Abstract
Gelsolin is a widely distributed actin binding protein involved in controlling cell morphology, motility, signaling and apoptosis. The role of gelsolin in tumor progression, however, remains poorly understood. Here we show that expression of green fluorescent pro tein (GFP)-tagged gelsolin in MDCK-AZ, MDCKtsSrc or HEK293T cells promotes invasion into collagen type I. In organ culture assays, MDCK cells expressing gelsolin–GFP invaded pre-cultured chick heart fragments. Gelsolin expression inhibited E-cadherin-mediated cell aggregation but did not disrupt the E-cadherin–catenin complex. Co-expression of dominant-negative Rac1N17, but not RhoAN19 or Cdc42N17, counteracted gelsolin-induced invasion, suggesting a requirement for Rac1 activity. Increased ARF6, PLD or PIP5K 1α activity canceled out gelsolin-induced invasion. Furthermore, we found that invasion induced by gelsolin is dependent on Ras activity, acting through the PI3K–Rac pathway via the Ras guanine nucleotide exchange factor Sos-1. These findings establish a connection between gelsolin and the Ras oncogenic signaling pathway.
Keywords: actin binding protein/collagen invasion/gelsolin/Ras signaling
Introduction
Vertebrate cells harbor an extensive repertoire of actin binding proteins which, by virtue of their specific interaction with monomeric or filamentous actin, enable the cell to control diverse physiological processes including cell morphology, cell motility, wound repair, cytokinesis, endocytosis and differentiation. Tumor-associated processes such as invasion and metastasis are known to be critically dependent on dynamic alterations in the organization of the actin cytoskeleton. Dysregulation of actin-based motility is regarded as a prominent factor in cell transformation, and is very probably associated with tumorigenesis (Kassis et al., 2001). This may arise either through modulation of the properties of actin regulatory proteins, or direct modulation of their expression levels. The latter is corroborated by gene-expression profiling studies using DNA microarrays. Clark et al. (2000) identified a set of genes selectively upregulated in metastatic mouse and human melanoma cells. Several of these genes encode proteins that regulate the actin cytoskeleton directly or indirectly and include thymo sin β4, α-actinin-1, calmodulin, α-catenin and IQGAP1. Kobayashi et al. (2002) recently showed that upregulation of thymosin β4 correlated with tumorigenicity of malignant mouse fibrosarcoma cells.
Gelsolin is the prototype and founding member of a family of actin binding proteins involved in controlling the organization of the actin cytoskeleton in cells (reviewed in Sun et al., 1999). Upon activation by calcium, they fragment and cap the fast-growing end of actin filaments. Removal of gelsolin from the (+) end of actin filaments (uncapping) favors actin polymerization and is controlled by phospholipids such as phosphatidylinositol 4,5-bisphosphate (PIP2) (Hartwig et al., 1995; Tolias et al., 2000) or lysophosphatidic acid (Meerschaert et al., 1998). Cells deficient in gelsolin exhibit defective chemotaxis and wound healing, and are defective in neurite retrac tion (Witke et al., 1995; Lu et al., 1997). Conversely, overexpression of gelsolin increases membrane ruffling and chemotaxis (Cunningham et al., 1991).
Although the biochemistry of gelsolin and the structure–function relationship with respect to calcium, PIP2 and actin binding have been well characterized, its potential value as a prognostic marker and its direct role in tumorigenesis remain unclear. A number of studies have shown downregulation of gelsolin in breast cancers of humans, mice and rats (Asch et al., 1996, 1999), lung cancers of heavy smokers (Dosaka-Akita et al., 1998), prostate cancers (Lee et al., 1999) and ovarian cancer (Afify and Werness, 1998). Shieh et al. (1999) found high expression of gelsolin in a subset (14%) of human early stage non-small cell lung cancers. However, it is unclear whether altered gelsolin expression levels in tumors are merely a side-effect, or reflect a more general and direct causal relationship with tumorigenesis. Only in the case of bladder cancer has inhibition of tumor growth by transfection of gelsolin been demonstrated (Tanaka et al., 1995, 1999).
To assess whether modulation of cytosolic gelsolin levels triggers changes in cellular behavior, we investigated whether gelsolin promotes invasion of cells. We demonstrate that cells overexpressing gelsolin invade into a collagen type I matrix, but also into pre-cultured chick heart fragments. We show that these events are dependent on the Ras–PI3K–Rac signal transduction pathway. Our findings establish a causal relationship between gelsolin expression and in vitro invasion by way of signaling through Ras.
Results
Gelsolin expression induces invasion of MDCK-AZ cells
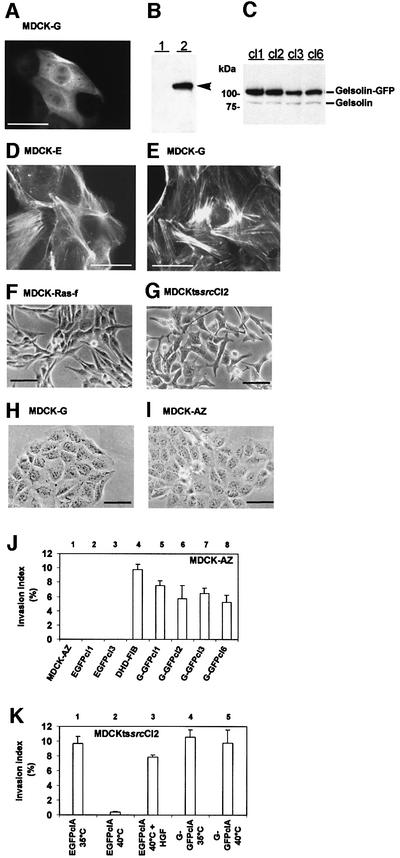
Collagen invasion. Expression of green fluorescent protein-tagged gelsolin (G-GFP) in MDCK-AZ cells resulted in an evenly distributed cytosolic localization of the fusion protein, with no particular enrichment in sub cellular compartments or regions (Figure 1A). Western blots on cytosolic extracts revealed the presence of a 105 kDa protein in MDCK-AZ cells that express G-GFP (MDCK-G cells) (Figure 1B). When parental MDCK-AZ or MDCK-G cells were stained for F-actin, no significant changes in the overall organization of the actin cytoskeleton, or in the distribution and quantity of actin stress fibers, were noticed (Figure 1D and E), suggesting that expression of G-GFP does not induce massive F-actin depolymerization through its F-actin severing activity in resting MDCK-AZ cells. Furthermore, MDCK-G cells (Figure 1H) displayed no overt alterations in cell morphology in comparison with MDCK-AZ cells (Figure 1I), unlike MDCK-Ras-f cells or a temperature-sensitive mutant of the v-Src kinase (Figure 1F and G).
Fig. 1. Gelsolin expression promotes collagen type I invasion of MDCK-AZ cells and MDCKtssrcCl2 cells. (A) Epifluorescence microscopy image of MDCK-G cells. (B) Western blot analysis on MDCK-AZ cytosolic proteins (lane 1) or MDCK-G cytosolic proteins (lane 2), probed with anti-GFP antibodies. G-GFP is indicated by an arrowhead in (B). (C) Western blot analysis on MDCK-G cytosolic proteins (clones 1–3 and 6) probed with a monoclonal anti-gelsolin antibody. Endogenous and GFP-tagged gelsolin are indicated. (D) Texas Red–phalloidin staining of MDCK-AZ cells expressing EGFP. (E) Texas Red–phalloidin staining of MDCK-G cells. (F–I) Phase- contrast images. Fibroblastic phenotype of MDCK-Ras-f cells (F) and MDCKtssrcCl2 cells, grown at 35°C (G). (H) MDCK-G cells. (I) MDCK-AZ cells. Bar in (A), (D) and (E) = 40 µm; bar in (F), (G), (H) and (I) = 50 µm. (J) Collagen invasion index levels of four independent MDCK-G clones. MDCK-AZ and MDCK-E cells were used as negative controls; DHD-FIB is a positive control. (K) Collagen invasion of MDCKtssrcCl2 G-GFP cells. EGFP-expressing MDCKtssrcCl2 cells are only invasive at the v-Src permissive temperature. MDCKtssrcCl2 G-GFP cells are invasive at the v-Src permissive and non-permissive temperatures. Data are means ± SE of three independent experiments.
