Abstract
Matrix metalloproteinases (MMPs), together with their endogenous inhibitors (TIMPs) form an enzymatic system that plays an important role in a variety of physiological and pathological conditions. These proteins are also expressed in the brain, especially under pathological conditions, in which glia as well as invading inflammatory cells provide the major source of the MMP activity. Surprisingly little is known about the MMP function(s) in adult neuronal physiology. This review describes available data on this topic, which is presented in a context of knowledge about the MMP/TIMP system in other organs as well as in brain disorders. An analysis of the MMP and TIMP expression patterns in the brain, along with a consideration of their regulatory mechanisms and substrates, leads to the proposal of possible roles of the MMP system in the brain. This analysis suggests that MMPs may play an important role in the neuronal physiology, especially in neuronal plasticity, including their direct participation in the remodeling of synaptic connections—a mechanism pivotal for learning and memory.
Keywords: dystroglycan/extracellular matrix/hippocampus/neuronal plasticity/TIMP
Matrix metalloproteinases and their endogenous inhibitors
Matrix metalloproteinases (MMPs) constitute a family of enzymes with >20 members identified to date, which are all extracellular (predominantly pericellular, and some membrane-bound) endopeptidases requiring Zn2+ for their enzymatic activity (Woessner and Nagase, 2000). Their major targets are proteins of the extracellular matrix (ECM). MMPs are grouped together because of their sequence kinship and consequent structural and enzymatic similarities (Massova et al., 1998). All of the MMPs are produced with an N-terminal signal peptide, which is removed after directing their synthesis into the endoplasmatic reticulum. They all contain a propeptide that has to be cleaved off to reveal enzymatic activity, and their catalytic domains, responsible also for the substrate recognition, are similar, with a conserved Zn2+ binding site. The vast majority of MMPs also have a hemopexin-like domain at the C-terminus that is mainly responsible for binding to certain substrates as well as other interacting proteins, including specific inhibitors (TIMPs—tissue inhibitors of metalloproteinases). A membrane-bound subclass is characterized by a single-pass transmembrane domain and a short cytoplasmic C-terminal tail or a hydrophobic region that may function as a GPI (glycophosphatidyl inositol) anchoring signal (Sternlicht and Werb, 2001). The relations among MMPs extend beyond the structure. All of them require enzymatic activation (by means of the propeptide cleavage) and this is achieved by the action of serine proteinases, such as plasmin as well as other MMPs, and involves an autocatalytic step (Yong et al., 1998, 2001; Woessner and Nagase, 2000).
The TIMPs (-1, -2, -3 and -4) are small (20–22 kDa) proteins that can bind MMPs and block their activities (Woessner and Nagase, 2000). Unfortunately, only very limited knowledge is available about physiological specificity of different TIMPs towards various MMPs. In addition to their effects on the MMPs, a growth-promoting action, which is unlikely to be explained on the basis of blocking the proteolysis, has also been ascribed to some TIMPs (Woessner and Nagase, 2000).
Physiological significance of the MMP/TIMP system outside the brain
Understanding of the physiological role played by the MMPs outside the brain may greatly aid in the elucidation of their function in this organ as well. Hence, it is noteworthy that the role of MMPs has been very well documented in embryonic development and tissue morphogenesis (for reviews, see Vu and Werb, 2000; Sternlicht and Werb, 2001). The evidence for developmental significance of the MMPs and TIMPs comes from studies on their expression patterns as well as from functional experiments with inhibitors and null mutations. Paramount evidence for MMP role in physiology has been collected in the case of implantation, mammary and bone development as well as wound healing. Vu and Werb (2000) proposed that MMP activity during development might be required for: (i) degradation of the ECM in order to allow cell migration; (ii) alteration of the ECM microenvironment to modify cell behavior; and (iii) modulation of the activity of biologically important molecules by direct cleavage, release from bound stores or modifying their inhibitors.
MMPs in diseases of the nervous system
A great deal of data has been collected regarding the MMP/TIMP system in a variety of brain pathologies. In particular, the MMPs have been implicated in gliomas (tumors of glial origin), viral infections, inflammation, multiple sclerosis (MS), Alzheimer’s disease, amyotrophic lateral sclerosis, brain trauma and ischemia (see Rosenberg, 1995; Rooprai and McCormick, 1997; Yong et al., 1998, 2001; Lukes et al., 1999; for a review, see Leppert et al. 2001). In the context of MS MMP-1, -2, -3 and -9 were immunolocalized to brain macrophages/microglia and astrocytes (Yong et al., 1998, 2001; Leppert et al., 2001). The levels of those enzymes could be increased by various inflammatory cytokines in vitro as well as in animals with experimental allergic encephalomyelitis (EAE) serving as a model for MS. Furthermore, chemical inhibitors of MMP activity were shown to ameliorate the severity of the EAE (Yong et al., 2001). In case of brain ischemia in rats and mice, expression and activation of the MMP-2 was observed in neurons, glia and the endothelium, whereas MMP-9 was found elevated in neurons and glia as well as myelinated fiber tracts, and MMP-3 was found in neurons (Rosenberg et al., 1996; Clark et al., 1997; Mun-Bryce and Rosenberg, 1998; Gasche et al., 1999; Heo et al., 1999; Rivera et al., 2002). Inhibition of MMPs with substances displaying especially high activity against gelatinases (MMP-2 and -9), as well as injection of a neutralizing antibody against MMP-9 and a targeted disruption of the MMP-9 gene, all diminished the severity of the damaging consequences of ischemia to the brain (Romanic et al., 1998; Rosenberg et al., 1998; Asahi et al., 2000, 2001; Jiang et al., 2001).
