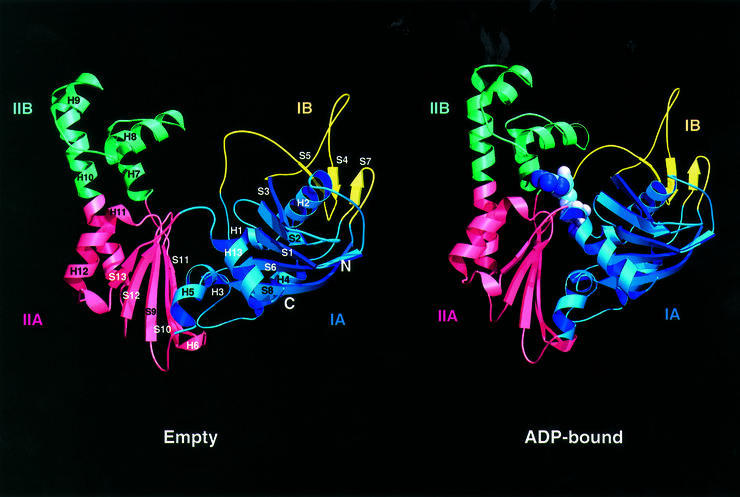
The elucidation of the crystal structure of ParM revealed that it is indeed closely related to actin and MreB. ParM has the characteristic fold of the actin family of proteins (Figure 3): two domains (I and II) with a nucleotide-binding pocket in the interdomain cleft (Kabsch and Holmes, 1995). Each domain is divided into two subdomains (A and B). The larger subdomains (IA and IIA) share a common fold that consists of a five-strand β-sheet surrounded by three α-helices. In general, the two smaller subdomains are more variable in size and structure.
Fig. 3. Ribbon plot of ParM in the empty form (left) and the ADP-containing form (right). ParM, as a member of the actin family of proteins, consists of two domains (I and II), which are divided into two subdomains (A and B). ADP binds in the interdomain cleft, which closes upon nucleotide binding through a rigid body movement of domains I and II. Images were created with MOLSCRIPT and RASTER3D (Kraulis, 1991; Merritt and Bacon, 1997).
Once ParM binds ADP, the interdomain cleft closes as domains I and II approach each other as rigid bodies (Figure 3). The domain movement upon ADP binding is ∼25°, with the connecting helix H5 (residues 152–154) acting as a mechanical hinge (as determined with the program Dyndom; Hayward et al., 1997). The rigid body movement validates the original nomenclature that divided the actin fold into two main domains (Bork et al., 1992), rather than the four domains conventionally assigned. The movement of the domains between nucleotide-free and -bound actin is smaller than the 25° rotation seen in ParM based on the nucleotide-free Arp2/3 and the structures of actin with nucleotide (Schutt et al., 1993; Otterbein et al., 2001; Robinson et al., 2001; Sablin et al., 2002). The domain movements in ParM are similar in magnitude to what has been suggested to occur within a tilted state of F-actin (Galkin et al., 2002).
ParM and the actin family of proteins
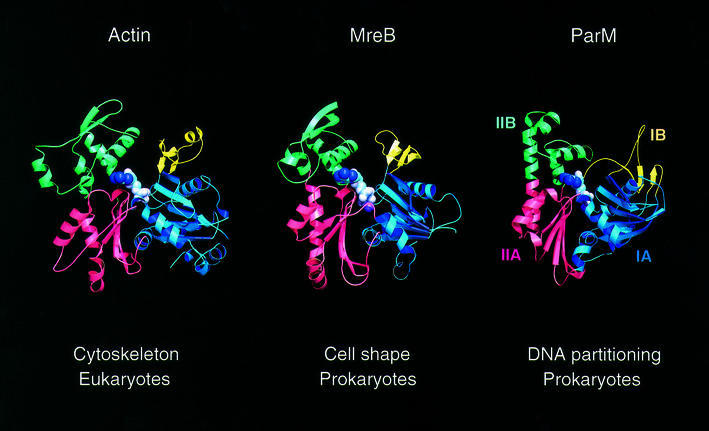
A three-dimensional similarity search (Holm and Sander, 1993) with the structure of ParM revealed MreB and actin as closest relatives (r.m.s.d. of 3.3 Å over 261 Cα atoms for actin; r.m.s.d. of 3.7 Å over 255 Cα atoms for MreB; see the legend to Figure 4). The other members of the actin family of proteins, HSC70 and FtsA, are more distantly related to ParM. The N-terminal region of chaperone HSC70 [constitutive Hsp70 protein, Protein Data Bank (PDB) entry 1ba1; Flaherty et al., 1990] is very similar to actin, MreB and ParM (it superimposes on ParM with an r.m.s.d. of 3.1 Å over 267 Cα atoms and a Z-score of 21.4), but the C-terminal region is unrelated to the actin family of proteins. Bacterial cell division protein FtsA has the second subdomain located at the opposite side of subdomain IA (van den Ent and Löwe, 2000). The remaining part of the protein superimposes reasonably well on ParM (r.m.s.d. of 3.3 Å over 248 Cα atoms and a Z-score of 19).
