Abstract
Trichostatin A (TSA) inhibits all histone deacetylases (HDACs) of both class I and II, whereas trapoxin (TPX) cannot inhibit HDAC6, a cytoplasmic member of class II HDACs. We took advantage of this differential sensitivity of HDAC6 to TSA and TPX to identify its substrates. Using this approach, α-tubulin was identified as an HDAC6 substrate. HDAC6 deacetylated α-tubulin both in vivo and in vitro. Our investigations suggest that HDAC6 controls the stability of a dynamic pool of microtubules. Indeed, we found that highly acetylated microtubules observed after TSA treatment exhibited delayed drug-induced depolymerization and that HDAC6 overexpression prompted their induced depolymerization. Depolymerized tubulin was rapidly deacetylated in vivo, whereas tubulin acetylation occurred only after polymerization. We therefore suggest that acetylation and deacetylation are coupled to the microtubule turnover and that HDAC6 plays a key regulatory role in the stability of the dynamic microtubules.
Keywords: acetylation/HDAC6/microtubule/stability/tubulin
Introduction
The N-terminal part of histones is the target of various post-translational modifications, including phosphorylation, methylation and acetylation. Recently, it has been proposed that these histone modifications create a specific epigenetic code known as the ‘histone code’ (Cheung et al., 2000; Strahl and Allis, 2000; Turner, 2000). The ‘histone code’ hypothesis proposes that a specific combination of histone modifications provides information that is interpreted by machinery capable of locally modifying the structure and function of chromatin. Histone acetylation is believed to play a central role in this ‘histone code’ system (Turner, 1993). Many histone acetyltransferases (HATs) and histone deacetylases (HDACs) are, respectively, activators and repressors of transcription (Kuo and Allis, 1998). Besides chromatin-related functions, protein acetylation seems to be a signal directly controlling the activity of key cellular regulators (Kouzarides, 2000). HATs and HDACs have been shown to control the state of acetylation of many non-histone proteins, among which some are cytoplasmic (Kouzarides, 2000; Sterner and Berger, 2000). Although the significance of the nuclear protein acetylation is under intensive investigation, almost nothing is known about the cytoplasmic protein acetylation. However, all members of class II HDACs show a regulated intracellular localization and could be found in both the cytoplasm and the nucleus (Khochbin et al., 2001). Class II HDACs are enzymes whose catalytic domain is related to that of yeast histone deacetylase HDA1 (Grozinger et al., 1999; Miska et al., 1999; Verdel and Khochbin, 1999; Wang et al., 1999; Kao et al., 2000). Among the members of this family, HDAC6 is a unique enzyme that is essentially cytoplasmic (Verdel et al., 2000). Moreover, it harbors a conserved zinc finger capable of directly interacting with ubiquitin in its C-terminal region (Seigneurin-Berny et al., 2001). The purification of an HDAC6-containing complex from mouse testis cytosolic extracts showed the associa tion of HDAC6 with two proteins involved in the control of protein ubiquitylation, VCP/p97 and PLAP/UFD3 (Seigneurin-Berny et al., 2001). The presence of HDAC6, as well as other members of class II HDACs, in the cytoplasm strongly suggests that they participate in the control of the acetylation of cytoplasmic proteins (Khochbin et al., 2001).
We took advantage of the difference in sensitivity of several HDACs to HDAC inhibitors to identify specific HDAC substrates, with a particular interest in HDAC6. Indeed, we have identified HDACs as the targets of trichostatin A (TSA) and trapoxin (TPX), both of which are microbial metabolites that induce cell differentiation, cell cycle arrest and reversal of transformed cells morphology (Yoshida et al., 1995). Recently, we showed that, although all class I and II HDACs were strongly inhibited by TSA, HDAC6 was specifically resistant to TPX (Furumai et al., 2001). We expected that this difference between TSA and TPX in the inhibition of HDAC activity would allow the identification of specific substrates for HDAC6, the acetylation of which would be enhanced by TSA but not by TPX. These investigations led to the identification of α-tubulin as a substrate for HDAC6. Tubulin acetylation occurs at the ε-amino group of a conserved lysine residue (Lys40) near the N-terminus (MacRae, 1997; Rosenbaum, 2000). However, the enzymes responsible for the α-tubulin acetylation and deacetylation are neither purified nor cloned.
In this paper, after identifying α-tubulin as a substrate for HDAC6 in vitro and in vivo, we show that α-tubulin acetylation reduces the rate of depolymerization of the cytoplasmic microtubules by depolymerizing agents. We also show that tubulin deacetylation by HDAC6 promotes its disassembly. Our data suggest that the cycle of tubulin acetylation and deacetylation is linked to the microtubule dynamics and has a role in regulating the stability of the microtubules. These findings may provide a basis for understanding the biological function of microtubule acetylation.
Results
Identification of α-tubulin as a protein specifically acetylated in TSA-treated cells
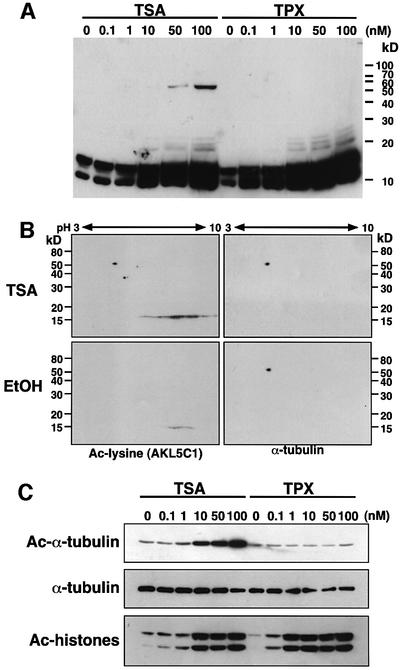
We looked for acetylated proteins accumulated in TSA-treated but not in TPX-treated cells by using an anti-acetylated lysine monoclonal antibody AKL5C1 (Figure 1A). Both TSA and TPX treatments caused marked enhancement of histone acetylation at low nanomolar concentrations. In contrast, the acetylation of a 54 kDa protein was specifically enhanced by TSA treatment but not by TPX. In this particular experiment, only this 54 kDa protein was detected as being specifically acetylated in the TSA-treated cells.