Several independent MDCK-G clones (western blots are shown in Figure 1C) were tested for invasion into collagen type I. Whereas parental MDCK-AZ or EGFP-expressing MDCK-AZ cells (MDCK-E, control) lacked any invasive potential (Figure 1J, 1–3), all MDCK-G clones tested were found to invade the collagen matrix (Figure 1J, 5–8). Similar findings were obtained with independent MDCKtssrcCl2 clones (Figure 1K). EGFP-expressing MDCKtssrcCl2 cells were invasive only at the v-Src permissive temperature of 35°C (Figure 1K, 1 and 2). As reported previously for MDCKtssrcCl2 cells (Kotelevets et al., 1998), collagen invasion of EGFP-expressing MDCKtssrcCl2 cells at 40°C (the v-Src non-permissive temperature) could be induced by addition of hepatocyte growth factor (HGF) (Figure 1K, 3). Significantly, at 40°C, G-GFP-expressing MDCKtssrcCl2 cells were invasive in the absence of HGF (Figure 1K, 5). Taken together, these findings indicate that elevated cytosolic levels of gelsolin can trigger invasion.
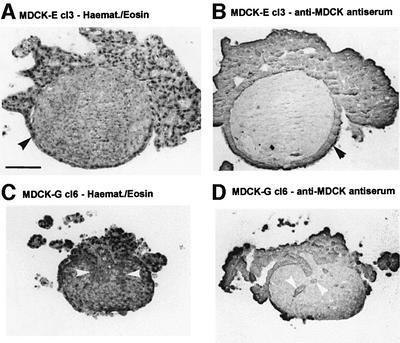
Invasion into chick heart fragments (PHF). To further assess the invasive capacity of MDCK-G cells, we performed stringent assays involving confrontation experiments with embryonic chick heart fragments. In addition to several collagen types, chick heart extracellular matrix (ECM) contains fibronectin and laminin (Bracke et al., 2001b). Confrontation treatments of MDCK-E cells with embryonic chick heart tissue resulted in formation of an epithelioid cell layer around the tissue (Figure 2A and B, black arrowheads). However, no invasion of chick heart fragments was observed. By contrast, MDCK-G cl6 cells failed to form an epithelioid layer and were also detected inside the tissue (Figure 2C and D, white arrowheads), indicating that gelsolin expression promotes invasion of MDCK-AZ cells into chick heart fragments.
Fig. 2. Invasion of MDCK-G cells in pre-cultured chick heart fragments. Sections of confrontation cultures of MDCK-E cl3 control cells (A and B) and MDCK-G cl6 (C and D) cells, fixed after 7 days and stained with hematoxylin–eosin (A and C) or with antiserum against MDCK cells (B and D). Black arrowheads in (A) and (B) mark MDCK-E cells. White arrowheads in (C) and (D) mark invasive MDCK-G cells. Bar = 100 µm.
Gelsolin expression inhibits aggregation of MDCK-AZ cells
The effect of gelsolin expression on E-cadherin-mediated cell aggregation was investigated by both slow (under static conditions) and fast (Gyrotory shaking) aggregation assays in the absence or presence of DECMA-1, a monoclonal E-cadherin neutralizing antibody.
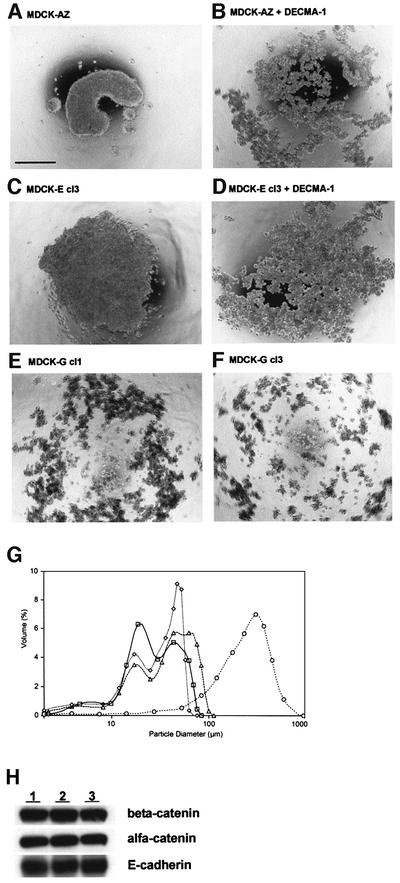
In slow aggregation assays, MDCK-AZ and MDCK-E cells aggregated as relatively large cell clusters in the absence of DECMA-1 (Figure 3A and C). Addition of DECMA-1 antibody resulted in the formation of numerous smaller cell colonies (Figure 3B and D). Interestingly, MDCK-G cells formed colonies with a size compar able to MDCK-E cells in the absence of DECMA-1 (Figure 3E and F, compared with D), suggesting that gelsolin expression affects cell aggregation similarly to DECMA-1. These observations were further investigated by fast aggregation assays where the particle diameter was measured with an LS particle size analyzer. Figure 3G shows that the particle diameter of MDCK-E is much larger than that of MDCK-G in the absence of DECMA-1 antibody (Figure 3G, compare circles with triangles). Treatment of MDCK-E cells with DECMA-1 antibody (Figure 3G, diamonds) drastically reduced their particle diameter to a size comparable to MDCK-G. To test whether gelsolin perturbs E-cadherin-mediated formation of cell–cell contacts, we investigated whether MDCK-G cells contain an intact E-cadherin–β-catenin–α-catenin complex. Immunoprecipitation of β-catenin from MDCK-G cells showed that associated α-catenin and E-cadherin levels in MDCK-G cells were similar to those found in MDCK-AZ or MDCK-E cells (Figure 3H), suggesting that gelsolin does not promote alterations in expression level of either of these components, or competitively interferes with formation of the complex.
Fig. 3. Gelsolin overexpression inhibits E-cadherin-mediated cell aggregation. (A–F) Slow aggregation assay. Phase-contrast images are shown of MDCK-AZ cells in the absence (A) or presence (B) of DECMA-1 antibody. (C) and (D) depict MDCK-E cl3 cells in the absence (C) or presence (D) of DECMA-1 antibody. (E) and (F) depict MDCK-G cl1 and MDCK-G cl3, respectively. Bar = 500 µm. (G) Fast aggregation assay. Plotted curves of relative volume distribution (Y-axis) as a function of particle diameter (X-axis) are shown for MDCK-E cl3 without DECMA-1 antibody after 30 min (open circle), MDCK-E cl3 with DECMA-1 antibody at 0 min (open square) and 30 min (open diamond) and MDCK-G cl1 without DECMA-1 antibody at 30 min (open triangle). (H) Western blots showing MDCK-G cells contain an intact E-cadherin–catenin complex. β-catenin was immunoprecipitated from MDCK-AZ (lane 1), MDCK-E cl3 (lane 2) or MDCK-G cl3 (lane 3) lysates and probed with anti-β-catenin antiserum (top panel). The blot was stripped and reprobed with anti-α-catenin (middle panel) or pan anti-E-cadherin antibodies (bottom panel).