In conclusion, the disease studies underscore the importance of the MMPs in brain dysfunctions, as well as clearly showing how important it is to investigate the cellular origins of the MMP activities within this organ, where non-neuronal cells, including the infiltrating inflammatory ones, provide the major component of pathology-related MMP overexpression. As Yong et al. (2001) have already pointed out, an intriguing question remains: to what extent the activation of MMPs in the response to brain-damaging treatments is detrimental to the tissue or, in contrast, represents a repair reaction.
MMP/TIMP expression patterns in the brain
Overall, the MMPs are expressed at basal conditions at very low levels in various tissues. Similarly, only a few of the MMPs were shown to be expressed in the unstimulated brain (Yong et al., 2001). For instance, Pagenstecher et al. (1997) and Vecil et al. (2000) used an RNase protection assay to show that among MMP-1, -2, -3, -7, -9, -10, -11, -12, -13 and -14 only five, MMP-2, -9, -11, -12 and -14, were expressed, and mostly at rather low levels, in the mouse brain. Sekine-Aizawa et al. (2001) used a RT–PCR approach to show that MMP-24 (also known as MT5 MMP; see also Pei, 1999) comprises 60% of the total rat brain population of the MMPs lacking a fibronectin-like domain, followed by MMP-14 (23%), MMP-15 (13%), MMP-13 (3.3%), MMP-3 (1.3%) and MMP-8 (0.7%).
Two brain structures investigated in more detail with regard to MMP expression are the cerebellum and the hippocampus. Sekine-Aizawa et al. (2001) found that most of the brain MMP-24 expression originates from the cerebellum, where its mRNA is especially abundant in the granule and Purkinje neurons. A similar conclusion was obtained with regard to the protein distribution in both rat and mouse cerebellum (Hayashita-Kinoh, 2001; Sekine-Aizawa et al., 2001). Using gelatinase assay, Vaillant et al. (1999) observed low levels of MMP-9 in the adult rat cerebellum and higher levels of the MMP-2. Abundant expression in the adult Purkinje neurons was also observed for MMP-9, -2 and -3 (Vaillant et al., 1999). MMP-3 and -9 were also present in the granular neurons of the adult cerebellum (Vaillant et al., 1999). It is noteworthy that the expression of MMP-3 and -9 was at high levels in the cerebellum of 15-day-old rats, i.e. at the time of intense synaptogenesis. As far as the subcellular distribution is concerned, Sekine-Aizawa et al. (2001) reported that MMP-24 was localized predominantly to neuronal cell soma and dendrites, whereas Vaillant et al. (1999) found predominant expression of MMP-2, -3 and -9 proteins only in the neuronal cell bodies.
In addition to the cerebellum, the MMP-24 mRNA and protein were also clearly detected in the rat and mouse hippocampus (in the neurons of the dentate gyrus as well as CA1, CA2 and CA3 subfields) (Jaworski, 2000; Hayashita-Kinoh, 2001; Sekine-Aizawa et al., 2001). In the human hippocampus, Backstrom et al. (1996) detected MMP-9 mRNA and protein in the pyramidal neurons of the CA subfields, but not in the granule neurons of the dentate gyrus. Relatively low levels of the MMP-24 mRNA and protein could also be found in the pyramidal cortical neurons, and some neurons of the thalamus as well as in the olfactory bulb (Jaworski, 2000; Sekine-Aizawa et al., 2001). More extensive analysis of MMP-2 and -9 expression at the level of mRNA and protein in the hippocampus indicated that whereas MMP-2 appeared to be mostly glial in origin, the MMP-9 was expressed mainly by neurons (Szklarczyk et al., 2002).
In addition to MMPs, also TIMPs are found in the brain (Pagenstecher et al., 1997; Vaillant et al., 1999; Jaworski, 2000). In the cerebellum TIMP-4 mRNA is particularly abundant, followed by TIMP-1. On the other hand, TIMP-2 mRNA is present all over the brain, although with lower levels in the cerebellum, while TIMP-3 appears to be present predominantly in the choroid plexus (Fager and Jaworski, 2000). Within the cerebellum, the TIMP-1, -2 and -3 are expressed by the Purkinje cells, with the latter two also expressed by granule neurons (Vaillant et al., 1999). TIMP-3 (protein, but not mRNA) was the only one detected in the dendrites (Vaillant et al., 1999).