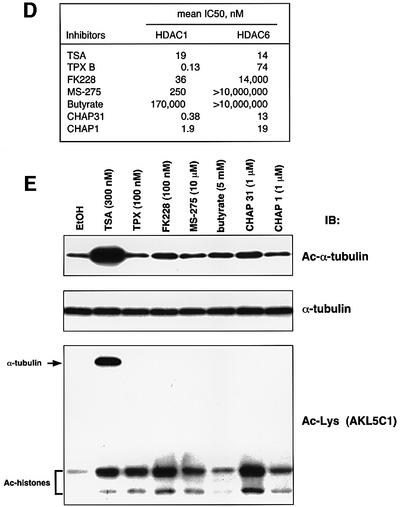
Fig. 1. Acetylation of α-tubulin induced by TSA. (A) A protein with an apparent molecular mass of 54 kDa was strongly acetylated in response to TSA. NIH 3T3 cells were treated with various concentrations of either TSA or TPX B for 6 h. Acetylated proteins were analyzed by immunoblotting using an anti-acetylated lysine antibody (clone AKL5C1). (B) Identification of p54 as α-tubulin. Cell lysates prepared from NIH 3T3 cells that had been treated with either TSA (upper) or EtOH (lower) were subjected to two-dimensional PAGE and immunoblotted with an anti-acetylated lysine antibody (AKL5C1; left). The same blots were reprobed with an anti-α-tubulin antibody (clone B-5-1-2; right). (C) Acetylation of Lys40 in α-tubulin in TSA-treated cells. Cell lysates used in (A) were immunoblotted with anti-acetylated α-tubulin (clone 6-11B-1), anti-α-tubulin (B-5-1-2) or anti-acetylated lysine antibodies (AKL5C1). Note that TPX effectively enhanced the acetylated level of histones but not of α-tubulin. (D) Inhibitory potency of HDAC inhibitors against HDAC1 and HDAC6. The 50% inhibitory concentration (IC50) of each inhibitor was determined as described previously (Furumai et al., 2001). (E) Correlation between the ability of HDAC inhibitors to inhibit HDAC6 and to induce in vivo α-tubulin acetylation. NIH 3T3 cells were cultured for 6 h with each inhibitor at a concentration sufficient to induce histone acetylation, and then the levels of acetylation of the α-tubulin (Ac-α-tubulin), the total α-tubulin (α-tubulin) and acetylated proteins (Ac-Lys) were determined by immunoblotting.
p53, importin-α and α-tubulin have been reported as potentially acetylated proteins with similar molecular sizes (L’Hernault and Rosenbaum, 1985; Gu and Roeder, 1997; Bannister et al., 2000). We therefore compared their mobility with that of the 54 kDa protein in a two-dimensional gel electrophoresis followed by immunoblotting. Only α-tubulin comigrated with the 54 kDa protein detected in the TSA-treated cells (Figure 1B). To confirm the identity of the 54 kDa protein, we used an anti-acetylated α-tubulin antibody 6-11B-1 (Piperno and Fuller, 1985) that recognizes a short region encompassing acetylated Lys40 in α-tubulin (Figure 1C). This antibody allowed us to show that the acetylation of α-tubulin was markedly enhanced by TSA treatment, whereas the total α-tubulin level determined with the pan-tubulin antibody B-5-1-2 was unchanged. In contrast, TPX, even at high concentrations, did not increase the acetylation level of the α-tubulin, although histone hyperacetylation was induced at a concentration 10-fold lower than that of TSA.
Effect of other HDAC inhibitors on tubulin acetylation
A number of natural and synthetic compounds have been described as HDAC inhibitors (Nakajima et al., 1998; Richon et al., 1998; Saito et al., 1999; Furumai et al., 2001; Komatsu et al., 2001). We next analyzed the correlation between the ability of these compounds to inhibit HDAC6 and to induce tubulin acetylation. First, the IC50 values of various HDAC inhibitors for HDAC6 inhibition were determined (Figure 1D). TSA inhibited both HDAC1 and HDAC6 to a similar extent. Although TPX strongly inhibited HDAC1 at sub-nanomolar concentrations, it failed to inhibit HDAC6 at these concentrations. Like TPX, all other inhibitors such as CHAPs, butyrate and FK228 were weaker inhibitors of HDAC6 activity than of HDAC1 activity. An immunoblot analysis showed that not only TPX but also FK228, MS-275, butyrate, CHAP31 and CHAP1 were very weak at inducing α-tubulin acetylation in cells, although they were capable of inducing histone hyperacetylation (Figure 1E). Thus, TSA is a unique compound in its abilities both to induce an α-tubulin acetylation in vivo and to inhibit HDAC6 activity in vitro. This suggested that HDAC6 is involved in α-tubulin deacetylation.
In vivo deacetylation of α-tubulin by HDAC6
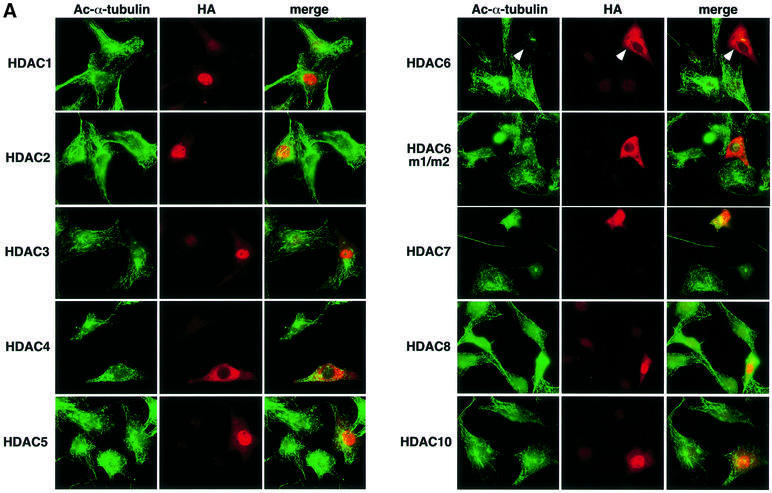
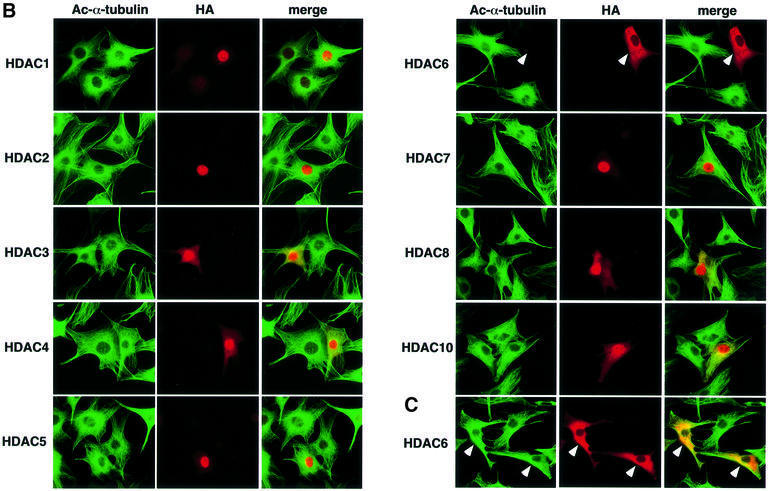
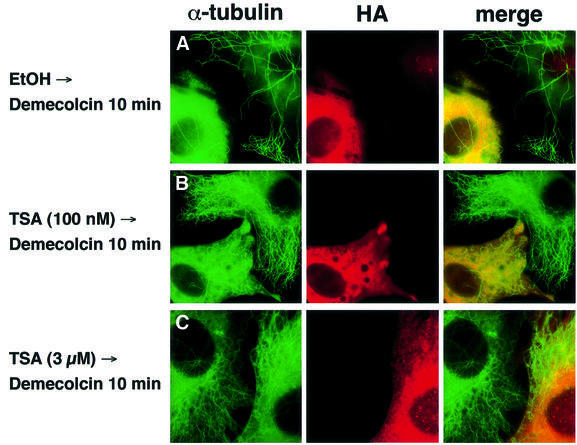
