Abstract
Store-operated Ca2+ channels, which are activated by the emptying of intracellular Ca2+ stores, provide one major route for Ca2+ influx. Under physiological conditions of weak intracellular Ca2+ buffering, the ubiquitous Ca2+ releasing messenger InsP3 usually fails to activate any store-operated Ca2+ entry unless mitochondria are maintained in an energized state. Mitochondria rapidly take up Ca2+ that has been released by InsP3, enabling stores to empty sufficiently for store-operated channels to activate. Here, we report a novel role for mitochondria in regulating store-operated channels under physiological conditions. Mitochondrial depolarization suppresses store-operated Ca2+ influx independently of how stores are depleted. This role for mitochondria is unrelated to their actions on promoting InsP3-sensitive store depletion, can be distinguished from Ca2+-dependent inactivation of the store-operated channels and does not involve changes in intracellular ATP, oxidants, cytosolic acidification, nitric oxide or the permeability transition pore, but is suppressed when mitochondrial Ca2+ uptake is impaired. Our results suggest that mitochondria may have a more fundamental role in regulating store-operated influx and raise the possibility of bidirectional Ca2+-dependent crosstalk between mitochondria and store-operated Ca2+ channels.
Keywords: Ca2+ influx/mitochondria/store-operated Ca2+ channels
Introduction
Store-operated (capacitative) Ca2+ influx, in which a fall in the Ca2+ content of the endoplasmic reticulum (ER) opens Ca2+ channels in the plasma membrane, is one of the more widespread mechanisms whereby mammalian non-excitable cells can increase their intracellular free calcium concentration (Putney, 1986; Parekh and Penner, 1997). In many cell types, store-operated Ca2+ entry is manifested as a non-voltage-gated, inwardly rectifying Ca2+ current called ICRAC (Hoth and Penner, 1992; Parekh and Penner, 1997).
In addition to their role as the main energy-producing centres of eukaryotic cells, much recent work has established that mitochondria can and do take up Ca2+ rapidly following physiological levels of cell stimulation, and then subsequently release this Ca2+ slowly back into the cytosol (Pozzan et al., 1994; Duchen, 2000; Rutter and Rizzuto, 2000). Mitochondrial Ca2+ uptake occurs via a relatively low affinity ruthenium red-sensitive uniporter that is driven by the large inner mitochondrial membrane potential (Pozzan et al., 1994). Mitochondria can take up Ca2+ that has been released from intracellular stores by the opening of either InsP3- or ryanodine-sensitive Ca2+ channels on the stores (Rizzuto et al., 1993, 1998; Jouaville et al., 1995; Hajnoczky et al., 1999; Tinel et al., 1999; Montero et al., 2000; Park et al., 2000). Alternatively, they can sequester Ca2+ that enters the cell via voltage-gated or store-operated CRAC channels (Lawrie et al., 1996; Babcock et al., 1997; Hoth et al., 1997; Gilabert and Parekh, 2000; Park et al., 2000).
Changes in mitochondrial Ca2+ dynamics have numerous effects on cell physiology. Buffering of cytosolic Ca2+ by respiring mitochondria is seen in many diverse cell types, where it shapes the profile of the cytosolic Ca2+ signal (Duchen, 2000; Rutter and Rizzuto, 2000). An increase in mitochondrial matrix Ca2+, following rapid Ca2+ uptake, stimulates key rate-limiting dehydrogenase enzymes of the Krebs cycle (McCormack et al., 1990; Hajnoczky et al., 1995), resulting in an increase in ATP production (Jouaville et al., 1999). In the polarized pancreatic acinar cell, mitochondria form a belt that surrounds the apical area, the latter containing the zymogen-containing secretory granules (Tinel et al., 1999). Mitochondrial Ca2+ uptake in the belt region restricts Ca2+ signals from propagating out of the apical zone and hence exocytosis is restricted to the apical area. In chromaffin cells, mitochondria seem to exist in functional units with ryanodine-sensitive Ca2+ release channels on the stores and voltage-operated Ca2+ channels in the plasma membrane (Montero et al., 2000). Following stimulation, rapid intra-mitochondrial Ca2+ transients in excess of 1 mM occur and this sequestration of Ca2+ determines the pattern of the secretory response.
Respiring mitochondria are also very important in determining the pattern of activation and inactivation of CRAC channels (Gilabert and Parekh, 2000; Gilabert et al., 2001). Under physiological conditions of weak intracellular Ca2+ buffering, the ubiquitous second messenger InsP3 generally fails to evoke any detectable whole-cell (macroscopic) ICRAC (Broad et al., 1999; Fierro and Parekh, 2000; Glitsch and Parekh, 2000). It appears that InsP3, in spite of releasing Ca2+ from the stores, fails to empty the stores sufficiently and/or long enough for ICRAC to activate (Parekh et al., 1997). However, if precautions are taken to maintain mitochondria in an energized state, InsP3 is now able to activate ICRAC in physiological buffer (Gilabert and Parekh, 2000; Gilabert et al., 2001). Mitochondria take up some of the Ca2+ that has been released from the stores and this results in greater store depletion and hence activation of the current. In addition, mitochondrial Ca2+ uptake reduces the extent of Ca2+-dependent inactivation of CRAC channels and slows its rate of development.
Here, we find that mitochondria play a central role in the ability of CRAC channels to activate under physiological conditions, even when stores are emptied irreversibly and independently of InsP3 receptors. The involvement of mitochondria under these conditions is unrelated to actions in promoting store depletion. The requirement for respiring mitochondria can also be distinguished from Ca2+-dependent inactivation of the CRAC channels. Instead, our results suggest that mitochondria seem to have a more fundamental role in regulating CRAC channels than has been hitherto suspected. Although the underlying mechanism is as yet unclear, it is not affected by anti-oxidants, acidification of the cytosol, nitric oxide or the permeability transition pore. One possibility is that mitochondria release factor(s), in a Ca2+-dependent manner, which are particularly important in regulating CRAC channels under physiological conditions. Our results support a novel role for mitochondria in regulating the activity of the widely expressed CRAC channels.