Fig. 4. Comparison of filament-forming proteins of the actin family of proteins. Eukaryotic actin is shown here in the ADP form (PDB entry 1J6Z). MreB is involved in the maintenance of cell shape in non-spherical bacteria (here shown in complex with AMPPNP; PDB entry 1JCG). ParM forms actin-like filaments in E.coli that are required for segregation of plasmid R1. It is shown here in the ADP form. Colour codes are the same as for Figure 3 (with subdomain IA in blue, IB in yellow, IIA in red and IIB in green). Subdomains IA, IB, IIA and IIB correspond to subdomains 1, 2, 3 and 4 in actin. ParM can be superimposed on 261 Cα atoms of actin (PDB entry 1yag-A) with an r.m.s.d. of 3.3 Å and a Z-score of 20.4. Similarly, it can be superimposed on MreB (PDB entry 1JCF) with an r.m.s.d. of 3.7 Å, for 255 Cα atoms, and a Z-score of 20.3 (Holm and Sander, 1993). As comparison, 310 Cα atoms of MreB superimpose on actin with an r.m.s.d. of 3.7 Å and a Z-score of 29.2 (van den Ent et al., 2001a).
Despite the structural similarity between ParM and actin/MreB, the sequence identity is low. A structure-based sequence alignment between ParM and actin (see Supplementary data available at The EMBO Journal Online) shows that <12% of the amino acids in ParM are identical to actin. The sequence similarity is equally low between ParM and MreB (11%), and only slightly higher between MreB and actin (15%; van den Ent et al., 2001a). The highest conservation is found in the residues that line the nucleotide-binding pocket, an observation that led to the prediction that ParM and MreB belong to the actin family of proteins (Bork et al., 1992). The active site of ParM containing Mg-ADP (see Supplementary data) can be superimposed on that of ADP-bound actin (Otterbein et al., 2001) with the main domains in approximately similar relative orientations. In both proteins, the magnesium ion is coordinated via an aspartate. Mutagenesis of this aspartate in ParM (D170A or D170E) impairs plasmid segregation (Jensen and Gerdes, 1997). Although the mutant protein still polymerizes, the filaments are no longer dynamic (Møller-Jensen et al., 2002). This indicates that the disassembly of the filaments requires ATP hydrolysis, whereas filament formation, as such, is independent of nucleotide hydrolysis.
Although the overall fold of ParM resembles actin (Figure 4), ParM has some unique features, which were unexpected for a protein whose filaments are almost indistinguishable from F-actin. Surprisingly, the differences are in regions that are involved in protofilament contacts. The first major difference is in subdomain IB. A long loop at the beginning of this subdomain is the most obvious difference. It partially overlaps with the DNase I-binding loop in actin. However, the loop in ParM does not change conformation upon ADP binding, which is in contrast to the DNase I-binding loop in actin (Kabsch et al., 1990; Egelman, 2001; Otterbein et al., 2001). Moreover, the overall fold of subdomain IB is unique for ParM. It is composed of a single sheet (7p4a5p) and lacks the α-helix found in the equivalent subdomains of MreB and ADP-bound actin. Unexpectedly, the third strand, S7, in ParM comes from an insertion in subdomain IA, a peculiarity not seen in any other member of the actin family. It has been shown for actin that subdomain IB adopts various conformations, dependent on the nucleotide state of the protein and the divalent cation bound by the active site (Belmont et al., 1999; Otterbein et al., 2001; Sablin et al., 2002). In the initial structure of actin co-crystallized with DNase I, the conformation of this subdomain is very similar to that of MreB (5a4p6a), with the DNase I-binding loop inserted between strands S4 and S5.