MMP/TIMP expression in the stimulated brain
Besides being actively produced by various non-neuronal (glial and infiltrating) cells in the brain in response to a variety of pathogenic insults (see above), MMP-9 and TIMP-1 were found to also be elevated in neurons in response to enhanced activity. Nedivi et al. (1993) were the first to find (by means of subtractive cloning) increased levels of TIMP-1 mRNA in the hippocampal dentate gyrus following seizures (seizures are often used as a convenient model system to investigate enhanced neuronal activity driven by the major excitatory neurotransmitter, glutamate). Rivera et al. (1997) and Jaworski et al. (1999) extended this observation, by documenting elevated TIMP-1 mRNA and protein expression in all the hippocampal subfields, thus suggesting that it is a neuronal response to the enhanced activity. Furthermore, Rivera et al. (1997) found that seizures elevate TIMP-1 immunoreactivity in hippocampal neurons and astrocytes.
In addition, seizures activate MMP-9 at all levels—enzymatic activity, protein and mRNA expression (Zhang et al., 1998; Szklarczyk et al., 2002). Enzymatic activity (as well as the abundance of the protein contained within a membrane-bound fraction) is elevated already at 6 h after the seizures, whereas mRNA expression peaks at 24–72 h, suggesting that elevated gene expression might be a response to, rather than cause for, the active protein release. Most interestingly, the enhanced mRNA expression was observed both in the neuronal cell bodies as well as in the dendritic layer, suggesting an activity-driven translocation of the MMP-9 mRNA (Szklarczyk et al., 2002). Such a phenomenon could have important consequences for neuronal plasticity, including learning and memory, as it has already been observed for mRNAs that are apparently locally translated at the activated, selected synapses (for a review, see Job and Eberwine, 2001; Steward and Schuman, 2001). It is believed that such local mRNA translation could provide a mechanism for synapse-specific control of protein synthesis, which may underlie delineation of synaptic specificity in long-term memory formation (see Frey and Morris, 1998; Martin et al., 2000).
Direct connection between MMP-9 expression and learning has recently been suggested by Wright et al. (2002) reporting that MMP-9 enzymatic activity is enhanced in the rat hippocampus in the course of spatial learning. Possible involvement of MMP-9 in brain physiology is also supported by the results of Taishi et al. (2001), who reported that manipulation with sleep and ambient temperature may affect the MMP-9 mRNA levels in the cerebral cortex and hippocampus. Parallel analysis of brain-derived neurotrophic factor (BDNF), tissue-type plasminogen activator (tPA) and immediate early gene—arc mRNA expression—all implicated previously in neuronal plasticity, and all responsive in various ways to the modulation of sleep and temperature conditions as shown by Taishi et al. (2001), led the authors to suggest that the aforementioned genes, sleep and synaptic plasticity are somehow linked.
Regulation of neuronal MMP expression and activity
As limited data are available on MMPs and TIMPs in neuronal physiology of the adult brain, important clues may come from extensive knowledge collected on regulation of this enzymatic system in other cells. MMPs are very elaborately controlled, especially at the levels of gene expression and enzymatic activation to produce a functional form, as well as through the inhibition by TIMPs (Sternlicht and Werb, 2001). In certain cases, there are also mechanisms affecting specifically the mRNA stability, protein secretion as well as specific degradation and clearance (Sternlicht and Werb, 2001). Particularly revealing are results on regulation of the MMPs and TIMPs by factors that have already been shown to play pivotal roles in the brain.
There are many instances when neuronal cell surface receptors, bound by specific ligands, affect function of the AP-1 transcription factor (Herdegen and Leah, 1998). AP-1 is a dimer made of Jun (c-Jun, JunB and JunD) or Jun and Fos (c-Fos, FosB, ΔFosB, Fra-1 and Fra-2) proteins. In the brain, a transient activation of AP-1 is intimately linked to neuronal plasticity (for example, see Kaczmarek, 1993; Kaczmarek and Chaudhuri, 1997; Kaczmarek, 2002). However, despite multiple efforts, our understanding of AP-1 in the brain is very limited. Thus, it is very interesting to note that in other organs AP-1 has been shown to play a major role in regulating the expression of a number of MMP genes. For MMP-9, the evidence for control exerted by AP-1 is paramount, although a number of other transcription factors/regulatory elements appear to play a role too. However, it has to be stressed that the MMP (including MMP-9) gene regulation may occur in a cell-specific manner (Sato and Seiki, 1993; Benbow and Brinckerhoff, 1997). Unfortunately, apparently no extensive analyses of the neuronal regulation of the MMP gene expression have been reported.