Results
Mitochondrial depolarization reduces calcium entry after store depletion
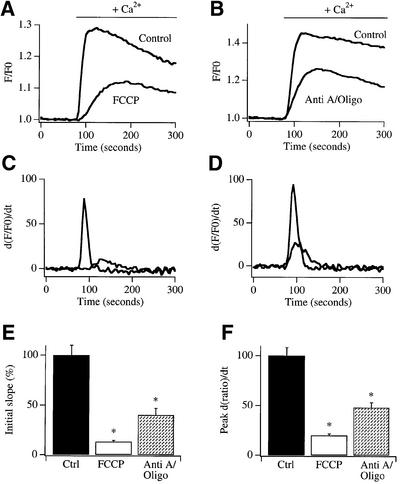
Cells loaded with the Ca2+-sensitive fluorescent dye Fura 2 were exposed to the SERCA pump blocker thapsigargin in Ca2+-free external solution for 10–20 min in order to empty the intracellular Ca2+ stores and open the CRAC channels in the plasma membrane. Thereafter, external Ca2+ was readmitted. This resulted in a rapid increase in cytosolic Ca2+, which then decayed slowly (see controls in Figure 1A and B). However, if mitochondria were depolarized after store depletion by exposure to either the protonophore FCCP (5 µM, which collapses the proton motive force across the inner mitochondrial membrane) or a mixture of antimycin A and oligomycin (to inhibit complex III of the respiratory chain and the mitochondrial F0F1 ATP synthase, respectively), subsequent readmission of Ca2+ generated a smaller Ca2+ signal, which developed more slowly (Figure 1A and B). We measured the initial rate of rise of the Ca2+ signal (initial slope) as this is a better indicator of the number of open CRAC channels than the amplitude of the peak signal. The slope was significantly reduced by either FCCP or antimycin A plus oligomycin (Figure 1E), compared with control recordings from the same cell preparations. To obtain more quantitative information on the effects of mitochondrial depolarization upon Ca2+ influx, the Ca2+ entry signal for both control cells and those exposed to either FCCP (Figure 1A) or antimycin A plus oligomycin (Figure 1B) was differentiated, and the corresponding graphs are shown in Figure 1C and D, respectively. The peak of the differentiated signal is another indicator of the maximal rate of Ca2+ entry and, like the slope, this parameter was also significantly reduced by mitochondrial depolarization (Figure 1F).
Fig. 1. Mitochondrial depolarization reduces the rate of Ca2+ entry in intact cells. After depleting stores with thapsigargin in Fura 2-loaded cells, readmission of external Ca2+ (2 mM) resulted in a rapid increase in cytosolic Ca2+ concentration (control). This was substantially reduced by depolarizing mitochondria with either 5 µM FCCP (A) or antimycin A (5 µg/ml) plus oligomycin (0.5 µg/ml) (B). The differentiated signals are shown in (C) and (D). (E) Bar chart comparing the initial slope of the Ca2+ signal upon Ca2+ readmission in control cells with those pre-exposed to either FCCP or antimycin A plus oligomycin. Numbers of cells (n) for each condition are: control (ctrl) 40, FCCP 38, antimycin A plus oligomycin (Anti A/Oligo) 35. (F) Bar chart plotting the peak rate of Ca2+ increase (obtained from the differentiated signal) for the conditions shown. *p < 0.01.
Because thapsigargin was used to deplete the stores irreversibly and mitochondrial depolarization occurred after thapsigargin had emptied the stores, the role of mitochondria is clearly distal to store emptying. This is fundamentally different from the effects of mitochondria in enabling InsP3 to activate ICRAC under physiological conditions (Gilabert and Parekh, 2000). In this latter case, by taking up some of the Ca2+ released by InsP3, mitochondria promote greater store depletion and hence activation of ICRAC.
Known Ca2+-dependent inactivation mechanisms cannot fully explain the inhibition of Ca2+ influx following mitochondrial depolarization
The reduction in Ca2+ influx following mitochondrial depolarization in Figure 1 cannot be explained solely by Ca2+-dependent inactivation of CRAC channels. In rat basophilic leukaemia (RBL-1) cells, there are at least three mechanisms whereby an increase in cytosolic Ca2+ can lead to inactivation of ICRAC. Ca2+-dependent fast inactivation occurs at a site within a few nanometres of the pore (Fierro and Parekh, 1999a) and is unaffected either by energizing mitochondria (Gilabert and Parekh, 2000) or by depolarizing them (extent of inactivation at the end of a 200 ms hyperpolarizing pulse to –100 mV was 53.0 ± 1% after exposure to antimycin A plus oligomycin, versus 50.0 ± 1% for control cells; five cells each condition). Hence Ca2+-dependent fast inactivation is unlikely to be involved here. Ca2+-dependent store refilling deactivates the current but this is prevented by thapsigargin (Bakowski et al., 2001). Because we had exposed cells continuously to thapsigargin (Figure 1), it is unlikely that refilling took place to any appreciable extent. Ca2+ entry-dependent but store-independent inactivation requires a more global increase in Ca2+ (being suppressed by the slow chelator EGTA) and develops slowly with a half-time of around 60 s (Gilabert and Parekh, 2000). One would therefore expect it to be manifest after a prominent initial rate of calcium entry, but this was clearly not the case (Figure 1). Although these well-characterized Ca2+-dependent inactivation mechanisms seem unlikely to fully account for the inhibition of Ca2+ influx that occurs following mitochondrial depolarization, we nevertheless considered other potential Ca2+-dependent mechanisms. One possibility is that mitochondria rapidly take up much of the Ca2+ that has been released from the stores by thapsigargin, but then release this Ca2+ upon depolarization. This release may result in a form of Ca2+-dependent slow inactivation of the channels. Two arguments are hard to reconcile with this. First, application of FCCP to cells pre-exposed to thapsigargin in Ca2+-free solution failed to produce any detectable Ca2+ increase (9/10 cells, data not shown). Hence it appears unlikely that mitochodrial depolarization results in a global Ca2+ increase following slow store emptying with thapsigargin. Secondly, dialysis with a pipette solution containing 1 µM Ca2+ did not prevent thapsigargin from evoking clear ICRAC (our unpublished observations). Therefore, in order for mitochondrial Ca2+ release to inactivate CRAC channels, an increase in cytosolic Ca2+ levels exceeding 1 µM globally would be required. It is conceivable that mitochondria take up Ca2+ following slow release from the stores and then release this Ca2+ locally, following depolarization, just below the plasma membrane such that the Ca2+ concentration exceeds 1 µM and inactivates the CRAC channels. But this explanation does not sit easily with the finding that fast Ca2+-dependent inactivation, reflecting local feedback of permeating Ca2+ on the CRAC channels, is not affected by either energizing (Gilabert and Parekh, 2000) or depolarizing mitochondria.
Collectively, the effects of mitochondrial depolarizing agents suggest a new role for mitochondria in regulating CRAC channel activity in intact cells. The following experiments were designed to establish the nature of this regulation.