Expression of dominant-negative Rac1 (Rac1N17) abolishes gelsolin-induced collagen invasion
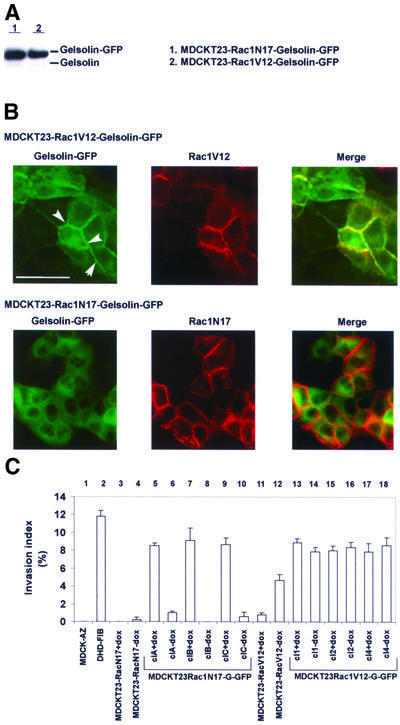
Small GTPases play a critical role in cell transformation (Schmitz et al., 2000). Rac, for example, is a downstream effector of Ras in cell transformation (Qiu et al., 1995). Azuma et al. (1998) demonstrated that Rac expression is upregulated in fibroblasts derived from gelsolin-deficient mice and that these fibroblasts showed defects in motility behavior. To investigate the contribution of Rac activity in gelsolin-induced invasion, we employed MDCKT23 cells expressing activated Rac1 (MDCKT23-Rac1V12) or dominant-negative Rac (MDCKT23-Rac1N17) in a doxycyclin-regulated manner (Jou and Nelson, 1998). MDCKT23-Rac1N17 and MDCKT23-Rac1V12 cell lines that stably express G-GFP were generated and we termed these clones MDCKT23-Rac1N17-Gelsolin-GFP and MDCKT23-Rac1V12-Gelsolin-GFP, respectively.
Western blots on lysates from MDCKT23-Rac1N17-Gelsolin-GFP and MDCKT23-Rac1V12-Gelsolin-GFP clones revealed that gelsolin expression levels were com parable to those obtained in MDCK-AZ cells (Figure 4A). Gelsolin localized in cell–cell contacts in MDCKT23-Rac1V12-Gelsolin-GFP (Figure 4B, top panel, white arrowheads), and co-localized with Rac1V12, a phenomenon that was not observed in MDCKT23-Rac1N17-Gelsolin-GFP cells (Figure 4B, lower panels; see also Supplementary figure 1 available at The EMBO Journal Online). Gelsolin expression had no appreciable effect on the invasive properties of MDCKT23-Rac1V12 cells in the absence or presence of doxycyclin (Figure 4C, 13–18). However, expression of Rac1N17 abrogated gelsolin-induced collagen invasion in a doxycyclin-regulated manner (Figure 4C, 5–10). Thus, Rac activity appears to be a critical factor in the pathway leading to gelsolin-mediated invasion.
Fig. 4. Rac1N17 inhibits invasion induced by gelsolin in MDCKT23 cells. (A) Western blot showing gelsolin expression levels in stable transfected MDCKT23-Rac1N17-Gelsolin-GFP (clC; lane 1) and MDCKT23-Rac1V12-Gelsolin-GFP (cl1; lane 2). The blot was probed with anti-gelsolin antibody. (B) G-GFP is targeted to cell–cell contacts in MDCKT23-Rac1V12-Gelsolin-GFP cells. Upper three panels: gelsolin co-localizes with activated Rac1. Left: G-GFP staining pattern in MDCKT23-Rac1V12 cells. Middle: staining pattern of activated Rac1. Right: merged image showing co-localization between G-GFP and Rac1V12 (yellow). Gelsolin localizes at cell–cell contacts (arrowheads), and co-localization is strong in cells with high Rac1V12 expression. Lower three panels: G-GFP does not co-localize with inactivated Rac1 (Rac1N17). Left: G-GFP staining pattern. Middle: Rac1N17 staining. Right: merged image. Bar = 50 µm. (C) Rac1N17 blocks gelsolin-mediated invasion of collagen gels. Induction of Rac1N17 expression in three representative MDCKT23-Rac1N17-Gelsolin-GFP clones (‘-dox’) counteracts invasion (compare lanes 6, 8 and 10 with 5, 7 and 9, respectively). Induction of Rac1V12 expression in three representative MDCKT23-Rac1V12-Gelsolin-GFP clones does not affect invasion index levels (compare lanes 14, 16 and 18 with 13, 15 and 17, respectively). Lanes 3 and 4, parental MDCKT23-Rac1N17 cells; lanes 11 and 12, parental MDCKT23-Rac1V12 cells. Lane 1, MDCK-AZ cells (negative control). Lane 2, positive control. Results are representative of three independent experiments (mean ± SE).
Gelsolin-induced invasion of HEK293T cells
We also investigated whether gelsolin promotes invasion of other cell types. To this end we performed collagen invasion assays using HEK293T cells. This approach enabled us to reconstruct, in part, the signaling pathway leading to invasion through co-transfection experiments with cDNA constructs encoding signaling components, thereby circumventing the need to generate stable cell lines.
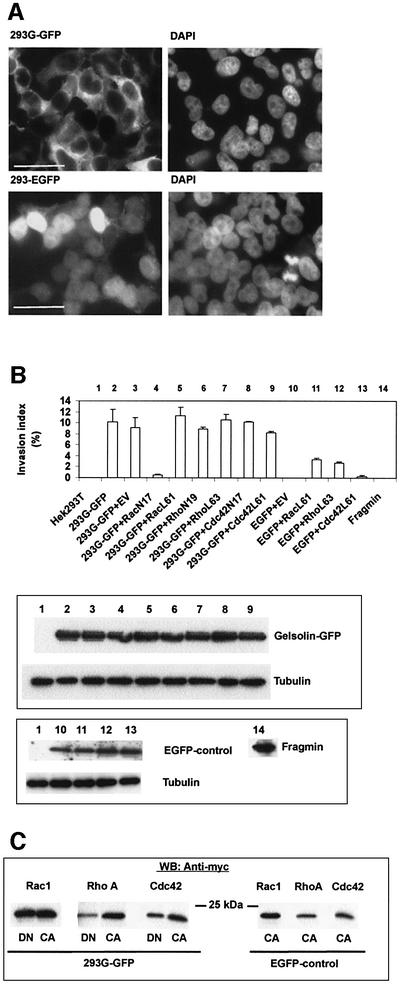
G-GFP in HEK293T cells (Figure 5A) showed the same subcellular distribution as in stable transfected MDCK-AZ or MDCKT23 cells. We calculated that 60–70% of all cells expressed the fusion protein (or EGFP) after 48 h, as judged by epifluorescence microscopy. Untransfected HEK293T, or HEK293T cells expressing EGFP alone, did not invade the collagen matrix (Figure 5B, 1 and 10). However, HEK293T-Gelsolin-GFP cells (293G-GFP) were invasive (Figure 5B, 2 and 3). Similar findings were obtained with HCT8/E-11 colon adenocarcinoma cells, or with a gelsolin cDNA construct encoding untagged cytoplasmic gelsolin (data not shown). Interestingly, expression of fragmin, an actin binding protein from Physarum with properties similar to gelsolin (Constantin et al., 1998), did not promote collagen invasion of HEK293T cells (Figure 5B, 14).
Fig. 5. Co-transfection of Rac1N17, but not of RhoN19 or Cdc42N17, abrogates gelsolin-induced collagen type I invasion of HEK293T cells. (A) Epifluorescence images of HEK293T cells transiently transfected with G-GFP (upper left) or EGFP (lower left). Right panels show corresponding DAPI staining of nuclei. Bar = 50 µm. (B) Collagen invasion. Upper panel: invasion index levels (mean ± SE, n = 3) are shown for parental HEK293T cells (lane 1, negative control) and gelsolin-transfected HEK293T cells (293G-GFP, lane 2). Lanes 4–9 represent co-transfections between G-GFP and activated or inactivated Rac1 (lanes 4 and 5), RhoA (lanes 6 and 7) or Cdc42 (lanes 8 and 9). Lanes 3 and 10–13 are controls. Lane 14, fragmin does not induce invasion. EV, empty vector. Middle panel: western blot on cytosolic proteins from transfected HEK293T cells showing equal expression levels of G-GFP or tubulin. The numbers at the top correspond to numbers in the histogram. Lower panel: EGFP expression (upper autoradioagram) in cells that were double transfected with empty vector or activated GTPases. Lower autoradiogram: tubulin. Fragmin was visualized using affinity-purified anti-fragmin antibodies. (C) Western blots showing expression of Rac, Rho or Cdc42, co-transfected with G-GFP (left panel) or with EGFP (right panel). DN, dominant-negative; CA, constitutively active.