The two other major differences between ParM and actin/MreB may explain the smaller spacing between the subunits in the ParM protofilament. First, MreB and actin have a two-stranded β-sheet on top of the α-helices of subdomain IIB (Figure 4). The β-sheet makes intersubunit contacts along the protofilament of MreB, as has been observed in the crystal structure (van den Ent et al., 2001a), and is presumably also important for the assembly of F-actin (Holmes et al., 1990; Chen et al., 1993; Otterbein et al., 2001). In ParM, the β-sheet is replaced by a short helix (H9), a small loop and part of helix H10. Secondly, a short loop in ParM (connecting helix H10 and strand S12) substitutes a substantial helix in actin/MreB at the bottom of domain IIA (see the structure-based sequence alignment in Supplementary data), a helix that also forms part of the interface between the subunits of the MreB protofilament. The lack of this helix in sub domain IIA and the two-stranded β-sheet in subdomain IIB makes ParM shorter than MreB and actin.
Actin has an insertion in the long helix connecting subdomains IIA and IIB, named the hydrophobic plug. This loop is thought to hold the protofilaments together (Holmes et al., 1990; Chen et al., 1993). Since the hydrophobic plug is absent in MreB, it was speculated that it might actually induce the twist in F-actin. However, ParM also lacks the plug. Apparently, interactions other than the hydrophobic plug induce the twist and stabilize the filaments in ParM.
Atomic model of a ParM filament
Although the current resolution of the electron microscopy images is too low to show a ParM filament in detail, a putative model can be built based on the surface charge distribution of ParM and its similarities to actin/MreB.
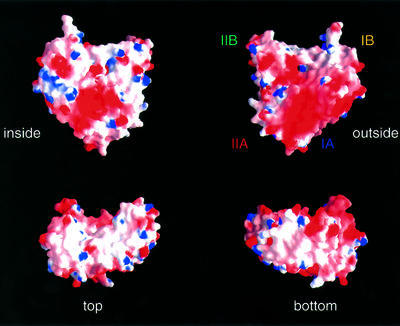
In the crystals of MreB, the protein is assembled into filaments, providing an accurate picture of the arrangement of the subunits in the protofilament for actin-like proteins and actin itself (van den Ent et al., 2001a). We assumed that the subunit arrangement in the ParM protofilament is analogous to MreB, therefore head to tail, and this matches with the surface charge distribution (Figure 5). The long, hydrophobic loop in subdomain IB (including residues 38–41) could slide into a hydrophobic pocket at the bottom of subdomain IA. From Figure 5, it is obvious that one of the flat surfaces is particularly acidic (right top corner of Figure 5). This region probably forms the outside of the filament, as has been postulated for actin. The inner surface is predominantly hydrophobic, which is expected for a protein–protein interface. Taking those assumptions into account, the crystal structure of ParM was assembled into a helical filament and superimposed on the density derived from the electron microscopy data (Figure 6). The polarity is fixed with the pointed end at the top compared with myosin-decorated F-actin, assuming that the same side of ParM forms the inside of the filament when compared with F-actin. As for the model of F-actin, it is currently not possible to accurately assign residues involved in the protofilament interface, because of the low-resolution data. However, it is obvious from both the structure-based sequence alignment between ParM and actin, and the variation in topology, that the interfaces must be different. The low degree of conservation of the residues in the protofilament interface was also noticed when the filaments of MreB and actin were compared, and co-evolution of residues at either site of the interface was proposed (van den Ent et al., 2001a).
Fig. 5. Surface charge distribution of ParM. The electrostatic potential [–6 kT (red) to +6 kT (blue)] is plotted onto the surface of ParM. The plots shown are a view from the outside of the filaments (‘outside’), and a view facing the second protofilament (‘inside’); ‘top’ and ‘bottom’ show the surface of the subunits after a 90° rotation around the x-axis, with respect to the plots in the upper half of the figure. Subdomains are labelled as in Figure 3. The figure was prepared using GRASP (Nicholls et al., 1991).