Mitochondrial depolarization suppresses ICRAC even when the membrane potential is controlled
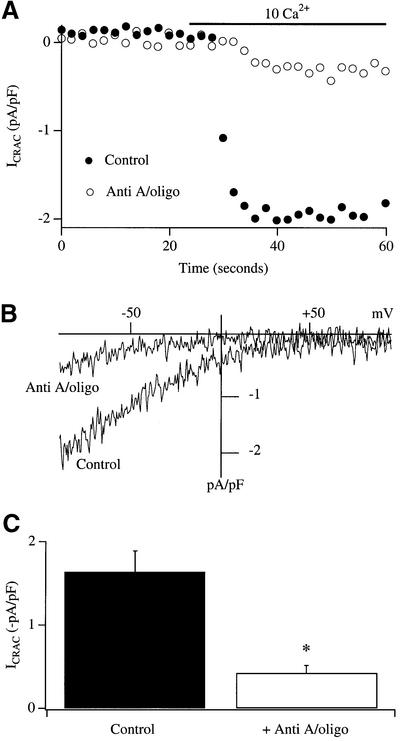
In the experiments of Figure 1, the cell membrane potential was not controlled but was free to fluctuate. It is therefore possible that mitochondrial depolarization somehow depolarizes the membrane potential, thereby reducing the electric driving force for Ca2+ entry through CRAC channels, as has been suggested for the effects of the protonophore (CCCP) on antigen-stimulated Ca2+ influx (Mohr and Fewtrell, 1987). To test this directly, we carried out patch–clamp experiments where the membrane potential was experimentally controlled. Figure 2 summarizes experiments using the perforated patch technique, which enables the current to be recorded with minimal perturbation of the cytoplasm under voltage clamp conditions. The protocol employed was identical to that used above for Fura 2-loaded cells and was as follows. Cells were first exposed to 2 µM thapsigargin in Ca2+-free external solution. Vehicle (DMSO) or antimycin A plus oligomycin was then added in Ca2+-free solution (in the presence of thapsigargin) and a seal was then formed. After obtaining background currents, Ca2+ was readmitted to the cell and the development of ICRAC was followed. A typical control recording is shown in Figure 2A (filled circles), the current–voltage relationship in Figure 2B, and aggregate data in Figure 2C (n = 5). However, if mitochondria were depolarized prior to the readmission of external Ca2+, the size of the current was significantly reduced (Figure 2; five cells, p < 0.002). Two conclusions can be drawn from these experiments. First, the reduction in Ca2+ influx following mitochondrial depolarization after store depletion cannot be explained simply by a reduction in electric driving force at the plasma membrane. Although this effect may contribute to the slightly greater reduction in the rate of Ca2+ influx seen after exposure to FCCP compared with antimycin A and oligomycin (Figure 1E), it is clearly not the dominant mechanism. Secondly, mitochondrial regulation of ICRAC is maintained in the perforated patch configuration and is likely therefore to be of physiological relevance.
Fig. 2. Mitochondrial depolarization reduces ICRAC in the perforated patch configuration. (A) Time course of ICRAC in a control cell is compared with one in which mitochondria were depolarized by a 20 min exposure to antimycin A plus oligomycin, prior to Ca2+ readmission. (B) The current–voltage relationships for the cells shown in (A) are depicted. These were taken after 50 s. (C) Aggregate data comparing the size of ICRAC between control cells and those with depolarized mitochondria. In these experiments, mitochondria were depolarized after store depletion with thapsigargin.
Mitochondria are required for thapsigargin to evoke ICRAC in the whole-cell configuration
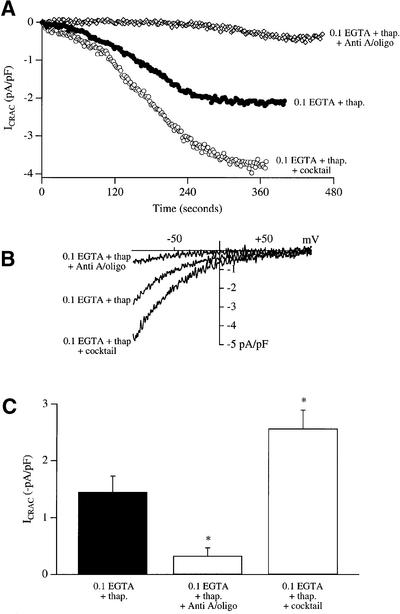
Thapsigargin is able to activate ICRAC routinely in weak intracellular Ca2+ buffer (0.1 mM EGTA) in the whole-cell patch–clamp configuration, whereas InsP3 is ineffective under these conditions (Fierro and Parekh, 2000). A typical recording of the activation of ICRAC by thapsigargin is shown in Figure 3A (filled circles, labelled 0.1 EGTA + thap.), the current–voltage relationship in Figure 3B, and aggregate data in Figure 3C (n = 7). The current developed relatively slowly as expected, since it is determined by the passive leakage of Ca2+ across the ER. Strikingly, if mitochondria were depolarized with antimycin A and oligomycin prior to exposure to thapsigargin in weak Ca2+ buffer, the subsequent size of the current was dramatically reduced (open diamonds in Figure 3A, ramp I–V in Figure 3B and pooled data in Figure 3C, n = 9; p < 0.001 compared with control). In the whole-cell configuration, mitochondrial Ca2+ uptake can be maintained by including a cocktail solution that maintains mitochondria in an energized state (Gilabert and Parekh, 2000). To see if the size of ICRAC evoked by thapsigargin could be enhanced by energized mitochondria, we dialysed cells with thapsigargin plus mitochondrial cocktail in weak buffer. Under these conditions, the size of the current was increased significantly (Figure 3, n = 8, p < 0.01 relative to control). Mitochondrial Ca2+ uptake plays an important role in the ability of ICRAC to activate under physiological conditions of weak intracellular Ca2+ buffering, even when the current is evoked by depleting stores with thapsigargin.
Fig. 3. Mitochondrial Ca2+ buffering regulates the extent of ICRAC evoked by thapsigargin in weak Ca2+ buffer. (A) Time courses of development of ICRAC following dialysis with thapsigargin alone (filled circles), thapsigargin plus cocktail (open circles) and thapsigargin alone after exposure to antimycin A and oligomycin (open diamonds) are shown. (B) Current–voltage relationships for the cells shown in (A) are depicted, taken when the whole-cell currents had reached steady state. (C) Bar chart plotting mean data for the three conditions. *p < 0.01.
Ca2+ release from intracellular stores is not compromized by mitochondrial depolarization
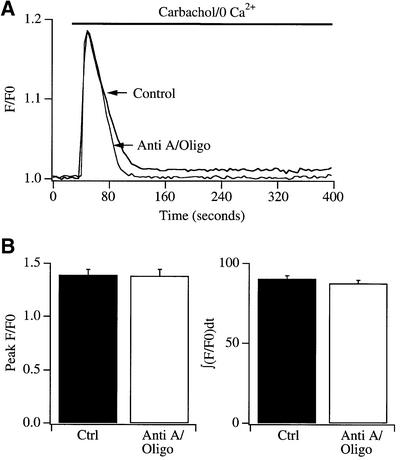
The results of Figure 3 are consistent with those summarized in Figures 1 and 2, although the experimental protocols employed differ slightly. In Figures 1 and 2, mitochondria were depolarized after store depletion, whereas in Figure 3, they were depolarized prior to store emptying. We considered that mitochondrial depolarization might somehow interfere with the ability of stores to empty. If so, then this would result in less store depletion and hence could account for the reduced ICRAC in the experiments of Figure 3. To examine this, we compared the size of the Ca2+ release transient from InsP3-sensitive stores between control cells and those exposed to antimycin A and oligomycin. In these experiments, we monitored Ca2+ release from stores by stimulating cell surface muscarinic receptors with carbachol instead of thapsigargin. Thapsigargin-evoked Ca2+ release in RBL cells is extremely variable in rate, rendering it difficult to quantify accurately (Parekh et al., 1997). Because the agonist- and thapsigargin-sensitive stores overlap completely in RBL cells (Ali et al., 1994; our unpublished data), receptor stimulation is a reasonable approach to use to probe the store Ca2+ content. Results are summarized in Figure 4. A typical control recording and one taken after exposure to antimycin A and oligomycin for 30 min are shown in Figure 4A, and averaged data for the peak increase in Ca2+ in Figure 4B (left panel). We also integrated the fluorescent signal arising from Ca2+ release from the stores (Figure 4B, right panel), but there was no significant difference between control cells and those pre-exposed to antimycin A and oligomycin for either method of analysis. It is important to note that such cytosolic Ca2+ measurements cannot give precise information about the amount of Ca2+ released from the stores. In the experiments of Figure 4, the rise in cytosolic Ca2+ concentration depends not only on the extent of Ca2+ release but also on both cytoplasmic Ca2+ buffering and the rate of Ca2+ extrusion via plasma membrane Ca2+ ATPases. It is possible that a reduced Ca2+ release from the stores could be matched exactly by reduced Ca2+ extrusion in the presence of antimycin/oligomycin, and this would produce an unchanged cytosolic Ca2+ transient. Nevertheless, the fact that the rate and extent of agonist-evoked Ca2+ release were largely unimpaired indicates that the reduction in ICRAC in cells pre-treated with antimycin A and oligomycin (Figure 3), following dialysis with thapsigargin, is unlikely to reflect an inability to release Ca2+ from the stores.