On the other hand, the most advanced example for AP-1 control of gene expression in the adult brain has been provided for TIMP-1. It has been known that the TIMP-1 gene promoter contains a non-canonical AP-1 responsive element, playing a role in gene regulation in non-neuronal cells (Alitalo et al., 1990; Campbell et al., 1991; Edwards et al., 1992; Bugno et al., 1995; Logan et al., 1996; Botelho et al., 1998). Jaworski et al. (1999) combined a variety of approaches to show that the gene encoding TIMP-1 may be an AP-1 target in the rodent hippocampus in response to seizures. The evidence was based on the following findings: (i) the AP-1 transcription factor that accumulated in seizure-stimulated hippocampi (Kaminska et al., 1994) was capable of binding the TIMP-1 AP-1 responsive DNA regulatory element in a sequence-dependent manner, and this AP-1 DNA binding activity contained such proteins as c-Fos, c-Jun, JunB and JunD; (ii) increase in TIMP-1 mRNA levels following c-fos mRNA accumulation was dependent on prior protein synthesis, and was spatially correlated with c-Fos protein expression; (iii) TIMP-1 promoter was seizure-responsive in the hippocampi of transgenic TIMP-1-LacZ mice; (iv) glutamate was able to activate expression of a reporter gene, driven by TIMP-1 promoter containing an intact AP-1 site, in cultured neurons of the dentate gyrus, and mutation of this promoter element abolished the expression (Jaworski et al., 1999).
Once the MMP proteins are released, their activation occurs via cleavage of the propeptide. Plasmin, a serine proteinase plays a major role in this reaction (Sternlicht and Werb, 2001). Plasmin, in turn, is produced from plasminogen due to activity of tPA or urokinase plasminogen activator (uPA). It is conspicuous that all three—plasmin, tPA and uPA—have repeatedly been implicated in neuronal plasticity, including learning and memory, by virtues of their expression patterns as well as by functional studies involving specific inhibitors, transgenic mice and null mutants (Qian et al., 1993; Meiri et al., 1994; Seeds et al., 1995; Baranes et al., 1998; Madani et al., 1999; Yoshida and Shiosaka, 1999).
MMP substrates in the brain
The list of MMP substrates is long (for example, see Liu et al, 2000; Woessner and Nagase, 2000; Kridel et al., 2001; Yamada et al., 2001). However, very little is known about which of them could comprise the in vivo targets for MMPs. The situation gets even more complicated for the brain, as composition of the ECM differs markedly from the one typical to other organs. The extracellular space between neurons in adult brain lacks a basal lamina and apparently does not contain such classical MMP-susceptible ECM proteins as laminins and collagens (Sugita et al., 2001). Nevertheless, there are MMP targets that are available in the brain and could be involved in neuronal plasticity. Integrins appear to be especially appealing in this context, as several lines of evidence suggest that integrins play important roles in neuronal plasticity, including learning and memory (see Rohrbough, 2000; Chun et al., 2001). In addition, recently, Lee et al. (2001) have identified pro-NGF and pro-BDNF as MMP (MMP-3 and MMP-7) substrates. The role of the neurotrophins, including NGF and BDNF, in neuronal plasticity has been well documented.
The most fascinating hypothesis may, however, be proposed after considering recent observations that dystroglycan may be a physiological target for MMP activity (most probably MMP-2 or -9; see Yamada et al., 2001). Although this phenomenon was not pronounced in the brain, our recent results show that similar proteolytic breakdown of the β-dystroglycan also occurs in the rat hippocampus in response to kainate in a temporal pattern parallel to increased levels of the MMP enzymatic activity (Figure 1). Furthermore, recent evidence based on a brain-limited knockout of dystroglycan, shows that this protein plays an important role in LTP (long-term potentiation of synaptic efficacy), serving as a model of neuronal plasticity (Moore et al., 2002).
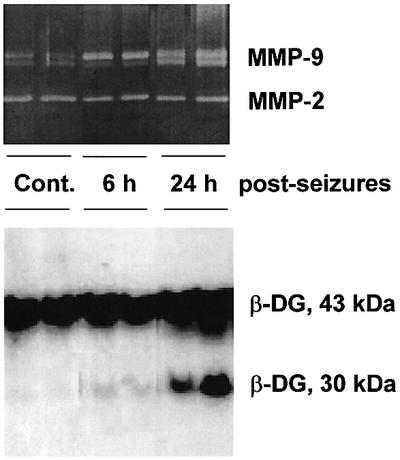
Fig. 1. Neuronal activity-driven limited proteolysis of β-dystroglycan. The adult Wistar rats were treated with 10 mg/kg sodium kainate (see Kaminska et al., 1994) and the animals displaying robust seizues were killed at either 6 or 24 h afterwards, along with the control ones (Cont.). The hippocampi were then dissected and the proteins extracted either for zymographic assay of MMP activity with gelatin as a substrate (see Szklarczyk et al., 2002) or for immunoblotting detection of β-dystroglycan (with the antibody kindly provided by Dr T.Petrucci; Rosa et al., 1996).