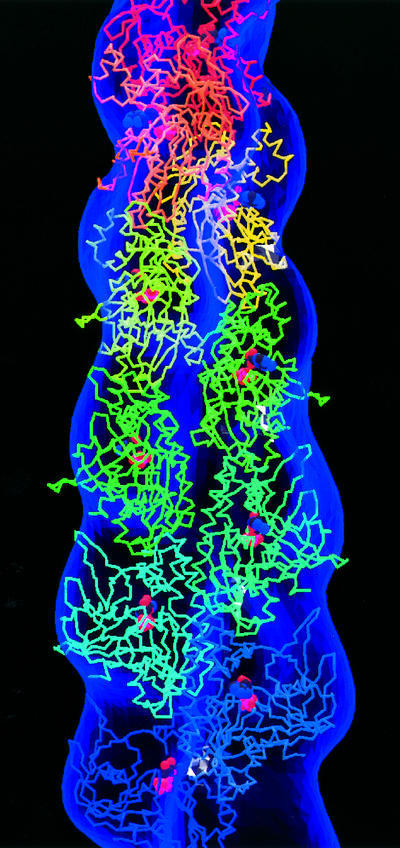
Fig. 6. Docking the crystal structure of ParM into the density derived from the negatively stained ParM filaments. ParM subunits are assembled head to tail into a protofilament. Each successive subunit is rotated by ∼166° and translated by 24.5 Å to create a helical filament. Every molecule of ParM is complexed with ADP and has a different rainbow colour, turning red toward the pointed end. The figure was created with MOLSCRIPT (Kraulis, 1991).
Actin-like filaments are involved in DNA partitioning
The elucidation of the ParM structure demonstrates that it is a close relative of the filamentous proteins MreB and actin. The most obvious differences are in regions that are likely to be involved in protofilament contacts. An amazing discrepancy between the two prokaryotic actin-like proteins is their propensity to form either straight (MreB) or twisted filaments (ParM) in vitro. Filament formation requires binding of ATP or a non-hydrolysable ATP analogue (van den Ent et al., 2001a; Møller-Jensen et al., 2002). ATP hydrolysis by ParM promotes filament dynamics presumably through a mechanism similar to that of F-actin, in which it is assumed that the energy released from the hydrolysis of ATP induces a conformational change in the protein, thereby weakening the bonds between the subunits in the filament. Subsequently, this would lead to depolymerization. An exceptional property of ParM is that it couples ATP hydrolysis to DNA partitioning in bacteria.
The actin-like properties of ParM explain its ability to form dynamic, fibrous structures observed by immunofluorescence microscopy (Møller-Jensen et al., 2002). It remains to be determined whether the mechanism used to carry the newly replicated plasmids toward the cell poles is similar to any of the two force-generating systems used by actin in eukaryotic cells. The DNA could move with the filaments of ParM via a treadmilling mechanism. The addition of new subunits to the ends of filaments may move the plasmid forward, a mechanism analogous to that occurring at the leading edge in amoeboid animal cells or in Listeria monocytogenes (Frishknecht and Way, 2001; Pantaloni et al., 2001). Alternatively, the ParM filaments could serve as a track on which motor proteins carry the plasmids to their destination, very much like the actin cytoskeleton, on which myosin moves its cargo. In either case, additional factors must be involved that determine the spatial organization of newly replicated plasmids and ensure directionality of plasmid distribution.
The crystallography and electron microscopy studies presented here show that ParM is a close relative of MreB and actin, both in its three-dimensional structure and in its propensity to form actin-like filaments. Actin has a variety of distinct roles in eukaryotic cells. MreB shares its role in the maintenance of cell shape. In contrast, ParM is required for plasmid partitioning, which adds a new function to bacterial actin-like filaments. A very interesting question raised by this finding is which of the force-generating mechanisms used by actin in eukaryotic cells is the basis for DNA partitioning in prokaryotes. The answer will provide us with important insights into this fundamental process in bacteria and may provide evidence for the prokaryotic ancestry of actin-based motility.