Fig. 4. Mitochondrial depolarization does not compromize Ca2+ release from InsP3-sensitive Ca2+ stores. (A) The Ca2+ signal is shown for a control cell and for one pre-treated with antimycin A and oligomycin for 30 min. Carbachol was applied in Ca2+-free external solution in order to selectively monitor Ca2+ release from the stores. (B) Aggregate data plotting the peak increase in the Ca2+ signal (left panel) and integrated Ca2+ response (right panel) are compared for the conditions indicated. n = 11 for each condition.
Mitochondrial depolarization does not interfere with maximal activation of ICRAC in strong buffer
Another possibility as to why mitochondrial depolarization suppresses the development of ICRAC might reflect a need for mitochondria in the activation mechanism of the current. To investigate this, we dialysed cells with a solution containing InsP3 in strong buffer (10 mM EGTA), which results in rapid and maximal activation of the current. We compared the size of ICRAC between control cells and those cells pre-incubated with antimycin A and oligomycin. ICRAC activated rapidly in both conditions and reached an overall extent not significantly different between the two conditions (Figure 5A and B). Hence mitochondrial depolarization per se does not impede the activation mechanism. Furthermore, these results argue against a direct CRAC channel-blocking action of antimycin A and oligomycin.
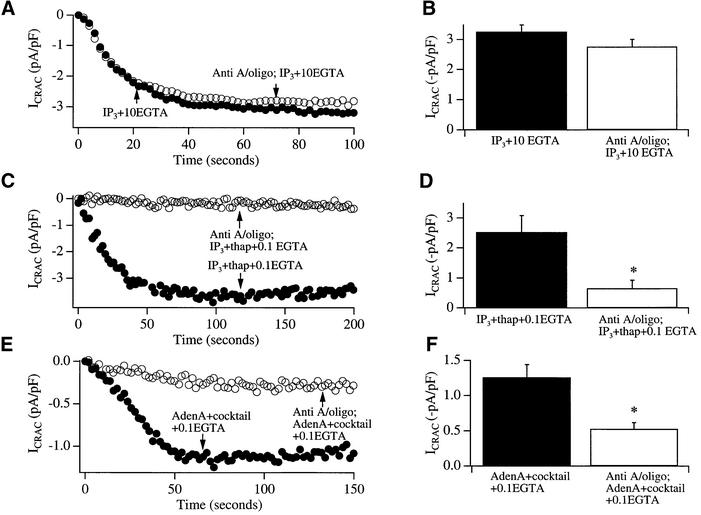
Fig. 5. Mitochondrial depolarization impairs ICRAC in weak, but not strong, intracellular Ca2+ buffer. (A) The time course of development of ICRAC in strong buffer (10 mM EGTA) is compared between a control cell (filled circles) and one exposed to antimycin A plus oligomycin (open circles). (B) Aggregate data for the two conditions (five cells each) are summarized. (C) The development of ICRAC in weak buffer (0.1 mM EGTA) is significantly reduced by antimycin A and oligomycin. In these experiments, stores were emptied by the combination of InsP3 (30 µM) and thapsigargin (2 µM), which activates ICRAC maximally in control cells. (D) Summary of pooled data for the two conditions (six cells each). (E) The development of ICRAC following store emptying by adenophostin A (1 µM) in the presence of mitochondrial cocktail is reduced by antimycin A and oligomycin. (F) Pooled data for the two conditions (six cells each).
Mitochondrial depolarization interferes with maximal activation of ICRAC in weak buffer
Although InsP3 is ineffective in activating ICRAC in weak buffer (0.1 mM EGTA), the combination of InsP3 with thapsigargin is very effective (Fierro and Parekh, 2000). The current is activated to its maximal extent, in that the amplitude of ICRAC is not significantly different between InsP3 and thapsigargin in 0.1 mM EGTA versus InsP3 in 10 mM EGTA, or InsP3 and thapsigargin in 10 mM EGTA (Fierro and Parekh, 2000; Glitsch and Parekh, 2000). Figure 5C and D summarizes experiments designed to test whether mitochondrial depolarization interfered with the activation of ICRAC by InsP3 and thapsigargin in weak buffer. Whereas dialysis with InsP3 and thapsigargin in 0.1 mM EGTA activated a large current (not significantly different from that in strong buffer; compare filled histograms in Figure 5B and D), pre-treatment with antimycin A and oligomycin significantly reduced the current by almost 80% (Figure 5C and D).
Mitochochondrial depolarization reduces the extent of ICRAC evoked by adenophostin A
The non-metabolizable fungal metabolite adenophostin A, which has a several-fold higher affinity for InsP3 receptors than InsP3, can activate ICRAC in weak buffer under conditions where InsP3 is largely ineffective (Parekh et al., 2002). As with InsP3, the size of the current in weak buffer is increased by maintaining mitochondria in an energized state. We examined whether mitochondrial depolarization impaired the activation of ICRAC by adenophostin A (Figure 5E and F). The extent of ICRAC by adenophostin A in the presence of mitochondrial cocktail was significantly reduced by antimycin A and oligomycin. Although just how adenophostin A activates ICRAC is unclear (Parekh et al., 2002), the present results demonstrate that mitochondrial depolarization regulates the overall extent of the current in weak buffer when stores are emptied with a structurally distinct analogue of InsP3.
The relatively infrequent activation of ICRAC by InsP3 in weak buffer can be suppressed by mitochondrial depolarization
Although InsP3 is generally unable to activate ICRAC in weak buffer, we have found on rare occasions (around 8% of the cells) that the current can develop partially. The mean amplitude of the current was –1.31 ± 0.14 pA/pF (six cells that responded under these conditions). If mitochondria were depolarized by pre-treatment with antimycin A and oligomycin, however, no such responses were seen in cells from the same preparations (mean amplitude –0.21 ± 0.06 pA/pF; six cells, p < 0.01). Presumably, in these controls cells, mitochondria are energized sufficiently to support some activation of ICRAC.