To see whether acetylated α-tubulin is the in vivo substrate of HDAC6, HDAC enzymes tagged with HA were overexpressed in NIH 3T3 cells, and the intracellular α-tubulin acetylation level of individual transfected cells was examined by immunofluorescent labeling using the antibody 6-11B-1 (Figure 2A). The acetylation of the tubulin network was greatly reduced in the HDAC6-transfected cells compared with that of the untransfected cells present in the same microscopic field. This decrease in tubulin acetylation was not observed with cells transfected with the enzymatically inactive HDAC6 mutant, which contained replacements of essential residues in both its deacetylase domains (HDAC6 m1/m2), indicating that the catalytic activity of HDAC6 is essential for the deacetylation of α-tubulin. All other HDACs, even HDAC10, the enzyme most related to HDAC6 (Guardiola and Yao, 2002), failed to deacetylate α-tubulin in vivo. Surprisingly, no increase in tubulin acetylation was observed in the HDAC6-overexpressing cells in the presence of 100 nM TSA, whereas microtubules in the untransfected cells or cells transfected with other HDACs were highly acetylated upon TSA treatment (Figure 2B). More than 1 µM TSA was required to enhance tubulin acetylation in the HDAC6-overexpressing cells (Figure 2C). These results clearly indicate that HDAC6 deacetylates microtubules in vivo and, moreover, that an elevated cellular concentration of HDAC6 confers a resistance to TSA at 100 nM, a concentration at which the endogenous HDAC6 is efficiently inhibited.
Fig. 2. In vivo deacetylation of α-tubulin by HDAC6. (A) Effects of HDAC expression on tubulin acetylation in individual cells. NIH 3T3 cells grown on coverslips were transfected with HA-HDACs. After fixation, cells were immunostained with anti-acetylated α-tubulin and anti-HA antibodies. The cells transfected with HDAC6 are indicated by arrowheads. (B) Effects of HDAC expression on tubulin acetylation in TSA-treated cells. NIH 3T3 cells grown on coverslips were transfected with HA-HDACs and then treated with 100 nM TSA for 6 h. The cells transfected with HDAC6 are indicated by arrowheads. (C) Inhibition of the HDAC6-mediated tubulin deacetylation by a high concentration of TSA. NIH 3T3 cells grown on coverslips were transfected with HA-HDAC6 and then treated with 3 µM TSA for 6 h. The cells transfected with HDAC6 are indicated by arrowheads.
In vitro deacetylation of α-tubulin by HDAC6
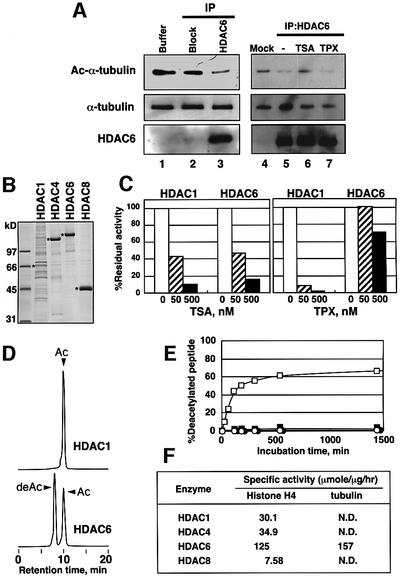
In order to confirm the capability of HDAC6 to deacetylate α-tubulin, an endogenous HDAC6-containing complex was purified from mouse testis by immunoprecipitation, taking advantage of the overexpression of HDAC6 in the testis (Grozinger et al., 1999; Verdel and Khochbin, 1999). The substrate was prepared by purifying paclitaxel-polymerized tubulin from TSA-treated cells (Vallee and Collins, 1986). When the HDAC6-containing complex was incubated with the acetylation-enriched tubulin, the amount of acetylated α-tubulin was reduced (Figure 3A, lane 3). The deacetylation of α-tubulin was not observed when the acetylated tubulin was incubated with material immunoprecipitated with an anti-HDAC6 antibody blocked by an excess of the peptide antigen (Figure 3A, lane 2). This in vitro deacetylation by HDAC6 was inhibited by TSA but not by TPX (Figure 3A, lanes 6 and 7, respectively). To further examine the substrate specificity of HDACs, His-tagged human HDAC enzymes were produced using the baculoviral expression system and affinity purified (Figure 3B). These enzymes were catalytically active, and their activity was inhibited by TSA (Figure 3C; data not shown). The recombinant HDAC6 was resistant to TPX, as was the enzyme prepared from mammalian cells (Figure 3C). Their ability to deacetylate α-tubulin was examined with the fluorescence-labeled 20 amino acid peptide containing acetylated Lys40 of α-tubulin (Dansylated 30-IQPDGQMPSDK (Ac)TIGGGDDSF-49). Since the specific activities of the tested HDACs to deacetylate 3H-labeled histones were different, enzyme concentrations were adjusted to equivalent total activities of the enzymes. As shown in Figure 3D, the HPLC analysis showed that HDAC6 could deacetylate the acetylated peptide. About 60% of the acetylated peptide was converted to a non-acetylated form within 3 h. In contrast, other HDACs had no deacetylating activity on the peptide (Figure 3D–F). The time-course experiments showed that the reaction proceeded linearly during the initial 2 h (Figure 3D). To compare the specific activities between histone and α-tubulin, their abilities to deacetylate the fluorescent-labeled 20 amino acid peptide containing acetylated Lys16 of histone H4 (Dansylated 1-SGRGKGGKGLGKGGAK(Ac)RHRK-20) were also examined (Figure 3F). Only HDAC6 could deacetylate both substrates to a similar extent. These results indicate that HDAC6 is specifically responsible for α-tubulin deacetylation both in vivo and in vitro.