Materials and methods
Protein expression and purification
Native ParM (35.7 kDa) was overexpressed in E.coli BL21(DE3) cells (Stratagene) from pMD137 (Dam and Gerdes, 1994). The pMD137 plasmid vector contains the parM gene (SWISSPROT accession No. P11904) in front of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Ptac promoter. Cells were grown at 30°C in 2× TY medium supplemented with 0.2% glucose and 50 µg/ml kanamycin until OD600 = 0.6, when protein expression was induced by addition of 1 mM IPTG. After 3 h of induction, the cells were harvested and frozen in liquid nitrogen. Cells were resuspended in lysis buffer (30 mM Tris–HCl pH 7.5, 25 mM KCl, 1 mM MgCl2, 1 mM DTT, 0.1% Triton X-100) containing 0.2 mg/ml lysozyme and 5 µg/ml DNase I, and sonicated. The cell extract was cleared by centrifugation for 60 min at 100 000 g and ParM was precipitated by addition of ammonium sulfate to a final concentration of 10% (w/v). Precipitated protein was resuspended in buffer A (30 mM Tris–HCl pH 7.5, 25 mM KCl, 1 mM MgCl2, 1 mM DTT). ParM was precipitated by addition of 10 mM MgCl2 and 10 mM ATP, followed by immediate centrifugation for 15 min at 100 000 g. Remaining ParM protein in the supernatant was precipitated by a second addition of 10 mM ATP. This allowed high recovery of the protein at high purity. Finally, ParM was gel filtrated on a HiPrep Sephacryl S200 column (Amersham-Pharmacia) using buffer A. The protein, eluting as a single peak, was frozen in liquid nitrogen and stored at –80°C.
Electron microscopy and image processing
ParM filaments for electron microscopy were prepared by incubating 3.5 µM protein in polymerization buffer (30 mM Tris–HCl pH 7.5, 100 mM KCl, 2 mM MgCl2, 1 mM DTT) with 2 mM ATPγS for 5 min at ambient temperature. Filaments were diluted 1:10 in polymerization buffer, applied to glow-discharged carbon-coated copper grids and negatively stained using 1% uranyl acetate. Electron microscopy was performed at 80 kV using a Philips EM208 transmission electron microscope. Images were photographed at a magnification of 32 000–50 000×. Images of single filaments, initially selected for their straightness and length, were digitized using a Zeiss SCAI CCD scanner at 7 µm resolution and compressed to 28 µm pixels. They were analysed using MRC image-processing software (Crowther et al., 1996). Filtered images were obtained by calculating Fourier transforms of straightened images (see Figure 2), masking off a box around each layer line and back-transforming. For three-dimensional reconstruction, data from nine layer lines, including the equator, were extracted from the Fourier transforms of filaments that gave good symmetrical patterns, and their phases and amplitudes were compared. The data from six best filaments, with a total length of ∼3300 nm, were averaged after their phases had been adjusted to bring them into the same orientation. The resolution of the reconstructed image is ∼32 Å.
SeMet-substituted ParM
SeMet-substituted ParM was expressed in BL21(DE3). Methionine biosynthesis was inhibited as described previously (Van Duyne et al., 1993). A preculture, grown in 2× TY medium with 50 µg/ml kanamycin, was used at 1000-fold dilution to inoculate 250 ml of M9 minimal medium supplemented with 0.4% glucose, 2 mM MgSO4, 1 µg/ml thiamine and 50 µg/ml kanamycin. After growth overnight, the culture was diluted into 12 l of minimal medium and grown to an OD600 of 0.3, when 50 mg/l L-SeMet (Acros Organics), 50 mg/l leucine, isoleucine and valine, and 100 mg/l lysine, threonine and phenylalanine (Fluka) were added. After 15 min, IPTG (1 mM) was added to induce protein expression for 12 h at 30°C. SeMet-substituted ParM was purified as described for the native protein, except that 5 mM DTT was added to all solutions. The incorporation of SeMet was confirmed by electrospray ionization mass spectrometry [ParM observed 35 764.6 Da (calculated 35 765.6 Da); SeMet ParM observed 36 045.2 Da (calculated 36 046.9 Da)].
Crystallization and data collection
Crystals were grown by the sitting-drop vapor diffusion technique. Native and SeMet-substituted ParM protein crystallized in 1.4–1.6 M Na3 citrate pH 6.5 and 80–100 mM guanidine–HCl. The SeMet crystals were improved by the addition of 0.6 µl of a 30 (w/v) solution of 6-aminocaproic acid to the 6 µl droplet. Droplets composed of one part ParM protein (10 mg/ml) and one part mother liquor were equilibrated at 19°C. Crystals belonged to space group P21212 with two molecules per asymmetric unit. The crystals were flash frozen in mother liquor containing 12% glycerol. Native ParM co-crystallized with ADP in 100 mM Na cacodylate pH 6.2, 400 mM NaCl, 400 mM Mg acetate and 11% PEG 8000 at 4°C. Prior to crystallization, the protein (10 mg/ml) was mixed with 1 mM ADP and 1 mM MgCl2. Crystals belonged to space group P41 with two molecules in the asymmetric unit. The crystals had a twinning fraction of 0.5 (twin operators h, –k, –l) and showed symmetry consistent with point group 422. Crystals were soaked in mother liquor containing 30% PEG 400 and frozen in liquid nitrogen.