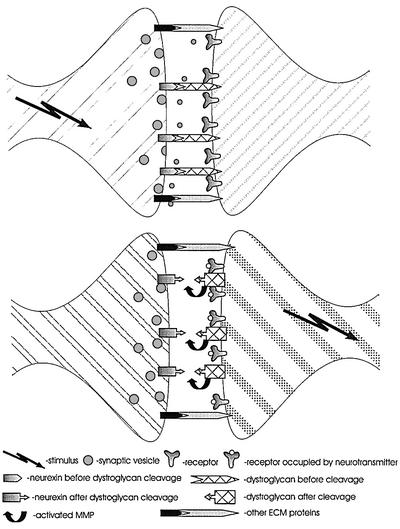
Dystroglycan is a molecule that extends from the postsynaptic side into the ECM. Inside the cell, dystroglycan binds dystrophin, which is involved in linking various proteins with the cytoskeleton. It has also been shown that dystroglycan comprises the major target for neurexins (Sugita et al., 2001). Neurexins (I, II, III: α and β) are apparent recognition molecules that extend from the presynaptic side into the ECM. Interestingly, Górecki et al. (1999) found that Neurexin IIα mRNA accumulated in the dentate gyrus of the hippocampus in response to enhanced neuronal activity, whereas mRNA for dystrophin (Dp71 isoform) disappeared from the same region after such treatment (Gorecki et al., 1998). These observations, combined with the fact that the dentate gyrus is selectively engaged in neuronal plasticity after kainate treatment, suggest a possible role for both proteins in plasticity phenomena. It is thus tempting to suggest that presynaptic membrane-bound neurexins interact directly with postsynaptic dystroglycan molecules, which in turn communicate through dystrophin with postsynaptic cytoskeleton and other proteins. Cleavage of dystroglycan by MMP (such as neuronal MMP-9) could produce conformation changes that affect both pre- (neurexin) and postsynaptic (dystroglycan–dystrophin) elements. The major question remains whether such structural changes affect cytoorganization and/or any signal transduction pathways on both sides of the synapse. If this is the case, this phenomenon could play the most important role in neuronal plasticity, serving a function of a retrograde messenger to be activated at the postsynaptic side in response to enhanced neuronal activity, and acting on the presynaptic side (Figure 2). Such signals have been intensely searched for, because of the conceptual framework provided by Hebb (1949) to explain learning. Hebb’s proposal demanded that both neurons, whose connection is to be strengthened, had to be simultaneously active.
Fig. 2. Schematic representation of putative retrograde messenger function exerted by the MMPs at the activated synapse. At the steady state, the presynaptic and postsynaptic sides are directly linked through transmembrane/ECM molecules, such as neurexin and dystroglycan (top panel); activity-driven release of the neurotransmitter results is stimulation of the postsynaptic side, which in turn releases active MMPs (bottom panel). The MMPs perform limited proteolysis of dystroglycan that results in allosteric modulation of both the neurexins and the remaining part of dystroglycan, which in turn produces specific structure/function changes in both the pre- and postsynaptic cytoplasmic environment (as shown in the figure by changes in the background pattern).
Concluding remarks: functions of the neuronal MMPs in the brain physiology
Despite their marked presence in the brain, especially in response to enhanced neuronal activity, as well as given important functional roles of MMPs in brain dysfunctions, very little is still known about physiological functions of these enzymes in the nerve tissue. Two enzymes that appear to be most interesting to study are MMP-24 in the cerebellum and MMP-9 in the hippocampus, along with all the TIMPs. The expression patterns and the regulatory mechanisms offer clues as far as the possible roles of these proteins are concerned. The massive activation and an increase in the mRNA as well as protein levels in the stimulated hippocampus appear to be a good indication for possible involvement of the MMP-9 in neuronal plasticity, i.e. experience-dependent reorganization of synaptic connections. In this context, it would be of great interest to analyze subcellular, including possible synaptic, distribution of this enzyme. The fact that TIMP-1 is controlled by AP-1 transcription factor in the hippocampus, along with an expected role for AP-1 in regulation of the neuronal MMP-9 gene (as already proven for non-neuronal cells) further supports the notion of a relation of this enzyme system with plasticity. Similarly, involvement of plasmin, which is controlled by tPA and uPA in MMP activation, is also suggestive as far as the role of the MMP/TIMP system in neuronal plasticity is concerned. Another clue for the MMP functions in the brain comes from identification of their substrates and interacting proteins with integrins, and dystroglycan appearing to be especially interesting for neuronal plasticity. Notably, those substrates perform either structural or signaling roles in neuronal functioning and thus, an involvement of the selected MMPs in control of ECM architecture as well as information transfer can be envisaged. In aggregate, it is safe to conclude that MMPs together with their endogenous inhibitors are expected to play a significant role in neuronal functions, including synaptic reorganization. It is up to forthcoming studies to verify this hypothesis.
Acknowledgments
Acknowledgements
The authors appreciate the support provided by State Committee for Scientific Research (KBN, Poland) through its commitment to the EU COSTB10 action ‘Brain Damage Repair’.