Fig. 3. In vitro deacetylation of α-tubulin by HDAC6. (A) Deacetylation of acetylated microtubules by HDAC6 from mouse testis. HDAC6 was isolated from mouse testis by immunoprecipitation using an anti-mouse HDAC6 antibody raised against an HDAC6 peptide (Verdel et al., 2000). As a control, the antigen peptide was added in excess to block the antibody binding to HDAC6 (Block). HDAC6 was incubated with acetylated microtubules isolated from TSA-treated cells in 20 µl for 3 h at 37°C in the presence or absence of TSA (100 nM) or TPX (100 nM). The success of the immunoprecipitation and the deacetylation of the microtubules were visualized by immunoblotting using the anti-mouse HDAC6 and anti-acetylated tubulin antibodies, respectively. (B) Purification of HDAC enzymes produced in insect cells via a baculovirus system. Asterisks denote the produced enzymes in the CBB stained gel of SDS–PAGE. HDAC4, HDAC6 and HDAC8 were efficiently expressed and highly purified. Although the production of HDAC1 was not efficient, the preparation had a sufficient activity to deacetylate 3H-histone. (C) Effects of TSA and TPX on the recombinant enzymes. The enzyme activities of HDAC1 and HDAC6 were determined with 3H-histone in the presence of various concentrations of TSA and TPX. (D) In vitro deacetylation of an acetylated tubulin peptide by recombinant human HDAC6. Recombinant human HDAC6 was incubated for 3 h with 0.5 mM Dns-tubulin peptide containing acetylated Lys40, and the reaction mixtures were analyzed with HPLC using a fluorescent detector. The retention time of the new peak (deAc) was identical to that of Dns-tubulin peptide without acetylation. (E) Enzyme specificity of deacetylation. The deacetylation of the Dns-acetylated tubulin peptide was analyzed over time with recombinant HDAC1, HDAC4, HDAC6 and HDAC8. The enzyme preparations used for the deacetylation assay were normalized based on their specific activities obtained with 3H-histone. The amounts of the deacetylated peptide after incubation with HDAC1 (open circles), HDAC4 (filled circles), HDAC6 (open squares) and HDAC8 (filled squares) for various lengths of time were plotted. (F) Specific activities of the recombinant enzymes for deacetylation of tubulin and histone H4 peptides. N.D., not detected.
TSA enhances acetylation of entire microtubules
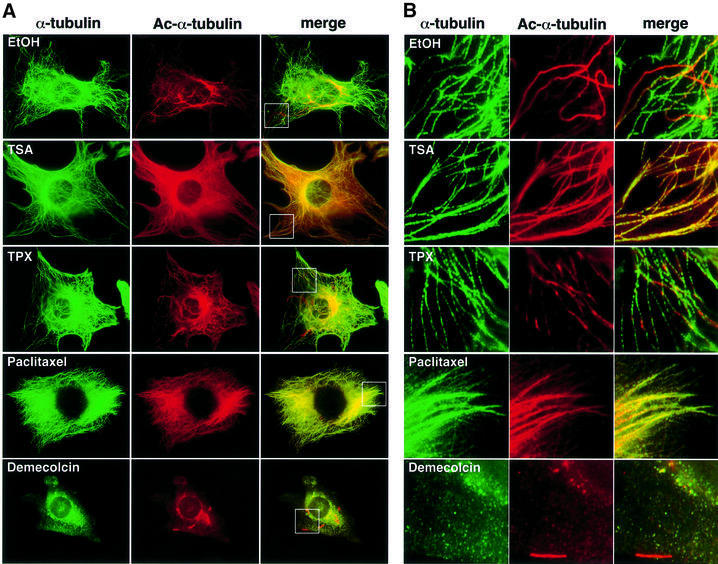
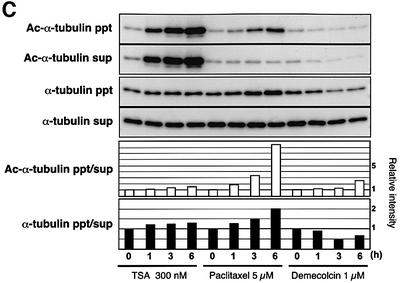
We next investigated whether TSA affects the morphology of the cytoplasmic microtubule network by immunofluorescent labeling of formaldehyde-fixed NIH 3T3 cells (Figure 4A and B). Immunofluorescent staining using the anti-acetylated α-tubulin antibody showed that acetylated α-tubulin was mostly localized in segments of a number of microtubules in untreated 3T3 cells (Figure 4A, first row). In TSA-treated cells, the microtubules were entirely labeled with the anti-acetylated α-tubulin antibody, even in the cell periphery region (Figure 4A and B, second row). Cell fractionation experiments demonstrated that α-tubulin in both the polymer and free subunits became highly acetylated by TSA treatment in a time-dependent manner (Figure 4C). Observation at a higher magnification revealed that the microtubules appeared to be partially bundled in the TSA-treated cells (Figure 4B, second row). In contrast, TPX did not cause any change in the intensity of the tubulin acetylation (Figure 4A and B, third row).
Fig. 4. Effect of TSA on microtubule morphology. (A) Immunostaining of cells treated with TSA, TPX, paclitaxel and demecolcin. NIH 3T3 cells were treated with the various drugs for 6 h. The cells were fixed and stained for tyrosinated α-tubulin (α-tubulin) and acetylated α-tubulin (Ac-α-tubulin). (B) Microtubule morphology in the cell edge region. The boxed regions in (A) were viewed at higher magnification. (C) NIH 3T3 cells were treated with either TSA, paclitaxel or demecolcin for the indicated time and lysed. Total cell lysates from the drug-treated cultures were separated into the precipitates and supernatants by 16 000 g centrifugation. The fractions were immunoblotted with anti-acetylated α-tubulin (upper two panels) and anti-α-tubulin (middle two panels) antibodies. The band intensities were measured using densitometry, and the precipitate/supernatant ratios were determined (lower two panels).