Co-transfection of Rac1L61 or Rac1N17 with G-GFP showed that Rac1N17 blocked invasion induced by the actin binding protein (Figure 5B, 4 and 5). The observation that RhoN19 did not counteract the effect of gelsolin in HEK293T cells (Figure 5B, 6) suggests that Rho is not implicated in the signal transduction pathway leading to invasion mediated by gelsolin. Furthermore, neither C3 transferase (3 µg/ml), an inhibitor of Rho, nor Y27632 (1 µM), an inhibitor of Rho-kinase (ROCK), blocked MDCK-G invasion to a significant degree (data not shown). Similarly, Cdc42N17 did not counteract gelsolin- induced invasion (Figure 5B, 8). Differences in collagen invasion were not due to lack of expression of small GTPases (Figure 5C).
Contribution of the ARF6-PLD/ARF6-PIP5K 1α pathways
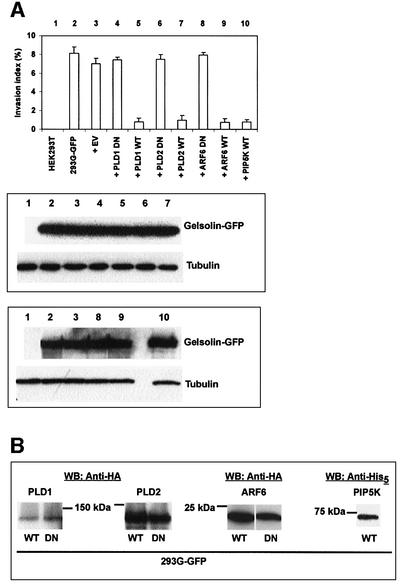
Tolias et al. (2000) demonstrated that Rac-induced uncapping of gelsolin from filament ends in thrombin-stimulated platelets is mediated by activation of phosphatidylinositol 4-phosphate 5-kinase 1α (PIP5K 1α). In HeLa cells, activation of PIP5K 1α by ADP ribosylation factor 6 (ARF6) required the product of PLD activity, phosphatidic acid (Honda et al., 1999). Santy and Casanova (2001) showed that ARNO acts as an exchange factor for ARF6, and that ARF6 can lead to activation of Rac but also to activation of phospholipase D (PLD). Considering the role of Rac1 in gelsolin invasion, we investigated the contribution of the ARF6-PIP5K 1α and ARF6-PLD pathways in HEK293T cells.
Co-expression of dominant-negative PLD1 or PLD2 with G-GFP did not affect invasion of 293G-GFP cells (Figure 6A, top panel, 4 and 6). By contrast, overexpression of wild-type PLD1 or PLD2 (Colley et al., 1997a,b) resulted in strong inhibition of invasion (Figure 6A, top panel, 5 and 7), suggesting that increased PLD activity antagonizes gelsolin invasion. Dominant-negative ARF6 had no effect on gelsolin invasion either, whereas co-expression of wild-type ARF6 with G-GFP abrogated invasion (Figure 6A, top panel, 8 and 9). Finally, wild-type PIP5K 1α also inhibited gelsolin invasion (Figure 6A, top panel, 10). Combined, these findings suggest that the ARF6 pathway leading to activation of Rac1-PIP5K 1α or PLD is not a prerequisite for gelsolin invasion. The lower panels in Figure 6A and B show equal expression levels of G-GFP and detection of PLD, ARF6 and PIP5K 1α epitope-tagged proteins.
Fig. 6. ARF6, PLD or PIP5 1α kinase activity is not required for collagen invasion induced by gelsolin. (A) Upper panel: histogram showing collagen invasion index levels (mean ± SE, n = 3) of HEK293T cells co-transfected with PLD1 (lanes 4 and 5), PLD2 (lanes 6 and 7), ARF6 (lanes 8 and 9) or PIP5K 1α (lane 10) and G-GFP. Lanes 1–3 represent controls. Middle panel: western blots showing expression of gelsolin and tubulin (control for equal loading) in PLD1 (4 and 5) or PLD2 (6 and 7) HEK293T cells co-transfected with G-GFP. Numbers at the top correspond to numbers in the top panel. Bottom panel: similar to above but for ARF6 (8 and 9) and PIP5K 1α (10). (B) Western blots showing expression of PLD1 and 2, ARF6 and PIP5K 1α.
Involvement of the Ras–PI3K kinase pathway in gelsolin-induced invasion
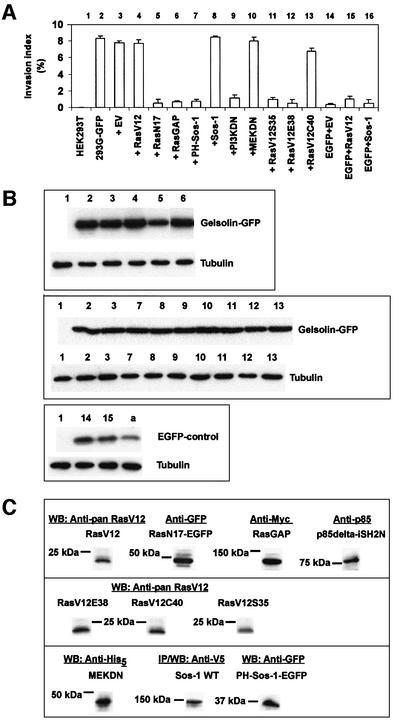
The Ras guanine nucleotide exchange factor (GEF) son of sevenless (Sos-1) couples Ras signaling to Rac via distinct associations with Grb2 and Eps8/E3b1, respectively (Innocenti et al., 2002). We explored the role of these signaling proteins in gelsolin invasion by co-transfection experiments. Co-expression of G-GFP and activated H-Ras (RasV12) did not significantly affect invasion index levels of gelsolin (Figure 7A, compare 4 with 2). Co-expression of G-GFP and dominant-negative Ras (RasN17) completely abrogated collagen invasion by gelsolin (Figure 7A, 5), suggesting that the signaling pathway leading to invasion induced by gelsolin requires Ras activity. Since expression of RasN17 downregulated expression of G-GFP but also EGFP [Figure 7B, compare 5 with 2–4 in the upper panel and compare (a) with 14 and 15 in the lower panel], we employed RasGAP, a GTPase-activating protein that converts Ras-GTP to Ras-GDP and inhibits Ras function (Iida et al., 2001). Co-expression of RasGAP with gelsolin also inhibited invasion (Figure 7A, 6).
Fig. 7. Collagen invasion mediated by gelsolin is dependent on Ras-PI3K activity. (A) Histogram. RasN17-EGFP (lane 5), RasGAP (lane 6), PH-Sos-1-EGFP (lane 7) and p85ΔiSH2N (‘PI3KDN’, lane 9) counteract invasion. WT Sos-1 (lane 8) or MEKDN (lane 10) shows no effect. RasV12C40 shows no inhibition of invasion (lane 13). RasV12S35 (lane 11) and RasV12E38 (lane 12) inhibit collagen invasion by gelsolin. Lanes 1–3 and 14–16 are controls. Values are representative of three independent experiments (mean ± SE). (B) Upper panel: western blots on double transfected HEK293T cells demonstrating expression of G-GFP (tubulin is the control). Numbers correspond to numbers in the histogram in (A). Middle panel: western blots showing G-GFP and tubulin for lanes 7–13 in the histogram. Lanes 1–3 are controls. Lower panel: controls showing expression of EGFP corresponding to lanes 14 and 15 in the histogram. (a) illustrates that expression of EGFP, like G-GFP [(B) upper panel, lane 5], is also downregulated when co-expressed with RasN17-EGFP. (C) Western blots showing expression of RasV12, RasN17-EGFP, RasGAP, p85ΔiSH2N, RasV12E38, RasV12C40, RasV12S35, MEKDN, WT Sos-1 and PH-Sos-1-EGFP in cells co-transfected with G-GFP.