References
- Alitalo R., Partanen,J., Pertovaara,L., Holtta,E., Sistonen,L., Andersson,L. and Alitalo,K. (1990) Increased erythroid potentiating activity/tissue inhibitor of metalloproteinases and jun/fos transcription factor complex characterize tumor promoter-induced megakaryo blastic differentiation of K562 leukemia cells. Blood, 75, 1974–1982. [PubMed] [Google Scholar]
- Asahi M., Asahi,K., Jung,J.C., del Zoppo,G.J., Fini,M.E. and Lo,E.H. (2000) Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J. Cereb. Blood Flow Metab., 20, 1681–1689. [DOI] [PubMed] [Google Scholar]
- Asahi M., Wang,X., Mori,T., Sumii,T., Jung,J.C., Moskowitz,M.A., Fini,M.E. and Lo,E.H. (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci., 21, 7724–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom J.R., Lim,G.P., Cullen,M.J. and Tokes,Z.A. (1996) Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-β peptide (1–40). J. Neurosci., 16, 7910–7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranes D., Lederfein,D., Huang,Y.Y., Chen,M., Bailey,C.H. and Kandel,E.R. (1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron, 21, 813–825. [DOI] [PubMed] [Google Scholar]
- Benbow U. and Brinckerhoff,C.E. (1997) The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol., 15, 519–526. [DOI] [PubMed] [Google Scholar]
- Botelho F.M., Edwards,D.R. and Richards,C.D. (1998) Oncostatin M stimulates c-Fos to bind a transcriptionally responsive AP-1 element within the tissue inhibitor of metalloproteinase-1 promoter. J. Biol. Chem., 273, 5211–5218. [DOI] [PubMed] [Google Scholar]
- Bugno M., Greave,L., Gatsios,P., Koj,A., Heinrich,P.C., Travis,J. and Kordula,T. (1995) Identification of the interleukin-6/oncostation M response element in the rat tissue inhibitor of metalloproteinases-1 promoter. Nucleic Acids Res., 23, 5041–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C.E., Flenniken,A.M., Skup,D. and Williams,B.R. (1991) Identification of a serum- and phorbol ester-responsive element in the murine tissue inhibitor of metalloproteinase gene. J. Biol. Chem., 266, 7199–7206. [PubMed] [Google Scholar]
- Chun D., Gall,C.M., Bi,X. and Lynch,G. (2001) Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience, 105, 815–829. [DOI] [PubMed] [Google Scholar]
- Clark A.W., Krekoski,C.A., Bou,S.S., Chapman,K.R. and Edwards,D.R. (1997) Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci. Lett., 238, 53–58. [DOI] [PubMed] [Google Scholar]
- Edwards D.R., Rocheleau,H., Sharma,R.R., Wills,A.J., Cowie,A., Hassell,J.A. and Heath,J.K. (1992) Involvement of AP1 and PEA3 binding sites in the regulation of murine tissue inhibitor of metalloproteinases-1 (TIMP-1) transcription. Biochim. Biophys. Acta, 1171, 41–55. [DOI] [PubMed] [Google Scholar]
- Fager N. and Jaworski,D.M. (2000) Differential spatial distribution and temporal regulation of tissue inhibitor of metalloproteinase mRNA expression during rat central nervous system development. Mech. Dev., 98, 105–109. [DOI] [PubMed] [Google Scholar]
- Frey U. and Morris,R.G. (1998) Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci., 21, 181–188. [DOI] [PubMed] [Google Scholar]
- Gasche Y., Fujimura,M., Morita-Fujimura,Y., Copin,J.C., Kawase,M., Massengale,J. and Chan,P.H. (1999) Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J. Cereb. Blood Flow Metab., 19, 1020–1028. [DOI] [PubMed] [Google Scholar]
- Górecki D.C., Lukasiuk,K., Szklarczyk,A.W. and Kaczmarek,L. (1998) Kainate-evoked changes in dystrophin mRNA levels in the rat hippocampus. Neuroscience, 84, 467–477. [DOI] [PubMed] [Google Scholar]
- Górecki D.C., Szklarczyk,A., Lukasiuk,K., Kaczmarek,L. and Simons,J.P. (1999) Differential seizure-induced and developmental changes of neurexin expression. Mol. Cell. Neurosci., 13, 218–227. [DOI] [PubMed] [Google Scholar]
- Hayashita-Kinoh H., Kinoh,H., Okada,A., Komori,K., Itoh,Y., Chiba,T., Kajita,M., Yana,I. and Seiki,M. (2001) Membrane-type 5 matrix metalloproteinase is expressed in differentiated neurons and regulates axonal growth. Cell Growth Differ., 12, 573–580. [PubMed] [Google Scholar]
- Hebb D.O. (1949) Organization of Behavior. Wiley, New York, NY. [DOI] [PubMed]
- Heo J.H., Lucero,J., Abumiya,T., Koziol,J.A., Copeland,B.R. and del Zoppo,G.J. (1999) Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab., 19, 624–633. [DOI] [PubMed] [Google Scholar]
- Herdegen T. and Leah,J.D. (1998) Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Brain Res. Rev., 28, 370–490. [DOI] [PubMed] [Google Scholar]
- Jaworski D.M. (2000) Developmental regulation of membrane type-5 matrix metalloproteinase (MT5-MMP) expression in the rat nervous system. Brain Res., 860, 174–177. [DOI] [PubMed] [Google Scholar]
- Jaworski J. et al. (1999) Neuronal excitation-driven and AP-1-dependent activation of timp-1 gene expression in rodent hippocampus. J. Biol. Chem., 274, 28106–28112. [DOI] [PubMed] [Google Scholar]
- Jiang X., Namura,S. and Nagata,I. (2001) Matrix metalloproteinase inhibitor KB-R7785 attenuates brain damage resulting from permanent focal cerebral ischemia in mice. Neurosci. Lett., 305, 41–44. [DOI] [PubMed] [Google Scholar]
- Job C. and Eberwine,J.(2001) Localization and translation of mRNA in dendrites and axons. Nat. Rev. Neurosci., 2, 889–898. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. (1993) Molecular biology of vertebrate learning: is c-fos a new beginning? J. Neurosci. Res., 34, 377–381. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. (2002) c-Fos in learning: beyond the mapping of neuronal activity. In Kaczmarek,L. and Robertson,H.A. (eds), Handbook of Chemical Neuroanatomy: Immediate Early Genes and Inducible Transcription Factors in Mapping of the Central Nervous System Function and Dysfunction. Elsevier, Amsterdam, The Netherlands, pp. 189–216.