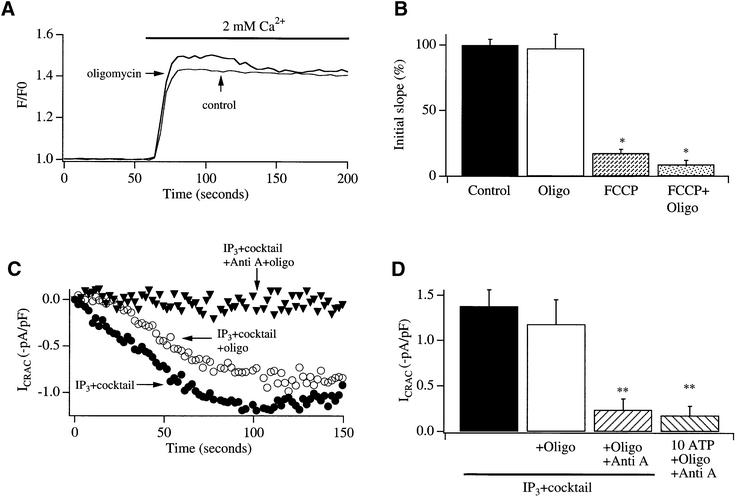
Mitochondrial effects on ICRAC in physiological buffer are not explained by changes in intracellular ATP
Mitochondrial depolarization results in reversal of the F0F1 ATP synthase and hence breakdown of cellular ATP. This can be prevented by inhibiting the ATP synthase with oligomycin. We carried out the following experiments to address whether the reduction in ICRAC by mitochondrial depolarization reflected a fall in intracellular ATP. First, we compared the rate of Ca2+ influx following Ca2+ readmission to cells with stores depleted by pre-treatment with thapsigargin in control cells, with those pre-exposed to oligomycin alone in the absence of mitochondrial depolarization. The rate of entry was not significantly different between the two conditions (Figure 6A and B). Secondly, after store depletion, Fura 2-loaded cells were exposed to oligomycin for 15 min and then to oligomycin plus FCCP. Ca2+ influx was still reduced following readmission of external Ca2+, and there was no significant difference between FCCP-treated cells and those first exposed to oligomycin and then FCCP and oligomycin (Figure 6B). Thirdly, pre-treatment with oligomycin did not suppress the activation of ICRAC following whole-cell dialysis with InsP3 in the presence of mitochondrial cocktail in weak buffer, whereas the current was inhibited by pre-exposure to antimycin A and oligomycin (Figure 6C). Fourthly, in cells pre-treated with anti mycin A and oligomycin, dialysis with InsP3 together with 10 mM Mg-ATP failed to activate any detectable ICRAC (Figure 6D). Hence high global levels of intracellular ATP did not seem to rescue the current when mitochondria were depolarized. Collectively, these results indicate that the reduction in ICRAC in weak buffer by mitochondrial depolarization is unlikely to be explained simply by changes in intracellular ATP levels.
Fig. 6. Changes in intracellular ATP levels do not seem to underlie the effects of mitochondrial depolarization on Ca2+ entry through CRAC channels. (A) Exposure to oligomycin (>15 min) after store depletion fails to affect the rate of Ca2+ entry compared with a control cell. (B) Aggregate data are depicted from experiments carried out as in Figure 1 (14 cells each). Also shown are are the rates of Ca2+ entry (initial slopes) for cells exposed to FCCP alone or oligomycin and then FCCP plus oligomycin following store depletion in Ca2+-free solution. The slopes were both significantly different from control, but not from each other. Oligomycin was added 15 min before FCCP plus oligomycin. (C) The activation of ICRAC in weak Ca2+ buffer is impaired by antimycin A plus oligomycin but not oligomycin alone. Cells were dialysed with InsP3 (30 µM) in the presence of mitochondrial cocktail. Pre-treatment with antimycin A and oligomycin (filled triangles) reduced the size of the current compared with a control recording (filled circles), but pre-treatment with oligomycin alone did not interfere with the development of ICRAC (open circles). (D) Aggregate data for the three conditions in (C) are shown (seven cells for control, seven for oligomycin and five for antimycin A plus oligomycin). Also shown are averaged data showing that the inhibition of ICRAC by pre-treatment with antimycin A plus oligomycin cannot be rescued by including 10 mM Mg-ATP in the pipette solution (six cells).
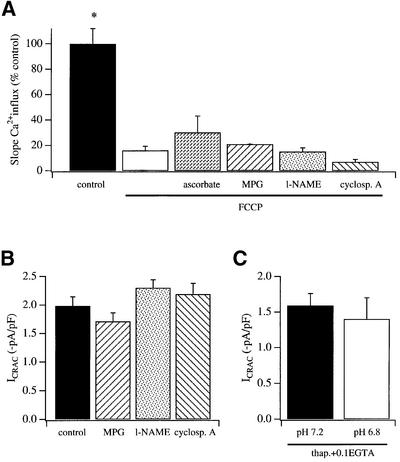
Effects of anti-oxidants, nitric oxide inhibitors, cyclosporin A and cytosolic pH on Ca2+ entry following mitochondrial depolarization
Mitochondrial depolarization can elicit a variety of intracellular changes, including oxidative stress, changes in the levels of nitric oxide (NO), opening of the permeability transition pore and acidification of the cytosol. We designed experiments to test whether such changes could account for the ability of mitochondrial depolarization to suppress ICRAC. Pre-exposure to the anti-oxidants ascorbate (3 mM) or 2-MPG (N-2- mercaptopropionylglycine; 2 mM), to the NO synthase blocker N-nitro-L-arginine methyl ester (l-NAME; 500 µM), or to the permeability transition pore inhibitor cyclosporin A (5 µM) all failed to rescue the rate of calcium influx in cells with depolarized mitochondria, when compared with FCCP alone (Figure 7A). The inability of these agents to rescue Ca2+ entry might be masked if the drugs were directly blocking CRAC channels themselves. To check this, we compared the extent of activation of ICRAC in strong buffer between control cells and those exposed to the various agents. However, the activation of the current was unimpaired (Figure 7B). Dialysis with an acidic intracellular pipette solution (pH 6.8) failed to interfere with the ability of thapsigargin to activate ICRAC in weak buffer (Figure 7C), indicating that the slight acidification of cytosolic pH following mitochondrial depolarization that has been described is unlikely to underlie the inhibition of the current.
Fig. 7. (A) Following mitochondrial depolarization with FCCP, the rate of Ca2+ influx (slope) was significantly reduced compared with control cells. We then examined whether agents known to interfere with signals generated by mitochondria could overcome the reduction in Ca2+ influx by FCCP. However, none of these agents increased the rate of Ca2+ influx significantly when compared with FCCP alone. The experimental protocol was to deplete stores by applying thapsigargin in Ca2+-free solution, and then expose cells to each agent (+thapsigargin) for >30 min before adding FCCP (+agent+thapsigargin) in Ca2+-free solution. The number of cells for each condition was as follows: control (21), FCCP (30), FCCP + ascorbate (11), FCCP + MPG (14), FCCP + l-NAME (25), FCCP + cyclosporin (12). (B) The amplitude of ICRAC was not affected by the agents tested. In these experiments, ICRAC was recorded in 10 mM external Ca2+ and stores were depleted by including InsP3 and 10 mM EGTA in the recording pipette. n = 5 cells for control, 3 for MPG, 6 for l-NAME and 5 for cyclosporin A. (C) Lowering pH from 7.2 (five cells) to 6.8 (seven cells) failed to affect the extent of activation of ICRAC. Stores were depleted by dialysing cells with thapsigargin in 0.1 mM EGTA. The current activated slowly (after a delay of ∼40 s), taking around 200 s to peak. The rate of development was similar for both conditions.