A potential role for Ras and Rac acting through the same pathway via Sos-1 was investigated by co-expression of the PH domain of Sos-1 with G-GFP. PH-Sos-1 has been reported to act in a dominant-negative manner towards Sos-1 (Chen et al., 1997). PH-Sos-1 counteracted invasion by gelsolin, whereas full-length Sos-1 showed no inhibitory effect (Figure 7A, 7 and 8), suggesting that invasion by gelsolin is dependent on a signaling mechanism whereby Ras and Rac act in the same pathway.
Ras signaling can bifurcate via PI3K and TIAM-1, leading to Rac activation (Sander et al., 1998), or the mitogen-activated protein kinase (MAPK) pathway through activation of Raf-1, which activates MEK (MAPKK) (Marshall, 1995). Dominant-negative MEK (Figure 7A, 10) or PD98059 (50 µM) (data not shown), a pharmacological inhibitor of MEK, did not abrogate gelsolin-induced invasion. Therefore, invasion mediated by gelsolin does not require signaling through the Raf–MEK–MAPK pathway. By contrast, co-expression of gelsolin with the p85 subunit of PI3K lacking its regulatory SH2 domain (p85ΔiSH2N; Hara et al., 1994), and which acts in a dominant-negative manner preventing PI3K activation, inhibited invasion (Figure 7A, 9). Wortmannin and LY294002, both potent and specific inhibitors of PI3K, also strongly inhibited invasion of MDCK-G cells (data not shown), suggesting that PI3K couples Ras signaling to Rac in the pathway leading to gelsolin-induced invasion. This was further investigated with RasV12 constructs containing additional point mutations in the Ras effector domain (amino acids 32–40). Previous studies have shown that RasV12C40 still activates PI3K, whereas RasV12S35 or RasV12E38 can activate Raf-1 (Withe et al., 1995; Rodriguez-Viciana et al., 1997). Co-transfection of these RasV12 effector mutants with G-GFP in HEK293T cells demonstrated that RasV12C40 did not inhibit collagen invasion by gelsolin, whereas RasV12S35 or RasV12E38 showed strong inhibition (Figure 7A, 11–13). These results further point to an important role for PI3K in gelsolin-induced invasion. An overview of all the transfection experiments is presented in Supplementary table I.
Discussion
Based on northern blotting, western blotting and immunocytochemistry experiments, downregulation of gelsolin has been demonstrated in various types of cancer. Whereas most non-small cell lung cancers express little or no gelsolin, a subset is characterized by high expression levels (Shieh et al., 1999); high focal expression of gelsolin correlated with lymphatic invasion. Rao et al. (2002) recently observed decreased gelsolin expression in premalignant and malignant lesions of urothelial carcinoma, but increased expression in the transition from non-invasive to invasive tumors. These authors argue that conversion of a non-invasive tumor to an invasive tumor may depend on acquiring a certain level of gelsolin expression and that epigenetic mechanisms may be involved in the regulation of gelsolin expression. Evidence in support of epigenetic mechanisms regulating expression of gelsolin has been reported in human breast cancer cells (Mielnicki et al., 1999). As a note of caution, not all tumor cells expressing high levels of gelsolin are invasive. For instance, gelsolin has been identified as a reliable marker for delineating the onset and progression of renal cystadenomas and carcinomas (Onda et al., 1999), but these tumors are not invasive.
Gelsolin is able to disrupt the actin cytoskeleton by virtue of its F-actin severing activity. Expression of gelsolin in fibroblasts causes a reduction in filamentous actin (Cunningham et al., 1991), which could result in reduced substrate-adhesive properties. However, several observations reported in this study do not support this. For instance, gelsolin(-GFP)-transfected MDCK cells showed no reduction in stress fibers or total actin levels. Also, MDCK-G cells showed the same, or reduced, motility levels in wound healing assays in comparison with MDCK-AZ cells (our unpublished observations). However, this observation requires confirmation through other motility assays. In chemoattractant or wound healing experiments, fibroblasts overexpressing gelsolin were found to display increased motility rates (Cunningham et al., 1991). Gelsolin may therefore affect motility differently in epithelial cells as compared with fibroblasts. Since fragmin was unable to induce collagen invasion, although it regulates actin dynamics in a calcium-dependent manner when expressed in CV1 or NIH 3T3 cells, we conclude that induction of collagen invasion is not a common trait of F-actin severing proteins, but appears closely related to an activity specific for gelsolin.
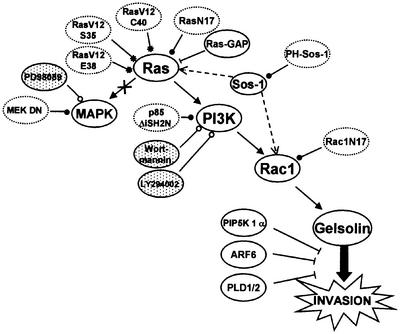
Gelsolin induced invasion of MDCK-AZ and HEK293T epithelial cells as well as HCT8/E-11 colon adenocarcinoma cells and this is dependent on signaling through the Ras–Rac pathway, with Ras acting through PI3K. A schematic overview is shown in Figure 8. No effect was observed by the MEK inhibitor PD98059 or dominant-negative MEK 1 (a component of the MAPK pathway), suggesting that the Raf–MEK–MAPK branch acting downstream of Ras is not involved. Loss-of-function mutants of Ras (Rodriguez-Viciana et al., 1997), the dominant-negative p85 subunit of PI3K (Hara et al., 1994) and specific PI3K inhibitors support a role for PI3K. A sustained hyperactivation of the PI3K pathway or the Raf–MAPK pathway was recently shown to be sufficient for tumorigenesis of Ras-transformed EpH4 mammary epithelial cells, whereas epithelial–mesenchymal transition and metastasis required Raf–MAPK activity in synergy with TGFβ receptor signaling (Janda et al., 2002). Gelsolin-induced invasion does not require activation of the MAPK pathway and this may suggest that elevated gelsolin levels would not lead to metastasis. Of note, in murine melanoma cells, Fujita et al. (2001) found that gelsolin suppresses metastasis.
Fig. 8. Schematic overview of the signal transduction pathway leading to gelsolin-induced invasion. Arrows and arcs point to activation and inactivation/blocking, respectively. Open and closed circles indicate pharmacological inhibitors (shaded) and transfection with dominant-negative constructs (dotted lines). Asterisks indicate Ras effector mutants. The crossed arrow means that this pathway is not implicated. The dotted arrow indicates direct binding. The thick arrow points to parallel or branching pathways. For details, see Discussion.
Rac1N17 inhibited gelsolin invasion, whereas RhoAN19 or Cdc42N17 was ineffective. This effect was independent of the level of gelsolin expression in stably transfected cells, suggesting that gelsolin’s activity is controlled by Rac. This finding further extends the observation that gelsolin is an essential downstream regulator of Rac (Azuma et al., 1998). Rac1N17 as well as Rac1V12 both localized at cell–cell contacts, as shown previously (Jou and Nelson, 1998), but gelsolin was targeted to cell–cell contacts only in Rac1V12-expressing MDCKT23 cells, suggesting that Rac1V12 is able to control the subcellular localization of gelsolin. It is unclear at present exactly if, and how, gelsolin translocation to cell–cell contacts contributes to the invasive phenotype. Although the E-cadherin–catenin complex remains intact in MDCK-G cells, we do not exclude the possibility that Rac-mediated gelsolin redistribution perturbs the interaction between the cell–cell adhesion complex and one of the many intracellular targets of catenin (Zhurinsky et al., 2000).