- Kaczmarek L. and Chaudhuri,A. (1997) Sensory regulation of immediate-early gene expression in mammalian visual cortex: implications for functional mapping and neural plasticity. Brain Res. Rev., 23, 237–256. [DOI] [PubMed] [Google Scholar]
- Kaminska B., Filipkowski,R.K., Zurkowska,G., Lason,W., Przewlocki,R. and Kaczmarek,L. (1994) Dynamic changes in composition of AP-1 transcription factor DNA binding activity in rat brain following kainate induced seizures and cell death. Eur. J. Neurosci., 6, 1558–1566. [DOI] [PubMed] [Google Scholar]
- Kridel S.J., Chen,E., Kotra,L.P., Howard.E.W., Mobashery.S. and Smith,J.W. (2001) Substrate hydrolysis by matrix metalloproteinase-9. J. Biol. Chem., 276, 20572–20578. [DOI] [PubMed] [Google Scholar]
- Lee R., Kermani,P., Teng,K.K. and Hempstead,B.L. (2001) Regulation of cell survival by secreted proneurotrophins. Science, 294, 1945–1948. [DOI] [PubMed] [Google Scholar]
- Leppert D., Lindberg,R.L.P., Kappos,L. and Leib,S.L. (2001) Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res. Rev., 36, 249–257. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhou,X., Shapiro,S.D., Shipley,J.M., Twining,S.S., Diaz,L.A., Senior,R.M. and Werb,Z. (2000) The serpin α1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell, 102, 647–655. [DOI] [PubMed] [Google Scholar]
- Logan S.K., Garabedian,M.J., Campbell,C.E. and Werb,Z. (1996) Synergistic transcriptional activation of the tissue inhibitor of metallproteinases-1 promoter via functional intercation of AP-1 and Ets-1 transcription factors. J. Biol. Chem., 271, 774–782. [DOI] [PubMed] [Google Scholar]
- Lukes A., Mun-Bryce,S., Lukes,M. and Rosenberg,G.A. (1999) Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol. Neurobiol., 19, 267–284. [DOI] [PubMed] [Google Scholar]
- Madani R., Hulo,S., Toni,N., Madani,H., Steimer,T., Muller,D. and Vassalli,J.D. (1999) Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J., 18, 3007–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.C., Barad,M. and Kandel,E.R. (2000) Local protein synthesis and its role in synapse-specific plasticity. Curr. Opin. Neurobiol., 10, 587–592. [DOI] [PubMed] [Google Scholar]
- Massova I., Kotra,L.P., Fridman,R. and Mobashery,S. (1998) Matrix metalloproteinases: structures, evolution, and diversification. FASEB J., 12, 1075–1095. [PubMed] [Google Scholar]
- Meiri N., Masos,T., Rosenblum,K., Miskin,R. and Dudai,Y. (1994) Overexpression of urokinase-type plasminogen activator in transgenic mice is correlated with impaired learning. Proc. Natl Acad. Sci. USA, 91, 3196–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.A. et al. (2002) Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature, 418, 422–425. [DOI] [PubMed] [Google Scholar]
- Mun-Bryce S. and Rosenberg,G.A. (1998) Matrix metalloproteinases in cerebrovascular disease. J. Cereb. Blood Flow Metab., 18, 1163–1172. [DOI] [PubMed] [Google Scholar]
- Nedivi E., Hevroni,D., Naot,D., Israeli,D. and Citri,Y. (1993) Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature, 363, 718–722. [DOI] [PubMed] [Google Scholar]
- Pagenstecher A., Stalder,A.K. and Campbell,I.L. (1997) RNAse protection assays for the simultaneous and semiquantitative analysis of multiple murine matrix metalloproteinase (MMP) and MMP inhibitor mRNAs. J. Immunol. Methods, 206, 1–9. [DOI] [PubMed] [Google Scholar]
- Qian Z., Gilbert,M.E., Colicos,M.A., Kandel,E.R. and Kuhl,D. (1993) Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature, 361, 453–457. [DOI] [PubMed] [Google Scholar]
- Rivera S., Tremblay,E., Timsit,S., Canals,O., Ben-Ari,Y. and Khrestchatisky,M. (1997) Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J. Neurosci., 17, 4223–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S., Ogier,C., Jourquin,J., Timsit,S., Szklarczyk,A., Miller,K., Gearing,A.J.H., Kaczmarek,L. and Khrestchatisky,M. (2002) Gelatinase B and TIMP-1 are regulated in a cell- and time-dependent manner in association with cell demise, neuroinflammation and repair processes after global forebrain ischemia. Eur. J. Neurosci., 15, 19–32. [DOI] [PubMed] [Google Scholar]
- Rohrbough J., Grotewiel,M.S., Davis,R.L. and Broadie,K. (2000) Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J. Neurosci., 20, 6868–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanic A.M., White,R.F., Arleth,A.J., Ohlstein,E.H. and Barone,F.C. (1998). Matrix metalloproteinase expression increases after cerebral focal ischemia in rats. Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke, 29, 1020–1030. [DOI] [PubMed] [Google Scholar]