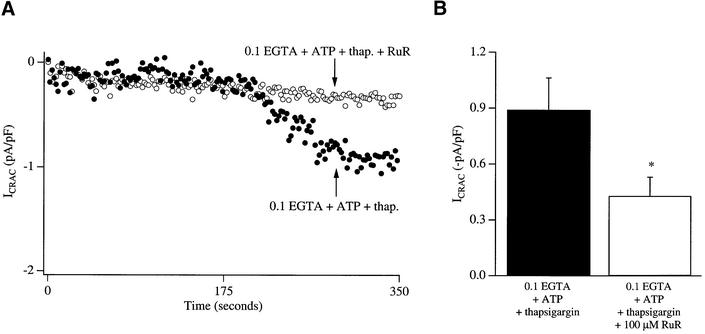
Ruthenium red interferes with the ability of thapsigargin to activate ICRAC in weak buffer
Depolarization of mitochondria reduces the electric driving force for Ca2+ uptake through the mitochondrial Ca2+-permeable uniporter and this impairs the ability of mitochondria to function as Ca2+ buffers. To determine whether reduced mitochondrial Ca2+ uptake could account for the inhibition of Ca2+ entry, we sought to separate Ca2+ uptake from mitochondrial depolarization by blocking the uniporter with ruthenium red. Dialysis with ruthenium red together with thapsigargin in weak buffer significantly reduced the size of the current compared with control recordings taken in the absence of the inhibitor (Figure 8). Unfortunately, specific inhibitors of the uniporter are lacking and ruthenium red has several additional targets. But ruthenium red does not interfere with the ability of ICRAC to activate in strong buffer (Fierro and Parekh, 1999b), ruling out non-specific actions on the activation mechanism.
Fig. 8. The mitochondrial Ca2+ uptake inhibitor ruthenium red reduces the ability of ICRAC to activate in weak intracellular Ca2+ buffer following store depletion with thapsigargin. (A) The time course of activation of ICRAC is compared between a control cell (filled circles; dialysed with thapsigargin and 0.1 mM EGTA) and one in which ruthenium red (100 µM) had been added to this pipette solution (open circles). (B) Aggregate data for the two conditions are summarized [nine cells for control and eight for ruthenium red (RuR)].
Discussion
Using a combination of fluorescent Ca2+ measurements in intact single cells, perforated patch recordings and whole-cell measurements of ICRAC, our major new finding is that functional mitochondria are required for the activation of CRAC channels, and this is observed using rather diverse methods for emptying the intracellular Ca2+ stores (InsP3, adenophostin A and thapsigargin). The involvement of mitochondria is especially prominent in weak intracellular Ca2+ buffer, suggesting that it is likely to be of considerable physiological importance.
Previously we have reported that mitochondrial Ca2+ uptake enables InsP3 to deplete the stores sufficiently for ICRAC to activate in weak Ca2+ buffer (Gilabert and Parekh, 2000; Gilabert et al., 2001). Because SERCA pumps in the ER membrane in RBL cells are located close to mitochondria (Csordas and Hajnoczky, 2001), and also because mitochondria sense microdomains of elevated Ca2+ that accompany opening of InsP3 receptors (Rizzuto et al., 1993, 1998), mitochondrial Ca2+ uptake would be expected to compete with SERCA for removing cytosolic Ca2+ as well as to reduce Ca2+-dependent inactivation of InsP3 receptors. These mechanisms, alone or in combination, would lead to more extensive store depletion and hence activation of ICRAC. Our new results demonstrate that the development of ICRAC is significantly impaired by mitochondrial depolarization even when stores are emptied with thapsigargin. Thapsigargin depletes stores slowly, by relying on an endogenous Ca2+ leakage pathway that seems to involve translocons (Lomax et al., 2002) but that does not require heparin-sensitive InsP3 receptors (Fierro and Parekh, 1999b; Lomax et al., 2002). Hence the mitochondrial requirement for the development of ICRAC is not restricted to InsP3-mediated store depletion but instead appears to be a more general property under physiological conditions in RBL cells; a model system for studying ICRAC.
How does mitochondrial depolarization suppress the extent of activation of ICRAC? The site of action is clearly distal to store emptying because, first of all, mitochondrial depolarization reduces ICRAC when administered after stores have already been emptied irreversibly with thapsigargin and, secondly, the amount of mobilizable Ca2+ within the InsP3-sensitive stores is not significantly affected by mitochondrial depolarization. But mitochondria are unlikely to be involved in the activation mechanism of CRAC channels per se because mitochondrial depolarization did not interfere with the ability of CRAC channels to develop in the presence of strong intracellular Ca2+ buffer, a condition that obviates a role for mitochondrial Ca2+ uptake (Gilabert and Parekh, 2000). The fact that mitochondrial depolarization impairs ICRAC in weak, but not strong, Ca2+ buffer imparts a Ca2+ dependence on the underlying mechanism. Furthermore, because ruthenium red reduces the ability of thapsigargin to activate ICRAC in weak buffer, the Ca2+ dependence might require Ca2+ entry into the mitochondrial matrix via the ruthenium red-sensitive Ca2+ uniporter. Mitochondrial depolarization would reduce the organelle’s role as an intracellular Ca2+ buffer and this could result in a larger and/or more sustained cytosolic Ca2+ increase following opening of CRAC channels. An increase in Ca2+ induces inactivation of CRAC channels, and this might explain the inhibition of ICRAC following mitochondrial depolarization. However, we do not think that such a mechanism can wholly account for the inability of ICRAC to activate following mitochondrial depolarization in weak intracellular Ca2+ buffer for the following reasons. First, Ca2+-dependent deactivation of CRAC channels, due to increased refilling of stores, is unlikely to be operating under our conditions because the stores have been emptied by blocking the SERCA pumps with thapsigargin, and the InsP3- and thapsigargin-sensitive stores overlap completely in these cells (Ali et al., 1994; our unpublished data). Secondly, Ca2+-dependent slow inactivation requires a global increase in Ca2+ and develops relatively slowly with a half-time of around 65 s (Gilabert and Parekh, 2000) in weak Ca2+ buffer. One might have expected therefore to see an initial increase in ICRAC followed by a subsequent slow inactivation as the Ca2+ levels rise to the level required to evoke such an inactivation process. However, this was not the case (see Figures 2 and 3). Furthermore, when Ca2+ was readmitted to cells maintained in thapsigargin/Ca2+-free external solution in the presence of depolarized mitochondria, the rate of Ca2+ entry was significantly reduced. No rapid initial increase followed by a reduced rate of influx was observed. Thirdly, changes in Ca2+-dependent fast inactivation are also unlikely to play a prominent role. Fast inactivation reflects negative feedback by permeating Ca2+ ions on the associated CRAC channel, and the binding site is thought to lie within 5 nm of the pore (Zweifach and Lewis, 1995; Fierro and Parekh, 1999a). Fast inactivation is still pronounced in the presence of the slow Ca2+ chelator EGTA. The rate and extent of fast inactivation were unaffected by either depolarizing mitochondria or energizing them with a mitochondrial cocktail solution included in the patch pipette solution (Gilabert and Parekh, 2000). Finally, Ba2+ influx in store-depleted cells (measured using fura 2) was suppressed by FCCP pre-treatment (data not shown). Since Ba2+ permeates CRAC channels but does not activate the three Ca2+-dependent inhibitory pathways described above, this provides further evidence against a major role for Ca2+-dependent inactivation in the reduction in the rate of Ca2+ influx following mitochondrial depolarization. It is possible that mitochondria first take up Ca2+ that is released slowly from the stores by thapsigargin, and then, upon their depolarization, release this Ca2+ locally onto CRAC channels, thereby inducing a form of Ca2+-dependent inactivation. Such local release would not be detectable in our global Ca2+ measurements but would have to attain levels well in excess of 1 µM, the latter concentration failing to inactivate CRAC channels in whole-cell recording. However, such a mechanism would require the mitochondria below the plasma membrane to take up Ca2+ that has been released from the stores and then release this Ca2+, but not to be able to take up Ca2+ entering through CRAC channels (as there is no change in Ca2+-dependent fast inactivation upon mitochondrial depolarization), which are presumably located in close proximity to such mitochondria. To date, there is no evidence to support the concept that mitochondria can selectively take up Ca2+ from one source but not another, despite being exposed to both. In fact, detailed studies by Montero et al. (2000) have found quite the opposite: mitochondria located close to the plasma membrane can avidly take up Ca2+ released from ryanodine-sensitive stores and Ca2+ channels in the plasma membrane.