We have shown that increased ARF6, PLD or PIP5K 1α activities counteract gelsolin-induced invasion. The observation that wild-type PIP5K 1α or PLD (1 or 2) inhibits invasion mediated by gelsolin may not be entirely unexpected given that activation of PIP5K 1α by ARF6 in HeLa cells requires phosphatidic acid, the product of PLD activity (Honda et al., 1999). Consequently, assuming that PIP5K 1α activation is linked with PLD in the cellular models studied here, it may follow that when PLD overexpression blocks gelsolin-induced invasion, overexpression of PIP5K 1α causes a similar effect. PLD may inhibit gelsolin through direct interaction. Gelsolin has been reported to physically interact with PLD (Steed et al., 1996) and to inhibit recombinant PLD1 and PLD2 activities in vitro (Banno et al., 1999), but no information is available regarding their interaction in cells. However, it seems unlikely that the lack of effect on gelsolin invasion by dominant-negative PLD would be due to disruption of its interaction with gelsolin. We therefore favor the hypothesis that phosphatidic acid (or an effector of the lipid) counteracts gelsolin invasion when expressed at high levels. Whereas phosphatidic acid does not affect gelsolin’s severing activity (in contrast to PIP2), we have observed a direct interaction (Kd in the low micromolar range) by tryptophan fluorescence changes (our unpublished observations). An alternative hypothesis is that phosphatidic acid enhances PIP5K 1α activity (in)directly, thereby increasing cellular PIP2 levels. High PIP2 concentrations could sequester gelsolin and prevent its interaction with another component of the invasion pathway. The counter-invasive effects on gelsolin by wild-type ARF6 and PIP5K 1α are consistent with this idea.
Invasion requires adhesion to the ECM and proteolytic degradation of ECM components, allowing cells to intravasate/extravasate (Mareel et al., 1993). This process is mediated by a group of related proteases termed matrix metalloproteinases (MMPs; Egeblad and Werb, 2002). In mesenchymal cells, Rac has been reported to upregulate collagen type I-induced MMP2 activation (Zhuge and Xu, 2001) and integrin-induced MMP-1 expression (Kheradmand et al., 1998). Engers et al. (2001), on the other hand, recently reported that Rac upregulates expression of tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 in human epithelial tumor cells. A possible role for gelsolin as an intermediary component between Rac and distinct MMPs requires further analysis.
In conclusion, we present evidence that the actin binding protein gelsolin can function as an effector of the Ras–PI3K–Rac invasion-signaling pathway. Although other components of the signal transduction pathway remain to be discovered, the implementation of gelsolin in gene therapy studies should be considered in light of the data presented here. Suppression of metastasis has been reported by ectopic gelsolin expression (Tanaka et al., 1999), but we show here that it can trigger invasion.
Materials and methods
Reagents
Wortmannin (10 nM) and PD98059 (50 µM) were from Calbiochem (Darmstadt, Germany). Clostridium botulinum C3 transferase was kindly provided by Dr C.Gespach (INSERM U482, France) and Y27632 was from Sanofi Recherche (Toulouse, France). 4′,6-diamidino-2-phenyl indole (DAPI) was purchased from Sigma (St Louis, MO). LY294002 was obtained from Biomol Research Laboratories (Devon, UK). Texas Red-X–phalloidin and Alexa Fluor-594 goat anti-mouse IgG conjugate were obtained from Molecular Probes (Eugene, OR). HiFi Platinum Taq polymerase was from Invitrogen (Merelbeke, Belgium) and restriction enzymes were from New England Biolabs (Hertfordshire, UK).
Antibodies
See Supplementary data.
cDNA cloning
A cDNA encoding full-length human cytoplasmic gelsolin (Finidori et al., 1992) was cloned as a BamHI–HindIII fragment into pEGFP-N1 (Clontech, CA) by PCR using pSV51-gelsolin plasmid DNA as a template. G-GFP cDNA was also cloned into pcDNA6 (Invitrogen) for transfection of MDCKT23Rac1V12/N17 cells to allow for blasticidin S selection. For cDNA cloning of SOS-1 and PH-SOS-1, total RNA was isolated from HEK293T cells using the Qiagen RNeasy Mini Kit (Qiagen) and mRNA was reverse transcribed into first strand cDNA with a 5′RACE kit (Invitrogen), used according to the manufacturer’s instructions. Fifty microliter PCR reactions were set up containing 2.5 U of Taq polymerase, 2 µl of template cDNA and 0.8 µM primer. Thirty-five cycles of PCR were performed using the following primers: 5′-GGATCCATGTTTTATAATGGAAGGAACTCTTACAC-3′ and 5′-TCAGTGTACTCCGGTACTGTAAAGATATCAATGC-3′ for PH-SOS-1; and 5′-GGGAAGCTTATGCAGGCGCAGCAGCTGCCCT ACG-3′ and 5′-GGGTCTAGAGGAAGAATGGGCATTCTCCAACA GTG-3′ for full-length SOS-1. Following PCR, PH-SOS-1 was cloned into pcDNA3.1/CT-GFP-TOPO® and SOS-1 was cloned into the pcDNA3.1V5/His TOPO® expression vector (Invitrogen). The pcDNA3.1-fragmin expression vector has been described previously (Constantin et al., 1998). All constructs were verified by sequencing. See Supplementary data for other constructs.
Cell cultures
MDCK-AZ (Vleminckx et al., 1991) and HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen), supplemented with 10% fetal bovine serum (FBS; Invitrogen), 0.05% l-glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin. MDCKT23 cells, expressing Rac1V12 or Rac1N17 under the control of the tetracycline-repressible transactivator (Jou and Nelson, 1998), were grown in DMEM supplemented with 200 µg/ml hygromycin B (Clontech, CA) and 20 ng/ml doxycyclin (Clontech). Expression of Rac1V12 or Rac1N17 was induced by removing doxycyclin (Tet-off) from the culture medium for 48 h. DHD-FIB rat colon myofibroblasts (Dimanche-Boitrel et al., 1994) were used as a positive control in collagen type I invasion assays. For microscopic analysis, HEK293T cells were plated on coverslips coated with rat tail collagen type I (Becton Dickinson, Bedford, MA).
Transfection and generation of stable cell lines
HEK293T cells were transiently transfected using calcium phosphate. Forty-eight hours after transfection, cells were used for collagen inva sion assays and western blotting. MDCKtssrcCl2, MDCK-AZ, MDCKT23Rac1N17 and MDCKT23Rac1V12 cells were stably transfected with pEGFP-N1-Gelsolin using LipofectAMINE reagent (Invitrogen) according to the manufacturer’s instructions. Control transfections were performed with pEGFP-N1. MDCKtssrcCl2 and MDCK-AZ cells were selected in 1 mg/ml G418 (Duchefa, Haarlem, The Netherlands) and MDCKT23Rac1N17/V12 cells were selected in the presence of 4 µg/ml blasticidin S (ICN, Aurora, OH) for 2 weeks. Individual colonies were picked out and amplified.
Western blot analysis
Cells were disrupted in ice-cold lysis buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1% Triton-X100, 1 mM PMSF) and a protease inhibitor cocktail mix (Roche Diagnostics, Mannheim, Germany). The lysate was sonicated and insoluble material was removed by centrifugation (20 000 g for 10 min at 4°C). Western blotting was performed as described in T’Jampens et al. (2002). Proteins were visualized by enhanced chemiluminescence detection (ECL kit; Amersham Pharmacia Biotech).
Immunoprecipitation
Cells were lysed in lysis buffer (see above) supplemented with 1% NP-40. One milligram of proteins was incubated with anti-β-catenin antiserum at 4°C for 4 h and subsequently incubated overnight with protein G–Sepharose (Amersham Pharmacia Biotech). The beads were washed three times, boiled for 5 min in Laemmli sample buffer (Laemmli, 1970) and proteins were fractionated by SDS–PAGE followed by western blotting.
Immunostaining and microscopy
Cells were washed with PBS and fixed with 3.7% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, incubated at 37°C with anti-Myc monoclonal antibody (1 h, 1:800) followed by Alexa Fluor-594 goat anti-mouse IgG (30 min at room temperature, 1:300) or incubated with Texas Red-X–phalloidin (30 min at room temperature, 1:200) or DAPI (0.4 µg/ml). Stained cells were examined using a Zeiss Axioplan II epifluorescence microscope equipped with a ×40 objective. Images were captured using a cooled CCD Axiocam Camera and KS100 software (Zeiss, Göttingen, Germany).