A MAD dataset as well as a native high-resolution dataset (APO) were collected on beamline ID14-4 (ESRF, Grenoble, France). Diffraction data from a frozen ParM–ADP crystal were collected at beamline 14-2 (SRS, Daresbury, UK). Crystallographic data are presented in Table II.
Structure determination and refinement
All data were indexed and integrated with MOSFLM (Leslie, 1991) and data were further processed using the CCP4 package (CCP4, 1994). Eight selenium sites were found using the program SOLVE (Terwilliger and Berendzen, 1999). The remaining four selenium sites were located using SHARP (de la Fortelle and Bricogne, 1997) and the initial phases were calculated with MLPHARE and SOLOMON (Abrahams and Leslie, 1996). A first model was built manually into the 2.6 Å MAD electron density map using the program MAIN (Turk, 1992). With this model, the 2.3 Å native crystal structure was solved by molecular replacement in CNS (Brünger et al., 1998). The ADP–ParM crystal structure was solved by molecular replacement after deletion of residues 29–69 of subdomain IB. Patterson correlation refinement allowing movement of domains I and II was essential. As the crystals were perfectly twinned, no attempt was made to de-twin the data prior to molecular replacement. All structures were built in MAIN2000 and refined using CNS1.1. Refinement statistics are summarized in Table III. Coordinates have been deposited in the PDB, entry code 1MWK (Apo form) and 1MWM (ADP-bound form).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Sew Peak-Chew for the mass spectrometry analyses and Thomas Leonard for critically reading the manuscript. J.M.-J. was financially supported by the Danish Biotechnology.
References
- Abrahams J.P. and Leslie,A.G.W. (1996) Methods used in the structure determination of bovine mitochondrial F-1 ATPase. Acta Crystallogr. D, 52, 30–42. [DOI] [PubMed] [Google Scholar]
- Addinall S.G. and Lutkenhaus,J. (1996) FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol. Microbiol., 22, 231–237. [DOI] [PubMed] [Google Scholar]
- Austin S. and Abeles,A. (1983) Partition of unit-copy miniplasmids to daughter cells.1. P1- and F-miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J. Mol. Biol., 169, 353–372. [DOI] [PubMed] [Google Scholar]
- Belmont L.D., Orlova,A., Drubin,D.G. and Egelman,E.H. (1999) A change in actin conformation associated with filament instability after P-i release. Proc. Natl Acad. Sci. USA, 96, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E.F. and Lutkenhaus,J. (1991) FtsZ ring structure associated with division in Escherichia coli. Nature, 354, 161–164. [DOI] [PubMed] [Google Scholar]
- Bork P., Sander,C. and Valencia,A. (1992) An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin and Hsp70 heat shock proteins. Proc. Natl Acad. Sci. USA, 89, 7290–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünger A. et al. (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- CCP4 (1994) The CCP4 Suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. [DOI] [PubMed] [Google Scholar]
- Chen X., Cook,R.K. and Rubenstein,P.A. (1993) Yeast actin with a mutation in the hydrophobic plug between subdomain-3 and subdomain-4 (L266D) displays a cold-sensitive polymerization defect. J. Cell Biol., 123, 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R.A., Henderson,R. and Smith,J.M. (1996) MRC image processing programs. J. Struct. Biol., 116, 9–16. [DOI] [PubMed] [Google Scholar]
- Dam M. and Gerdes,K. (1994) Partitioning of plasmid R1—10 direct repeats flanking the parA promoter constitute a centromere-like partition site parC, that expresses incompatibility. J. Mol. Biol., 236, 1289–1298. [DOI] [PubMed] [Google Scholar]
- de la Fortelle E. and Bricogne,G. (1997) Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Macromol. Crystallogr. A, 276, 472–494. [DOI] [PubMed] [Google Scholar]