Tubulin deacetylation and destabilization by HDAC6
It has been reported that Taxol, an agent that stabilizes microtubules, causes an increased acetylation of α-tubulin (Piperno et al., 1987). Indeed, paclitaxel enhanced α-tubulin acetylation of polymer microtubules but not unpolymerized tubulins in 3T3 cells, whereas demecolcin (colcemid), a depolymerizing agent, reduced the amount of acetylation (Figure 4C). We therefore compared the effect of paclitaxel and TSA on the microtubule morphology and acetylation (Figure 4A and B). Staining of total microtubules with the anti-α-tubulin antibody revealed that paclitaxel stabilizes the cytoplasmic microtubules, which normally disassemble and assemble at high rates, thereby forming bundled microtubules. As reported previously, immunostaining with the antibody 6-11B-1 showed that the microtubules are highly acetylated in the paclitaxel-treated cells. However, the microtubule morphology in paclitaxel-treated cells was totally different from that in the presence of TSA (Figure 4A and B, fourth row).
We further determined the relative polymer levels in drug-treated cells. The paclitaxel treatment greatly increased the polymer content (Figure 4C, α-tubulin ppt/sup) as well as the polymer acetylation relative to the free tubulin acetylation level (Figure 4C, Ac-α-tubulin ppt/sup), whereas demecolcin reduced the polymer level in cells. In the TSA-treated cells, both the polymer level and the polymer acetylation level were slightly but reproducibly increased. These results suggest that TSA-induced acetylation may affect the stability of microtubules.
Effect of acetylation on the drug-induced depolymerization
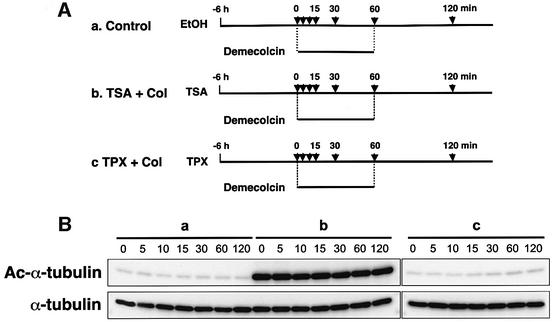
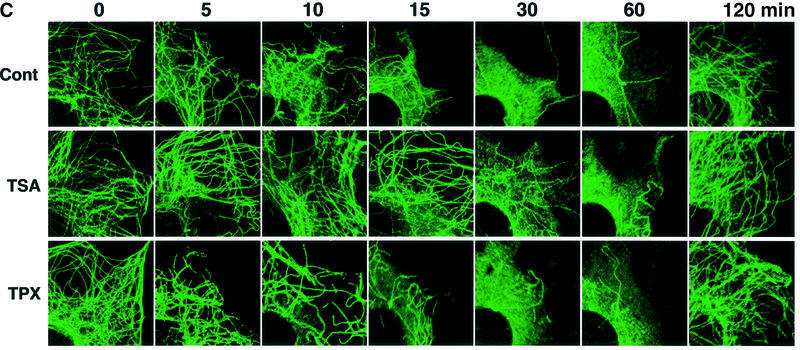
We next examined whether the TSA-induced α-tubulin acetylation affects the sensitivity of the interphase arrays of cytoplasmic microtubules to a microtubule-depolymerizing agent. Cells pretreated with ethanol (control), TSA or TPX for 6 h were exposed to 1 µM demecolcin for various lengths of time up to 60 min (Figure 5A), and the cellular levels of acetylated and total α-tubulin were monitored by immunoblotting (Figure 5B). Immunofluorescent staining of the cells with the α-tubulin antibody showed that, in the control culture, a large part of the cytoplasmic microtubule networks was disrupted by demecolcin within 15 min (Figure 5C, first row) and only a subset of stable microtubules was observed 30 min after demecolcin addition. TPX pretreatment did not affect this pattern of changes in the microtubule morphology by demecolcin (Figure 5C, third row). In contrast, the entirely acetylated microtubules in the TSA-treated cells showed a slow depolymerization, with most of the cytoplasmic microtubule network being intact after 15 min. Some of the microtubules remained intact even 30 min after demecolcin treatment. The microtubules were finally disrupted by 60 min (Figure 5C, second row). Since colchicine inhibits the assembly of tubulins by sub-stoichiometric binding of tubulin dimers rather than by direct dissociation of formed microtubules (Wilson, 1975), these results indicate that tubulin acetylation does not block the microtubule depolymerization itself but reduces its rate. The amount of acetylated α-tubulin in the TSA-treated cells did not change during the demecolcin treatment (Figure 5B), showing that the sequential treatment with TSA and demecolcin eventually led to the accumulation of acetylated, unpolymerized tubulin in the cytoplasm. Additionally, the removal of demecolcin resulted in the microtubule assembly of acetylated tubulin in TSA-treated cells without delay, suggesting that acetylated, unpolymerized tubulin has essentially the same ability to be assembled into microtubules as unmodified tubulin (data not shown).
Fig. 5. Delayed depolymerization of microtubules in TSA-treated cells. (A) Schematic representation of experimental procedures. Bars indicate the periods during which the cells were treated with drugs. Arrows indicate the time-points at which cells were taken for immunoblot analysis (B) and immunofluorescent microscopy (C). (B) Cellular levels of tubulin acetylation in the time-course experiments. The amounts of acetylated and total tubulin in the cells treated with various drugs in the time-course experiments designed in (A) were determined by immunoblotting with anti-acetylated α-tubulin (upper) and anti-α-tubulin (lower) antibodies. (C) Depolymerization and repolymerization during demecolcin treatment and removal. Microtubules were visualized by immunofluorescent staining with the anti-α-tubulin antibody. The microscopic images were taken at the time-points indicated in (A).
HDAC6 destabilizes the tubulin network in vivo
To see whether HDAC6 regulates the stability of the cytoplasmic microtubules, we overexpressed HDAC6 in NIH 3T3 cells and examined the sensitivity of the microtubule network of these cells to a drug-induced depolymerization by demecolcin. As shown in Figure 6A, the microtubules in HDAC6-overexpressing cells were almost completely disrupted 10 min after demecolcin addition, whereas some of the fibers still remained in untransfected cells. This result suggests that HDAC6 accelerates depolymerization by demecolcin. However, the effect of HDAC6 was subtle in this experiment, probably because depolymerization by demecolcin was too fast and the normal level of acetylation in the untransfected cells not high. To enhance this effect, the HDAC6-overexpressing cells were treated with 100 nM TSA, which is insufficient to inhibit HDAC6 in overexpressing cells but sufficient to block the endogenous enzyme activity in untransfected cells (Figure 2B). The HDAC6-transfected cultures pretreated with 100 nM TSA for 6 h were then treated with demecolcin for 10 min. In the presence of demecolcin, the cytoplasmic microtubule network in untransfected cells remained almost intact for at least 10 min. In clear contrast, the HDAC6-overexpressing cells almost completely lost their tubulin network (Figure 6B). Finally, treatment with 3 µM TSA, which induces hyperacetylation of tubulin in both HDAC6-transfected and untransfected cells, conferred resistance to demecolcin treatment even in HDAC6-overexpressing cells (Figure 6C). Thus, the acetylation status of the cytoplasmic microtubules seems to regulate their stability in the presence of demecolcin.