Invasion assays
Invasion into collagen type I was performed as described previously (Bracke et al., 2001a). Using a phase-contrast microscope controlled by a computer program, invasive and superficial cells were counted in 12 fields of 0.157 mm2. Trypan blue staining was performed to check cell viability. The invasion index expresses the percentage of invading cells into the gel over the total number counted. Invasion into pre-cultured embryonic chick-heart fragments was performed as described previously (Bracke et al., 2001b). Details on laminin and fibronectin in chick heart fragments are described in De Bruyne et al. (1988).
Cellular aggregation
Aggregation assays were performed as described previously (Boterberg et al., 2001). For slow aggregation, single-cell suspensions were seeded on top of a semi-solid agar medium with or without DECMA-1. After 24 h, aggregate formation was evaluated subjectively under an inverted phase-contrast microscope at a magnification of ×40. For the fast aggregation assay, single-cell suspensions were prepared with an E-cadherin-saving procedure. Particle diameters were measured in a Coulter LS200 counter (Coulter, Lake Placid, NY) and plotted as a percentage of the volume distribution.
Miscellaneous
Protein concentration was measured according to Bradford (1976) using bovine serum albumin as standard. SDS–PAGE (Laemmli, 1970) was performed on 7.5, 10 or 15% polyacrylamide mini slab gels according to Matsudaira and Burgess (1978).
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We acknowledge the contribution of colleagues who generously provided cDNA constructs. We also thank Drs K.Meerschaert for helpful discussions during the course of this project and Mr J.Roels van Kerckvoorde for preparing the PHF illustrations. This work was supported by grants from the Fund for Scientific Research-Flanders (FWO-Vlaanderen), the Interuniversity Attraction Poles of the Prime Ministers’ Services (IUAP/P5), the Concerted Actions Program (GOA), and Fortis Bank Verzekeringen. V.D.C. and J.G. are Postdoctoral Fellows of the FWO.
References
- Afify A.M. and Werness,B.A. (1998) Decreased expression of the actin-binding protein gelsolin in endometrial and ovarian adeno-carcinomas. Appl. Immunohistochem., 6, 30–34. [Google Scholar]
- Asch H.L., Head,K., Dong,Y., Natoli,F., Winston,J.S., Connolly,J.L. and Asch,B.B. (1996) Widespread loss of gelsolin in breast cancers of humans, mice, and rats. Cancer Res., 56, 4841–4845. [PubMed] [Google Scholar]
- Asch H.L., Winston,J.S., Edge,S.B., Stomper,P.C. and Asch,B.B. (1999) Down-regulation of gelsolin expression in human breast ductal carcinoma in situ with and without invasion. Breast Cancer Res. Treat., 55, 179–188. [DOI] [PubMed] [Google Scholar]
- Azuma T., Witke,W., Stossel,T.P., Hartwig,J.H. and Kwiatkowski,D.J. (1998) Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J., 17, 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno Y., Fujita,H., Ono,Y., Nakashima,S., Ito,Y., Kuzumaki,N. and Nozawa,Y. (1999) Differential phospholipase D activation by bradykinin and sphingosine 1-phosphate in NIH 3T3 fibroblasts overexpressing gelsolin. J. Biol. Chem., 274, 27385–27391. [DOI] [PubMed] [Google Scholar]
- Boterberg T., Bracke,M.E., Bruyneel,E.A. and Mareel,M.M. (2001) Cell aggregation assays. In Brooks,S.A. and Schumacher,U. (eds), Metastasis Research Protocols, Vol. 58. Humana Press, Totowa, NJ, pp. 33–45. [DOI] [PubMed]
- Bracke M.E., Boterberg,T., Bruyneel,E.A. and Mareel,M.M. (2001a) Collagen invasion assay. In Brooks,S.A. and Schumacher,U. (eds), Metastasis Research Protocols, Vol. 58. Humana Press, Totowa, NJ, pp. 81–89. [DOI] [PubMed]
- Bracke M.E., Boterberg,T. and Mareel,M.M. (2001b) Chick heart invasion assay. In Brooks,S.A. and Schumacher,U. (eds), Metastasis Research Protocols, Vol. 58. Humana Press, Totowa, NJ, pp. 91–102. [DOI] [PubMed]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Chen R.-H., Corbalan-Garcia,S. and Bar-Sagi,D. (1997) The role of the PH domain in the signal-dependent membrane targeting of Sos. EMBO J., 16, 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E.A., Golub,T.R., Lander,E.S. and Hynes,R.O. (2000) Genomic analysis of metastasis reveals an essential role for RhoC. Nature, 406, 532–535. [DOI] [PubMed] [Google Scholar]
- Colley W.C. et al. (1997a) Cloning and expression analysis of murine phospholipase D1. Biochem. J., 326, 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley W.C., Sung,T.C., Roll,R., Jenco,J., Hammond,S.M., Altshuller, Y., Bar-Sagi,D., Morris,A.J. and Frohman,M.A. (1997b) Phospho lipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeleton reorganization. Curr. Biol., 7, 191–201. [DOI] [PubMed] [Google Scholar]
- Constantin B., Meerschaert,K., Vandekerckhove,J. and Gettemans,J. (1998) Disruption of the actin cytoskeleton of mammalian cells by the capping complex actin-fragmin is inhibited by actin phos phorylation and regulated by Ca2+ ions. J. Cell Sci., 111, 1695–1706. [DOI] [PubMed] [Google Scholar]
- Cunningham C.C., Stossel,T.P. and Kwiatkowski,D.J. (1991) Enhanced motility in NIH 3T3 fibroblasts that overexpress gelsolin. Science, 251, 1233–1236. [DOI] [PubMed] [Google Scholar]
- De Bruyne G.K., Bracke,M.E., Plessers,L. and Mareel,M. (1988) Invasiveness in vitro of mixed aggregates composed of two human mammary cell lines MCF-7 and HBL-100. In Heppner,G.H., Salomon,J.-C. and Sordat,B. (eds), Invasion and Metastasis. S. Karger AG, Basel, Switzerland, pp. 253–265. [PubMed]
- Dimanche-Boitrel M.T., Vakaet,L.,Jr, Pujuguet,P., Chauffert,B., Martin,M.S., Hammann,A., Van Roy,F., Mareel,M. and Martin,F. (1994) In vivo and in vitro invasiveness of a rat colon-cancer cell line maintaining E-cadherin expression: an enhancing role of tumor-associated myofibroblasts. Int. J. Cancer, 56, 512–521. [DOI] [PubMed] [Google Scholar]
- Dosaka-Akita H., Hommura,F., Fujita,H., Kinoshita,I., Nishi,M., Morikawa,T., Katoh,H., Kawakami,Y. and Kuzumaki,N. (1998) Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res., 58, 322–327. [PubMed] [Google Scholar]
- Egeblad M. and Werb,Z. (2002) New functions for the matrix metallo proteinases in cancer progression. Nat. Rev. Cancer, 2, 161–174. [DOI] [PubMed] [Google Scholar]
- Engers R., Springer,E., Michiels,F., Collard,J.G. and Gabbert,H.E. (2001) Rac affects invasion of human renal cell carcinomas by up-regulating tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 expression. J. Biol. Chem., 276, 41889–41897. [DOI] [PubMed] [Google Scholar]
- Finidori J., Friedrich,E., Kwiatkowski,D.J. and Louvard,D. (1992) In vivo analysis of functional domains from villin and gelsolin. J. Cell Biol., 116, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H., Okada,F., Hamada,J., Hosokawa,M., Moriuchi,T., Koya,R.C. and Kuzumaki,N. (2001) Gelsolin functions as a metastasis suppressor in B16-BL6 mouse melanoma cells and requirement of the carboxyl-terminus for its effect. Int. J. Cancer, 93, 773–780. [DOI] [PubMed] [Google Scholar]
- Hara K. et al. (1994) 1-phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl Acad. Sci. USA, 91, 7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J.H., Bokoch,G.M., Carpenter,C.L., Janmey,P.A., Toker,A. and Stossel,T.P. (1995) Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell, 82, 643–653. [DOI] [PubMed] [Google Scholar]