- Egelman E.H. (2001) Molecular evolution: actin’s long lost relative found. Curr. Biol., 11, R1022–R1024. [DOI] [PubMed] [Google Scholar]
- Engh R.A. and Huber,R. (1991) Accurate bond and angle parameters for x-ray protein-structure refinement. Acta Crystallogr. A, 47, 392–400. [Google Scholar]
- Erickson H.P. (1998) Atomic structures of tubulin and FtsZ. Trends Cell Biol., 8, 133–137. [DOI] [PubMed] [Google Scholar]
- Flaherty K.M., Delucaflaherty,C. and McKay,D.B. (1990) 3-dimensional structure of the ATPase fragment of a 70k heat-shock cognate protein. Nature, 346, 623–628. [DOI] [PubMed] [Google Scholar]
- Frishknecht F. and Way,M. (2001) Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol., 11, 30–38. [DOI] [PubMed] [Google Scholar]
- Galkin V.E., VanLoock,M.S., Orlova,A. and Egelman,E.H. (2002) A new internal mode in F-actin helps explain the remarkable evolutionary conservation of actin’s sequence and structure. Curr. Biol., 12, 570–575. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Larsen,J.E.L. and Molin,S. (1985) Stable inheritance of plasmid R1 requires two different loci. J. Bacteriol., 161, 292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K., Moller-Jensen,J. and Jensen,R.B. (2000) Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol., 37, 455–466. [DOI] [PubMed] [Google Scholar]
- Gordon G.S. and Wright,A. (2000) DNA segregation in bacteria. Annu. Rev. Microbiol., 54, 681–708. [DOI] [PubMed] [Google Scholar]
- Hayward S., Kitao,A. and Berendsen,H.J.C. (1997) Model-free methods of analyzing domain motions in proteins from simulation: a comparison of normal mode analysis and molecular dynamics simulation of lysozyme. Proteins, 27, 425–437. [DOI] [PubMed] [Google Scholar]
- Heald R. (2000) Motor function in the mitotic spindle. Cell, 102, 399–402. [DOI] [PubMed] [Google Scholar]
- Holm L. and Sander,C. (1993) Protein-structure comparison by alignment of distance matrices. J. Mol. Biol., 233, 123–138. [DOI] [PubMed] [Google Scholar]
- Holmes K.C., Popp,D., Gebhard,W. and Kabsch,W. (1990) Atomic model of the actin filament. Nature, 347, 44–49. [DOI] [PubMed] [Google Scholar]
- Ireton K., Gunther,N.W. and Grossman,A.D. (1994) Spo0j is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol., 176, 5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R.B. and Gerdes,K. (1997) Partitioning of plasmid R1. The ParM protein exhibits ATPase activity and interacts with the centromere-like ParR–parC complex. J. Mol. Biol., 269, 505–513. [DOI] [PubMed] [Google Scholar]
- Jensen R.B. and Shapiro,L. (1999) Chromosome segregation during the prokaryotic cell division cycle. Curr. Opin. Cell Biol., 11, 726–731. [DOI] [PubMed] [Google Scholar]
- Jensen R.B., Lurz,R. and Gerdes,K. (1998) Mechanism of DNA segregation in prokaryotes: replicon pairing by parC of plasmid R1. Proc. Natl Acad. Sci. USA, 95, 8550–8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.J.F., Carballido-Lopez,R. and Errington,J. (2001) Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell, 104, 913–922. [DOI] [PubMed] [Google Scholar]
- Kabsch W. and Holmes,K.C. (1995) Protein motifs 2. The actin fold. FASEB J., 9, 167–174. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz,H.G., Suck,D., Pai,E.F. and Holmes,K.C. (1990) Atomic structure of the actin DNaseI complex. Nature, 347, 37–44. [DOI] [PubMed] [Google Scholar]
- Koonin E.V. (1993) A superfamily of ATPases with diverse functions containing either classical or deviant ATP-binding motif. J. Mol. Biol., 229, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]
- Laskowski R.A., MacArthur,M.W., Moss,D.S. and Thornton,J.M. (1993) Procheck—a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr., 26, 283–291. [Google Scholar]
- Leslie A.G.W. (1991) Recent Changes to the MOSFLM Package for Processing Film and Image Plate Data. SERC Laboratory, Daresbury, Warrington, UK.