Fig. 6. Enhanced depolymerization of microtubules in HDAC6-overexpressing cells. NIH 3T3 cells transfected with an HA-HDAC6 expression vector were pretreated with ethanol (A), 100 nM TSA (B) and 3 µM TSA (C) for 6 h and then exposed to demecolcin for 10 min. Microtubules were stained with the anti-α-tubulin antibody and the HDAC6-transfected cells were visualized with the anti-HA antibody.
Coupling of tubulin acetylation/deacetylation with the polymerization/depolymerization cycle
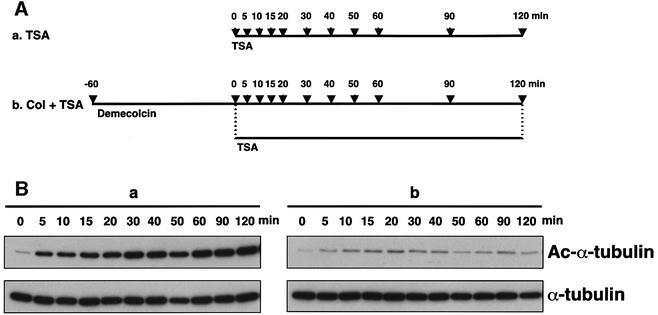
It has been reported that in vivo acetylation occurs from the ends of polymerized microtubules and is a slower process than microtubule assembly (Wilson and Forer, 1997). To test whether in vivo depolymerized tubulin is the substrate for tubulin acetyltransferase, we pretreated cells with demecolcin to depolymerize the microtubules and then inhibited HDAC6 by TSA (Figure 7A). If the unpolymerized tubulin can be acetylated by tubulin acetyltransferase, the acetylation of depolymerized tubulin should be increased in the presence of TSA. In the absence of demecolcin pretreatment, the acetylation of α-tubulin gradually increased and reached a plateau 2 h after TSA addition (Figure 7Ba; data not shown). However, the increased tubulin acetylation by TSA treatment was not observed when the microtubules were depolymerized (Figure 7Bb). These results clearly indicate, as suggested previously, that acetylation occurs only on the polymerized tubulin in vivo.
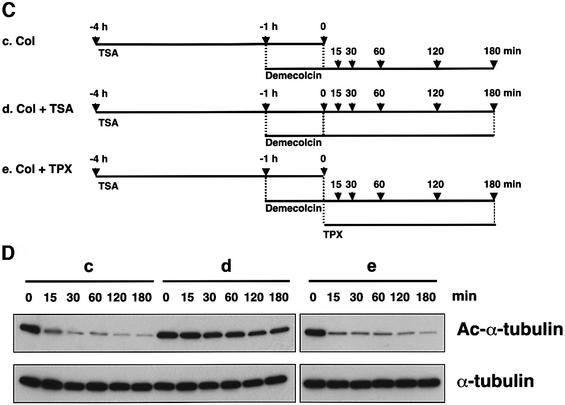
Fig. 7. Acetylation dependent on tubulin polymerization. (A) Schematic representation of experimental procedures for tubulin acetylation. Bars indicate the periods during which cells were treated with the drugs. Arrows indicate the time-points at which cells were taken for immunoblot analysis (B). (B) Effect of demecolcin pretreatment on TSA-induced tubulin acetylation. The amounts of acetylated and total tubulin in the cells in the time-course experiments designed as in (A) were determined by immunoblotting with anti-acetylated α-tubulin (upper) and anti-α-tubulin (lower) antibodies. (C) Schematic representation of experimental procedures for tubulin deacetylation. Bars indicate the periods during which the cells were treated with drugs. Arrows indicate the time-points at which cells were taken for immunoblot analysis (D). (D) Deacetylation of depolymerized tubulin. The amounts of acetylated and total tubulin in the cells treated with various drugs in the time-course experiments designed as in (C) were determined by immunoblotting with anti-acetylated α-tubulin (upper) and anti-α-tubulin (lower) antibodies.
We next asked whether unpolymerized tubulin could be deacetylated in vivo. To this end, we pretreated cells with TSA for 4 h to induce tubulin acetylation, and then the microtubules were depolymerized by demecolcin for 1 h, leading to the accumulation of depolymerized, acetylated tubulin (Figure 7C). Since the effect of TSA is reversible, if the depolymerized tubulin can be deacetylated by HDAC6, the acetylation of depolymerized tubulin should be decreased upon removal of TSA in the presence of demecolcin. As shown in Figure 7D, a very rapid deacetylation of the depolymerized tubulin was observed after TSA removal, and its level decreased to almost a background level within 15 min. This decrease in the acetylation was completely inhibited by the readdition of TSA but not by TPX (Figure 7D). These results suggest that polymerized tubulin is predominantly subject to acetylation, whereas unpolymerized tubulin is efficiently deacetylated by HDAC6.
Discussion
HDAC6 as a tubulin deacetylase
Following the discovery of nuclear HATs, a number of non-histone nuclear proteins have been identified as their substrates. Many of these substrates are involved in transcriptional regulation (Kouzarides, 2000). However, very little is known about the regulation of the acetylation of the cytoplasmic proteins. In the present study, we show that HDAC6 is responsible for the deacetylation of α-tubulin in cells. Three lines of evidence support this conclusion. First, TSA, which is capable of efficiently inhibiting HDAC6, induces α-tubulin acetylation in vivo, whereas other HDAC inhibitors, which are also weak inhibitors of HDAC6, fail to do so. Secondly, in vivo α-tubulin acetylation was markedly reduced by overexpression of HDAC6 but not by other HDACs. Thirdly, HDAC6 deacetylates both polymerized tubulin and the α-tubulin peptide containing the acetylated lysine residue in vitro. Our in vitro assays showed that the substrate specificity, rather than the intracellular localization, is responsible for the lack of α-tubulin deacetylation in vivo when HDACs other than HDAC6 were tested. Moreover, we can also rule out the involvement of the Sir2 family of enzymes in the α-tubulin deacetylation, because they are known to be insensitive to TSA (Imai et al., 2000).