- Rooprai H.K. and McCormick,D. (1997) Proteases and their inhibitors in human brain tumours: a review. Anticancer Res., 17, 4151–4162. [PubMed] [Google Scholar]
- Rosa G., Ceccarini,M., Cavaldesi,M., Zini,M. and Petrucci,T.C. (1996) Localization of the dystrophin binding site at the carboxyl terminus of β-dystroglycan. Biochem. Biophys. Res. Commun., 223, 272–277. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. (1995) Matrix metalloproteinases in brain injury. J. Neurotrauma, 12, 833–842. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A., Navratil,M., Barone,F. and Feuerstein,G.Z. (1996) Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J. Cereb. Blood Flow Metab., 16, 360–366. [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A., Estrada,E.Y. and Dencoff,J.E. (1998) Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke, 29, 2189–2195. [DOI] [PubMed] [Google Scholar]
- Sato H. and Seiki,M. (1993) Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene, 8, 395–405. [PubMed] [Google Scholar]
- Seeds N.W., Williams,B.L. and Bickford,P.C. (1995) Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science, 270, 1992–1994. [DOI] [PubMed] [Google Scholar]
- Sekine-Aizawa Y. et al. (2001) Matrix metalloproteinase (MMP) system in brain: identification and characterization of brain-specific MMP highly expressed in cerebellum. Eur. J. Neurosci., 13, 935–948. [DOI] [PubMed] [Google Scholar]
- Sternlicht M.D. and Werb,Z. (2001) How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol., 17, 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O. and Schuman,E.M. (2001) Protein synthesis at synaptic sites on dendrites. Annu. Rev. Neurosci., 24, 299–325. [DOI] [PubMed] [Google Scholar]
- Sugita S., Saito,F., Tang,J., Satz,J., Campbell,K. and Sudhof,T.C. (2001) A stoichiometric complex of neurexins and dystroglycan in brain. J. Cell Biol., 154, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk A., Lapinska,J., Rylski,M., McKay,R.D.G. and Kaczmarek,L. (2002) Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci., 22, 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taishi P., Sanchez,C., Wang,Y., Fang,J., Harding,J.W. and Krueger,J.M. (2001) Conditions that affect sleep alter the expression of molecules associated with synaptic plasticity. Am. J. Physiol. Regul. Integr. Comp. Physiol., 281, R839–R845. [DOI] [PubMed] [Google Scholar]
- Vaillant C., Didier-Bazes,M., Hutter,A., Belin,M.F. and Thomasset,N. (1999) Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J. Neurosci., 19, 4994–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecil G.G., Larsen,P.H., Corley,S.M., Herx,L.M., Besson,A., Goodyer,C.G. and Yong,V.W. (2000) Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J. Neurosci. Res., 61, 212–224. [DOI] [PubMed] [Google Scholar]
- Vu T.H. and Werb,Z. (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev., 14, 2123–2133. [DOI] [PubMed] [Google Scholar]
- Woessner J.F. and Nagase,H. (2000) Matrix Metalloproteinases and TIMPs. Oxford University Press, Oxford, UK.
- Wright W., Eniko,A., Kramar,E.A., Meighan,S.E. and Harding,J.W. (2002) Extracellular matrix molecules, long-term potentiation, memory consolidation and the brain angiotensin system. Peptides, 23, 221–246. [DOI] [PubMed] [Google Scholar]
- Yamada H. et al. (2001) Processing of β-dystroglycan by matrix metalloproteinase disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum. Mol. Genet., 10, 1563–1569. [DOI] [PubMed] [Google Scholar]
- Yong V.W., Krekoski,C.A., Forsyth,P.A., Bell,R. and Edwards,D.R. (1998) Matrix metalloproteinases and diseases of the central nervous system. Trends Neurosci., 21, 75–80. [DOI] [PubMed] [Google Scholar]
- Yong V.W., Power,C., Forsyth,P. and Edwards,D.R. (2001) Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci., 2, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. and Shiosaka,S. (1999) Plasticity-related serine proteases in the brain. Int. J. Mol. Med., 3, 405–409. [DOI] [PubMed] [Google Scholar]
- Zhang J.W., Deb,S. and Gottschall,P.E. (1998) Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur. J. Neurosci., 10, 3358–3368. [DOI] [PubMed] [Google Scholar]