- Löwe J. and Amos,L.A. (1998) Crystal structure of the bacterial cell-division protein FtsZ. Nature, 391, 203–206. [DOI] [PubMed] [Google Scholar]
- Löwe J. and Amos,L.A. (1999) Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J., 18, 2364–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt E.A. and Bacon,D.J. (1997) Raster3D: photorealistic molecular graphics. Macromol. Crystallogr. B, 277, 505–524. [DOI] [PubMed] [Google Scholar]
- Mohl D.A. and Gober,J.W. (1997) Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell, 88, 675–684. [DOI] [PubMed] [Google Scholar]
- Møller-Jensen J., Jensen,R.B. and Gerdes,H. (2000) Plasmid and chromosome segregation in prokaryotes. Trends Microbiol., 8, 313–320. [DOI] [PubMed] [Google Scholar]
- Møller-Jensen J., Jensen,R.B., Löwe,J. and Gerdes,K. (2002) Prokaryotic DNA segregation by an actin-like filament. EMBO J., 21, 3119–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer W.J. and Hartl,F.U. (1998) Protein folding in the cytosol: chaperonin-dependent and -independent mechanisms. Trends Biochem. Sci., 23, 68–73. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp,K.A. and Honig,B. (1991) Protein folding and association—insights from the interfacial and thermodynamic properties of hydrocarbons. Prot. Struct. Func. Genet. 11, 281–296. [DOI] [PubMed] [Google Scholar]
- Niki H. and Hiraga,S. (1998) Polar localization of the replication origin and terminus in Escherichia coli nucleoids during chromosome partitioning. Genes Dev., 12, 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N. and Yoshikawa,H. (1992) Genes and their organization in the replication origin region of the bacterial chromosome. Mol. Microbiol., 6, 629–634. [DOI] [PubMed] [Google Scholar]
- Ogura T. and Hiraga,S. (1983) Partition mechanism of F-plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell, 32, 351–360. [DOI] [PubMed] [Google Scholar]
- Otterbein L.R., Graceffa,P. and Dominguez,R. (2001) The crystal structure of uncomplexed actin in the ADP state. Science, 293, 708–711. [DOI] [PubMed] [Google Scholar]
- Pantaloni D., Le Clainche,C. and Carlier,M.F. (2001) Mechanism of actin-based motility. Science, 292, 1502–1506. [DOI] [PubMed] [Google Scholar]
- Robinson R.C., Turbedsky,K., Kaiser,D.A., Marchand,J.B., Higgs,H.N., Choe,S. and Pollard,T.D. (2001) Crystal structure of Arp2/3 complex. Science, 294, 1679–1684. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Justice,S. and Garcia-Lara,J. (1999) Bacterial cell division. Annu. Rev. Genet., 33, 423–448. [DOI] [PubMed] [Google Scholar]
- Sablin E.P., Dawson,J.F., VanLoock,M.S., Spudich,J.A., Egelman,E.H. and Fletterick,R.J. (2002) How does ATP hydrolysis control actin’s associations? Proc. Natl Acad. Sci. USA, 99, 10945–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt C.E., Myslik,J.C., Rozycki,M.D., Goonesekere,N.C.W. and Lindberg,U. (1993) The structure of crystalline profilin β-actin. Nature, 365, 810–816. [DOI] [PubMed] [Google Scholar]
- Sharpe M.E. and Errington,J. (1999) Upheaval in the bacterial nucleoid—an active chromosome segregation mechanism. Trends Genet., 15, 70–74. [DOI] [PubMed] [Google Scholar]
- Terwilliger T.C. and Berendzen,J. (1999) Automated MAD and MIR structure solution. Acta Crystallogr. D, 55, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. (1992) Weiterentwicklung eines Programms für Molekülgrafik und Elektrondichte-Manipulation und seine Anwendung auf verschiedene Protein-Strukturaufklärungen. PhD thesis, Technical University of Munich, Munich, Germany.
- van den Ent F. and Löwe,J. (2000) Crystal structure of the cell division protein FtsA from Thermotoga maritima. EMBO J., 19, 5300–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F., Amos,L.A. and Löwe,J. (2001a) Prokaryotic origin of the actin cytoskeleton. Nature, 413, 39–44. [DOI] [PubMed] [Google Scholar]
- van den Ent F., Amos,L. and Löwe,J. (2001b) Bacterial ancestry of actin and tubulin. Curr. Opin. Microbiol., 4, 634–638. [DOI] [PubMed] [Google Scholar]
- van Duyne G.D., Standaert,R.F., Karplus,P.A., Schreiber,S.L. and Clardy,J. (1993) Atomic structures of the human immunophilin Fkbp-12 complexes with Fk506 and rapamycin. J. Mol. Biol., 229, 105–124. [DOI] [PubMed] [Google Scholar]