An alternative explanation is that mitochondria release factors in a Ca2+-dependent manner and such factors regulate CRAC channel activity. These factors are clearly not required for the activation of ICRAC per se (since the current develops in strong buffer in spite of depolarized mitochondria) but may be more important under physiological conditions of weak intracellular Ca2+ buffering. The identity of such factors is, as yet, unclear. An increase in Ca2+ within the mitochondrial matrix stimulates key regulatory enzymes of the Krebs cycle, resulting in increased aerobic metabolism and hence production of intermediary metabolites and reducing equivalents (McCormack et al., 1990). In addition, Ca2+-dependent transporters like the aspartate/glutamate shuttle are found in the inner mitochondrial membrane (Palmieri et al., 2001). Mitochondrial release of glutamate, for example, has been reported to prime secretory granules for exocytosis in pancreatic beta cells (Maechler and Wollheim, 1999).
Ca2+ entering through CRAC channels can be taken up by mitochondria (Lawrie et al., 1996; Hoth et al., 1997; Gilabert and Parekh, 2000) and this likely increases mitochondrial metabolism and hence ATP levels, which may be important in ensuring adequate energy supply following cell stimulation. Our findings raise the intriguing possibility for a reciprocal Ca2+-dependent signal, emanating from mitochondria, which may regulate CRAC channels in the plasma membrane under physiological conditions. Further work is needed to establish whether such a link exists and, if so, its molecular identity.
Materials and methods
Cell preparation and solutions
RBL-1 cells, which were bought from Cell Bank at the Sir William Dunn School of Pathology, Oxford University, were grown in culture medium containing DMEM supplemented with 10% FCS and penicillin– streptomycin, as previously described (Fierro and Parekh, 2000). Cells were plated onto glass coverslips and used between 24 and 60 h after plating. Standard external solution contained: 145 mM NaCl, 2.8 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM d-glucose, 10 mM HEPES pH 7.4 with NaOH. In Ca2+-free solution, Ca2+ was simply omitted. For whole-cell patch–clamp recordings, the external solution contained: 145 mM NaCl, 2.8 mM KCl, 10 mM CaCl2, 2 mM MgCl2, 10 mM CsCl, 10 mM d-glucose, 10 mM HEPES pH 7.4 with NaOH. The standard pipette solution for whole-cell patch–clamp recordings contained: 145 mM Cs glutamate, 8 mM NaCl, 1 mM MgCl2, 2 mM Mg-ATP, 0.1 mM EGTA, 10 mM HEPES pH 7.2 with CsOH. In a few experiments, EGTA was raised to 10 mM (strong buffer), as indicated in the text. In some experiments (described in the text), the standard pipette solution was supplemented with a mitochondrial cocktail solution, which maintains mitochondria in an energized state (Gunter and Pfeiffer, 1990). This cocktail contained: 2 mM pyruvic acid, 2 mM malic acid, 1 mM NaH2PO4, 0.5 mM cAMP, 0.5 mM GTP, 0.5 mM MgCl2. The pipette solution for perforated patch recordings contained: 145 mM K glutamate, 8 mM NaCl, 5 mM MgCl2, 10 mM HEPES, amphotericin B (250 µg/ml, diluted 1:500 from a frozen DMSO stock solution pH 7.2 with KOH). All reagents were from Sigma (Poole, UK) except cyclosporin A and l-NAME (both from Calbiochem).
Whole-cell patch–clamp experiments
Patch–clamp experiments were conducted in the tight-seal whole-cell configuration at room temperature (20–25°C), as described previously (Glitsch and Parekh, 2000; Bakowski et al., 2001). Sylgard-coated, fire-polished pipettes had d.c. resistances of 2.9–4 MΩ when filled with standard pipette solution. A correction of +10 mV was applied for the subsequent liquid junction potential that arose from this glutamate-based internal solution. Mean series resistance was 7.0 ± 2.2 MΩ; ICRAC was measured by applying voltage ramps (–100 to +100 mV in 50 ms) at 0.5 Hz from a holding potential of 0 mV, as previously described (Glitsch and Parekh, 2000; Bakowski et al., 2001). The currents were corrected for leak by averaging the first few ramps after break-in and then subtracting this from all subsequent currents. Currents were filtered using an 8-pole Bessel filter at 2.5 kHz and digitized at 100 µs. Currents were normalized by dividing the amplitudes (measured from the voltage ramps at –80 mV) by the cell capacitance. Capacitative currents were compensated before each ramp by using the automatic compensation of the EPC 9 and EPC 9 –2 amplifiers.
Perforated patch recordings
Perforated patch recordings were carried out with amphotericin B. Mean series resistance in perforated patch recordings stabilized at 25.1 ± 4.4 MΩ within 5 min of forming a high-resistance seal (>10 GΩ). To deplete the stores fully in perforated patch recordings, external solution for whole-cell patch–clamp experiments was used but with Ca2+ omitted and 2 µM thapsigargin added. In some experiments, mitochondria were depolarized by applying antimycin A and oligomycin (added to Ca2+-free-containing external solution containing thapsigargin) after 10 min pre-incubation with thapsigargin/Ca2+-free external solution. ICRAC was recorded in the perforated patch configuration by subsequent readmission of calcium-containing external solution.