An endogenous cytoplasmic complex of HDAC6 has recently been purified from mouse testis, containing p97/Cdc48/VCP and PLAP/UFD3, and HDAC6 has been shown to bind ubiquitin (Seigneurin-Berny et al., 2001). HDAC6 is highly expressed in the testis, in which the cells are subject to dynamic changes in both protein turnover and microtubule configurations. During spermatogenesis, many proteins are degraded, and acetylated tubulin is redistributed from a cortical network to the axial filament, a precursor of the sperm tail (Moreno and Schatten, 2000). Therefore, HDAC6 may contribute to these two independent events.
Control of acetylation and microtubule stability
Tubulin proteins, the building blocks of microtubules, are subject to several types of evolutionarily conserved post-translational modifications. The biological importance of these modifications is not fully understood. Many laboratories have shown that a subset of stable cytoplasmic microtubules that was unable to be exchanged with free tubulin was enriched in tubulin acetylated on a single lysine residue and detyrosinated at their C-terminus (MacRae, 1997; Rosenbaum, 2000). These modified microtubules are much more resistant to drug-induced depolymerization than unmodified microtubules (LeDizet and Piperno, 1986; Piperno et al., 1987). The half-life of the stable microtubules was ∼1 h, as opposed to 5–10 min for the dynamic microtubules (Schulze et al., 1987). These stable microtubules were therefore, on average, older than the dynamic microtubules. Since acetylation appears to be a slow reaction that occurs on polymerized tubulin, unmodified microtubules are likely to be too dynamic to become acetylated (Schulze et al., 1987).
The role of tubulin acetylation in vivo has mainly been studied in two different ways. One of them is to examine the in vitro stability of acetylated micro tubules. Maruta et al. (1986) showed that the acetylation did not significantly affect the in vitro temperature- dependent polymerization or depolymerization of tubulin using calf brain tubulin acetylated by Chlamydomonas tubulin acetyltransferase. Billger et al. (1991) also demonstrated that polymerization of naturally highly acetylated tubulin purified from cod brain was sensitive to low concentrations of colchicine in the absence of microtubule-associated proteins (MAPs). However, it was slightly more resistant to disassembly, suggesting that acetylation somehow induces a resistance to colchicine. Interestingly, the acetylated microtubules became much more resistant to colchicine than the unmodified microtubules in the presence of not only cod MAPs but also bovine MAPs. Thus, it seems likely that acetylated microtubules have MAP-dependent colchicine stability.
The other approach is a more straightforward one that includes replacement of the α-tubulin lysine residue potentially acetylated with arginine in Tetrahymena. The mutants lacking detectable acetylated tubulin are indistinguishable from wild-type cells (Gaertig et al., 1995). These results indicate that acetylation per se does not play an essential role in some of the functions of microtubules, such as mitosis in the cell cycle. However, in the absence of knowledge of the enzymes involved in tubulin acetylation/deacetylation, it was difficult to assess the in vivo effect of tubulin hyperacetylation on microtubule functions. In the present study, thanks to the identification of a specific tubulin deacetylating enzyme and its inhibitor TSA, we could induce hyperacetylation as well as hypoacetylation of both polymerized and unpolymerized tubulin in cultured cells. In particular, we found that a low concentration (100 nM) of TSA was unable to induce tubulin hyperacetylation in HDAC6-overexpressing cells but could efficiently induce it in untransfected cells. This property allowed a direct comparison of the effects of tubulin hyperacetylation and hypoacetylation in the same cultures. Our results showed that depolymerization of acetylated cytoplasmic microtubules by demecolcin treatment was markedly slower than that of hypoacetylated microtubules. Interestingly, HDAC6 overexpression accelerated the depolymerization of these acetylated microtubules. However, the normally stable pool of microtubules is more resistant to demecolcin than the microtubules acetylated by TSA treatment. These observations suggest that acetylation affects the in vivo stability of the dynamic pool of microtubules but not of the stable microtubules. This hypothesis was also supported by the observation that detyrosination, another marker for the stable microtubules, was not affected by TSA treatment (A.Matsuyama, T.Shimazu and M.Yoshida, unpublished results). The increased stability of the dynamic pool of microtubules may be ascribed to the better organization of the microtubules at the cell edge of the TSA-treated cells than that of the control cells (Figure 4B). It is currently unclear whether the in vivo stabilization by acetylation is mediated by a MAP(s) that associates with acetylated microtubules. The regulatory role of tubulin acetylation in the stability of dynamic microtubules should be further examined. The main point of interest will be to see whether cell motility, vesicle transport and cell polarity, all of which are controlled by microtubules and associated motor proteins, are affected by tubulin acetylation.
Tubulin acetylation/deacetylation cycle and microtubule dynamics
It has been shown previously that acetylation preferentially occurs on tubulin polymers (Piperno et al., 1987). We also observed that TSA-induced tubulin acetylation was markedly suppressed when microtubules were disassembled by demecolcin, indicating that polymerization is a prerequisite for acetylation (Figure 7B). Moreover, depolymerized tubulin was rapidly deacetylated after releasing the TSA block (Figure 7D). We observed that recombinant HDAC6 produced in insect cells failed to deacetylate microtubules isolated from paclitaxel-treated cells but was capable of deacetylating the acetylated 20mer peptide (Figure 3; data not shown). However, some degree of microtubule deacetylation was observed in the presence of an endogenous HDAC6 complex isolated from mouse testis cytosolic extracts, which is a more active enzyme. These observations suggest that microtubules constitute a poor substrate for HDAC6 and that tubulin dimers are better substrates. A recent high-resolution model of the microtubule obtained from docking the electron crystallography has led to the prediction that the acetylation site in α-tubulin should be located in the interior of the microtubules (Nogales et al., 1999). If this is the case, the enzymes catalyzing acetylation and deacetylation should diffuse slowly along the lumen of the microtubules. Following polymerization, acetylation was shown to occur from the ends of the microtubule (Wilson and Forer, 1997). Since microtubules become stabilized and highly acetylated in paclitaxel-treated cells, it is plausible that deacetylation of the polymerized tubulin, if it happens, is a much slower reaction than acetylation. Considering the very rapid deacetylation of depolymerized tubulin (Figure 7D), it seems likely that deacetylation occurs preferentially on the tubulin dimers, whereas acetylation occurs on the polymers. This idea is also supported by the early observation that deacetylation of depolymerized tubulin was kinetically much faster than that of polymers (Black et al., 1989). We therefore suggest that acetylation and deacetylation are coupled to the microtubule dynamics. Further studies will be needed to prove this hypothesis. Thus, the identification of HDAC6 as a tubulin deacetylase and its inhibition by TSA will pave the way for more detailed studies on the biological role of tubulin acetylation.