- Honda A. et al. (1999) Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell, 99, 521–532. [DOI] [PubMed] [Google Scholar]
- Iida N., Namikawa,K., Kiyama,H., Ueno,H., Nakamura,S. and Hattori,S. (2001) Requirement of ras for the activation of mitogen-activated protein kinase by calcium influx, cAMP, and neutrophin in hippocampal neurons. J. Neurosci., 21, 6459–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti M., Tenca,P., Frittoli,E., Faretta,M., Tocchetti,A., Di Fiore, P.P. and Scita,G. (2002) Mechanisms through which Sos-1 coordinates the activation of Ras and Rac. J. Cell Biol., 156, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda E., Lehmann,K., Killisch,I., Jechlinger,M., Herzig,M., Downward,J., Beug,H. and Grunert,S. (2002) Ras and TGF-β cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol., 156, 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T.S. and Nelson,W.J. (1998) Effects of regulated expression of mutant RhoA and RacA small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol., 142, 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J., Lauffenburger,D.A., Turner,T. and Wells,A. (2001) Tumor invasion as dysregulated cell motility. Semin. Cancer Biol., 11, 105–117. [DOI] [PubMed] [Google Scholar]
- Kheradmand F., Werner,E., Tremble,P., Symons,M. and Werb,Z. (1998) Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science, 280, 898–902. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. et al. (2002) Thymosin-β4 regulates motility and metastasis of malignant mouse fibrosarcoma cells. Am. J. Pathol., 160, 869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelevets L., Noë,V., Bruyneel,E., Myssiakine,E., Chastre,E., Mareel,M. and Gespach,C. (1998) Inhibition by platelet-activating factor of Src- and hepatocyte growth factor-dependent invasiveness of intestinal and kidney epithelial cells. J. Biol. Chem., 273, 14138–14145. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee H.K., Driscoll,D., Asch,H., Asch,B. and Zhang,P.J. (1999) Downregulated gelsolin expression in hyperplastic and neoplastic lesions of the prostate. Prostate, 40, 14–19. [DOI] [PubMed] [Google Scholar]
- Lu M., Witke,W., Kwiatkowski,D.J. and Kosik,K.S. (1997) Delayed retraction of filopodia in gelsolin null mice. J. Cell Biol., 138, 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareel M.M., Van Roy,F.M. and Bracke,M.E. (1993) How and when do tumor cells metastasize? Crit. Rev. Oncog., 4, 559–594. [PubMed] [Google Scholar]
- Marshall M. (1995) Interactions between Ras and Raf: key regulatory proteins in cellular transformation. Mol. Reprod. Dev., 42, 493–499. [DOI] [PubMed] [Google Scholar]
- Matsudaira P.T. and Burgess,D.R. (1978) SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal. Biochem., 87, 386–396. [DOI] [PubMed] [Google Scholar]
- Meerschaert K., De Corte,V., De Ville,Y., Vandekerckhove,J. and Gettemans,J. (1998) Gelsolin and functionally similar actin-binding proteins are regulated by lysophosphatidic acid. EMBO J., 17, 5923–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielnicki L.M., Ying,A.M., Head,K.L., Asch,H.L. and Asch,B.B. (1999) Epigenetic regulation of gelsolin expression in human breast cancer cells. Exp. Cell Res., 249, 161–176. [DOI] [PubMed] [Google Scholar]
- Onda H., Lueck,A., Marks,P.W., Warren,H.B. and Kwiatkowski,D.J. (1999) Tsc2(+/–) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J. Clin. Invest., 104, 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R.G., Chen,J., Kirn,D., McCormick,F. and Symons,M. (1995) An essential role for rac in ras transformation. Nature, 374, 457–459. [DOI] [PubMed] [Google Scholar]
- Rao J., Seligson,D., Visapaa,H., Horvath,S., Eeva,M., Michel,K., Pantuck,A., Belldegrun,A. and Palotie,A. (2002) Tissue microarray analysis of cytoskeletal actin-associated biomarkers gelsolin and E-cadherin in urothelial carcinoma. Cancer, 95, 1247–1257. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Warne,P.H., Khwaja,A., Marte,B.M., Pappin,D., Das,P., Waterfield,M.D., Ridley,A. and Downward,J. (1997) Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell, 89, 457–467. [DOI] [PubMed] [Google Scholar]
- Sander E.E., van Delft,S., ten Klooster,J.P., Reid,T., van der Kammen, R.A., Michiels,F. and Collard,J.G. (1998) Matrix-dependent Tiam/Rac signaling in epithelial cells promotes either cell–cell adhesion or cell migration and is regulated by phosphatidylinositol 3-kinase. J. Cell Biol., 143, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santy L.C. and Casanova,J.E. (2001) Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol., 154, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A.A., Govek,E.E., Bottner,B. and Van Aelst,L. (2000) Rho GTPases: signaling, migration, and invasion. Exp. Cell Res., 261, 1–12. [DOI] [PubMed] [Google Scholar]
- Shieh D.B., Godleski,J., Herndon,J.E.,II, Azuma,T., Mercer,H., Sugarbaker,D.J. and Kwiatkowski,D.J. (1999) Cell motility as a prognostic factor in Stage I non-small cell lung carcinoma: the role of gelsolin expression. Cancer, 85, 47–57. [DOI] [PubMed] [Google Scholar]
- Steed P.M., Nagar,S. and Wennogle,L.P. (1996) Phospholipase D regulation by a physical interaction with the actin-binding protein gelsolin. Biochemistry, 35, 5229–5237. [DOI] [PubMed] [Google Scholar]
- Sun H.Q., Yamamoto,M., Mejillano,M. and Yin,H.L. (1999) Gelsolin, a multifunctional actin regulatory protein. J. Biol. Chem., 274, 33179–33182. [DOI] [PubMed] [Google Scholar]
- Tanaka M. et al. (1995) Gelsolin: a candidate for suppressor of human bladder cancer. Cancer Res., 55, 3228–3232. [PubMed] [Google Scholar]
- Tanaka M., Sazawa,A., Shinohara,N., Kobayashi,Y., Fujioka,Y., Koyanagi,T. and Kuzumaki,N. (1999) Gelsolin gene therapy by retrovirus producer cells for human bladder cancer in nude mice. Cancer Gene Ther., 6, 482–487. [DOI] [PubMed] [Google Scholar]
- T’Jampens D., Devriendt,L., De Corte,V., Vandekerckhove,J. and Gettemans,J. (2002) Selected BTB/POZ-kelch proteins bind ATP. FEBS Lett., 516, 20–26. [DOI] [PubMed] [Google Scholar]
- Tolias K.F., Hartwig,J.H., Ishihara,H., Shibasaki,Y., Cantley,L.C. and Carpenter,C.L. (2000) Type I α phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr. Biol., 10, 153–156. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet,L.,Jr, Mareel,M., Fiers,W. and van Roy,F. (1991) Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell, 66, 107–119. [DOI] [PubMed] [Google Scholar]
- Withe M.A., Nicolette,C., Minden,A., Polverino,A., Van Aelst,L., Karin,M. and Wigler,M.H. (1995) Multiple ras functions can contribute to mammalian cell transformation. Cell, 80, 533–541. [DOI] [PubMed] [Google Scholar]
- Witke W., Sharpe,A.H., Hartwig,J.H., Azuma,T., Stossel,T.P. and Kwiatkowski,D.J. (1995) Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell, 81, 41–51. [DOI] [PubMed] [Google Scholar]
- Zhuge Y. and Xu,J. (2001) Rac1 mediates type I collagen-dependent MMP-2 activation. Role in cell invasion across collagen barrier. J. Biol. Chem., 276, 16248–16256. [DOI] [PubMed] [Google Scholar]
- Zhurinsky J., Shtutman,M. and Ben-Ze’ev,A. (2000) Plakoglobin and β-catenin: protein interactions, regulation and biological roles. J. Cell Sci., 113, 3127–3139. [DOI] [PubMed] [Google Scholar]