Cytosolic calcium measurements
Calcium imaging experiments were carried out at room temperature using the IMAGO system from TILL Photonics (Bakowski et al., 2001). Cells were alternately excited at 356 and 380 nm (30 ms exposures) and images were acquired using the TILLVision software once every 2–4 s. The images were analysed off-line using IGOR Pro for Windows (Wavemetrics, Lake Oswego, OR). Cells were loaded with Fura 2-AM (1 µM) for 40 min at room temperature in standard external solution, as previously described (Bakowski et al., 2001). After loading, cells were washed three times and then left for 15 min to allow for further de-esterification. Results are presented as F/F0, where F0 denotes the ratio (356 nm/380 nm) prior to stimulation (averaged over 10 s) and F represents the change in the ratio as a function of time. Each image was corrected for background fluorescence.
Data are presented as mean ± SEM (number of experiments) and statistical evaluation was carried out using both Student’s t and Mann–Whitney non-parametric tests.
Acknowledgments
Acknowledgements
M.D.G. was supported by Wellcome Trust (Grant no. 034204). A.B.P. is a Lister Institute Senior Research Fellow.
References
- Ali H., Maeyama,K., Sagi-Eisenberg,R. and Beaven,M.A. (1994) Antigen and thapsigargin promote influx of Ca2+ in rat basophilic RBL-2H3 cells by ostensibly similar mechanisms that allow filling of inositol 1,4,5-trisphosphate-sensitive and mitochondrial Ca2+ stores. Biochem. J., 304, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock D.F., Herrington,J., Goodwin,P.C., Park,Y.B. and Hille,B. (1997) Mitochondrial participation in the intracellular Ca2+ network. J. Cell Biol., 136, 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski D., Glitsch,M.D. and Parekh,A.B. (2001) An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current ICRAC in RBL-1 cells. J. Physiol., 532, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad L.M., Armstrong,D.L. and Putney,J.W. (1999) Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback inhibition of calcium release-activated calcium current ICRAC. J. Biol. Chem., 274, 32881–32888. [DOI] [PubMed] [Google Scholar]
- Csordas G. and Hajnoczky,G. (2001) Sorting of calcium signals at the junctions of endoplasmic reticulum and mitochondria. Cell. Calcium, 29, 249–262. [DOI] [PubMed] [Google Scholar]
- Duchen M.R. (2000) Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell. Calcium, 28, 339–348. [DOI] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (1999a) Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J. Membr. Biol., 168, 9–17. [DOI] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (1999b) On the characterisation of the mechanism underlying passive activation of the Ca2+ release-activated Ca2+ current ICRAC in rat basophilic leukaemia cells. J. Physiol., 520, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro L. and Parekh,A.B. (2000) Substantial depletion of the intracellular Ca2+ stores is required for macroscopic activation of the Ca2+ release-activated Ca2+ current in rat basophilic leukaemia cells. J. Physiol., 522, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert J.-A. and Parekh,A.B. (2000) Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J., 19, 6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert J.-A., Bakowski,D. and Parekh,A.B. (2001) Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J., 20, 2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch M.D. and Parekh,A.B.(2000) Ca store dynamics determines the pattern of activation and inactivation of the store-operated Ca current ICRAC in response to InsP3 in rat basophilic leukaemia cells. J. Physiol., 523, 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter T.E. and Pfeiffer,D.R. (1990) Mechanisms by which mitochondria transport calcium. Am. J. Physiol., 258, C755–C786. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Robb-Gaspers,L.D., Seitz,M.B. and Thomas,A.P. (1995) Decoding of cytosolic calcium oscillations in the mitochondria. Cell, 82, 415–424. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Hager,R. and Thomas,A.P. (1999) Mitochondria suppress local feedback activation of inositol 1,4,5-trisphosphate receptors by Ca2+. J. Biol. Chem., 274, 14157–14162. [DOI] [PubMed] [Google Scholar]
- Hoth M. and Penner,R. (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature, 355, 353–356. [DOI] [PubMed] [Google Scholar]
- Hoth M., Fanger,C.M. and Lewis,R.S. (1997) Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol., 137, 633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville L.S., Ichas,F., Holmuhamedov,E.L., Camacho,P. and Lechleiter,J.D. (1995) Synchronization of calcium waves by mito chondrial substrates in Xenopus laevis oocytes. Nature, 377, 438–411. [DOI] [PubMed] [Google Scholar]
- Jouaville L.S., Pinton,P., Bastianutto,C., Rutter,G.A. and Rizzuto,R. (1999) Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl Acad. Sci. USA, 96, 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie A.M., Rizzuto,R., Pozzan,T. and Simpson,A.W.M. (1996) A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J. Biol. Chem., 271, 10753–10759. [DOI] [PubMed] [Google Scholar]
- Lomax R., Camello,C., Van Coppenolle,F., Petersen,O.H. and Tepikin,A.V. (2002). Basal and physiological Ca2+ leak from the endoplasmic reticulum of pancreatic acinar cells. Second messenger-activated channels and translocons. J. Biol. Chem., 277, 26479–26485. [DOI] [PubMed] [Google Scholar]
- Maechler P. and Wollheim,C.B. (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature, 402, 685–689. [DOI] [PubMed] [Google Scholar]
- McCormack J.G., Halestrap,A.P. and Denton,R.M. (1990) Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev., 70, 391–425. [DOI] [PubMed] [Google Scholar]
- Mohr F.C. and Fewtrell,C. (1987) The relative contributions of extracellular and intracellular calcium to secretion from tumor mast cells. Multiple effects of the proton ionophore carbonyl cyanide m-chlorophenylhydrazone. J. Biol. Chem., 262, 10638–10643. [PubMed] [Google Scholar]
- Montero M., Alonso,M.T., Carnicero,E., Cuchillo-Ibanez,I., Albillos,A., Garcia,A.G., Garcia-Sancho,J. and Alvarez,J. (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat. Cell Biol., 2, 57–61. [DOI] [PubMed] [Google Scholar]
- Palmieri L. et al. (2001) Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J., 20, 5060–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A.B. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Parekh A.B., Fleig,A. and Penner,R. (1997) The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell, 89, 973–980. [DOI] [PubMed] [Google Scholar]
- Parekh A.B., Riley,A.M. and Potter,B.V.L. (2002) Adenophostin A and ribophostin, but not inositol 1,4,5-trisphosphate or manno-adenophostin, activate the Ca2+ release-activated Ca2+ current, ICRAC, in weak intracellular Ca2+ buffer. Biochem. J., 361, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.K., Ashby,M.C., Erdemli,G., Petersen,O.H. and Tepikin,A.V. (2000) Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J., 19, 5729–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Putney J.W. (1986) A model for receptor-regulated calcium entry. Cell. Calcium, 7, 1–12. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini,M., Murgia,M. and Pozzan,T. (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science, 262, 744–747. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton,P., Carrington,W., Fay,F.S., Fogarty,K.E., Lifshitz, L.S., Tuft,R.A. and Pozzan,T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Rutter G.A. and Rizzuto,R. (2000) Regulation of mitochondrial metabolism by ER Ca2+ release; an intimate connection. Trends Biochem. Sci., 25, 215–221. [DOI] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Gerasimenko, O.V., Tepikin,A.V. and Petersen,O.H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A. and Lewis,R.S. (1995) Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J. Gen. Physiol., 105, 209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]