Materials and methods
Materials, cell lines and antibodies
TSA, TPX, CHAP1 and CHAP31 were prepared as described previously (Yoshida et al., 1990; Kijima et al., 1993; Furumai et al., 2001; Komatsu et al., 2001). FK228 and MS-275 were gifts from H.Nakajima and O.Nakanishi, respectively. NIH 3T3 and HL-60 cells were cultured in Eagle’s minimum essential medium and RPMI 1640 medium, respectively, supplemented with 10% fetal bovine serum. Sf9 cells were cultured in TNM-FH medium. The monoclonal mouse antibodies against α-tubulin (clone B-5-1-2) and acetylated α-tubulin (clone 6-11B-1) were purchased from Sigma. The monoclonal rat antibody against tyrosinated α-tubulin (clone MAS077) was from Harlan Sera laboratories. The monoclonal anti-acetylated lysine antibody AKL5C1 was raised against peptides comprising an acetylated lysine residue. The polyclonal antibody against mouse HDAC6 was as described previously (Verdel et al., 2000).
Synthetic peptides
The 20mer peptide fragments of α-tubulin (30-49), Dansylated-IQPDG QMPSDK(Ac)TIGGGDDSF, histone H4 (1-20), Dansylated-SGRGKG GKGLGKGGAK(Ac)RHRK, and their unacetylated reference 20mer peptides were synthesized with a Model 433A Peptide Synthesizer (Applied Biosystems). The MALDI-TOF MS for the purified peptides gave 2340 (M+H) for C100H146N24O37S2 (2339), 2298 (M+H) for C98H144N24O36S2 (2297), 2270 (M+H) for C96H164N37O25S (2269) and 2227 (M+H) for C94H161N37O24S (2226).
Plasmids, transfection and assay for tubulin deacetylase
HDAC6 m1/m2 is a deacetylase-negative version of mouse HDAC6 that was constructed to have amino acid replacements of D250N, D252N, H254V, H255V, D648N, H650V and H651V. For the construction of C-terminally HA-tagged HDAC1–4 and HDAC7–8, the PCR products amplified using the Gateway technology (Invitrogen) were integrated into the pcDNA3-cHA-ccdB2 vector, which was constructed by inserting the ccdB cassette and the HA cassette into pcDNA3. To synthesize the Gateway primer sets, we added 5′-GGGGACAAGTTTGTACAAA AAAGCAGGCTCCGGTACC-3′ to the 5′ ends of the following specific forward primers and 5′-GGGGACCACTTTGTACAAGAAAGCTGGG TCGAC-3′ to the reverse primers. Specific primer sets were 5′-ATGGCGCAGACGCAGGGCAC-3′ and 5′-GGCCAACTTGACC TCCTCCTTGAC-3′ for human HDAC1; 5′-ATGGCGTACAGTCAA GGAGG-3′ and 5′-GGGGTTGCTGAGCTGTTCTGATT-3′ for mouse HDAC2; 5′-ATGGCCAAGACCGTGGCCTA-3′ and 5′-AATCTCCAC ATCGCTTTCCTT-3′ for human HDAC3; 5′-ATGCTGGCCATGAA GCACCAG-3′ and 5′-CAGGGGCGGCTCCTCTTCCAT-3′ for human HDAC4; 5′-ATGCACAGCCCCGGCGCGGG-3′ and 5′-GAGGTTCA TGGGTTCTTCCTC-3′ for mouse HDAC7; and 5′-ATGGAGGAGCC GGAGGAACC-3′ and 5′-CACATGCTTCAGATTCCC-3′ for human HDAC8. Human HDAC10 was isolated by nested PCR using human kidney cDNA library (Gibco) as a PCR template in the course of our screening for novel HDAC cDNAs. The first PCR primer set (5′-GTTTGGGAACCCAGGGTGACCA-3′ and 5′-ATGCTCCCACC TTGGCCGATTTCAA-3′) and the second primer set (5′-AGGAG CGTCGACGAATTCCAGCCATGGGGACCGCGCTTGTGTA-3′ and 5′-CGCTATCTCGAGAATTCTATAGCCACCAGGTGAGGATGGC ACT-3′) were designed according to the putative HDAC10 sequence (DDBJ/EMBL/GenBank accession number CAB63048). The full-length cDNA sequence was identical to the human HDAC10 sequence (DDBJ/EMBL/GenBank accession numbers NM_032019, AF426160, AF393962 and AF407272). The HDAC10 open reading frame was PCR amplified using primers 5′-ATCGACGAATTCCAGCCATGGGG ACCGCGC-3′ and 5′-TGGCCGCTCGAGAGCCACCAGGTGAG GATG-3′ and inserted into the EcoRI–XhoI site of pcDNA3-cHA. NIH 3T3 cells were transiently transfected with each plasmid using the Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours after transfection, NIH 3T3 cells were treated with or without drugs for 6 h and then processed for immunostaining. To construct baculoviral expression plasmids for human HDACs, the full-length human HDAC1, HDAC6 and HDAC8 were cloned from HL-60 cells by RT–PCR using primer sets 5′-GCAGGAAGCCAGAGCTGGAG-3′ and 5′-CGAGCAAGATGG CGCAGAC-3′ for HDAC1; 5′-CCTCAACTATGACCTCAACCG-3′ and 5′-AGGCTGGAATGAGCTACAGC-3′ for HDAC6; and 5′-AGGAAGCCAGCTGCCACTTG-3′ and 5′-AAGCGGAAGAT GGAGGAGCC-3′ for HDAC8. To obtain the full-length HDAC4 clone, the 5′ region of HDAC4 was amplified from HL-60 cells by RT–PCR using 5′-TTATAGGAGGTCGACACTCCGCTC-3′ and 5′-GACGCCTCTGTTCAACTTGTGG-3′ as primers and ligated with a SalI–XbaI fragment of HDAC4 and a His6 linker. These fragments coding for HDACs with the His6 linker were subcloned into the NotI–XbaI site of the pVL1392 vector. Sf9 cells were transfected with the expression plasmids by using the Bac-N-Blue transfection kit (Invitrogen). The recombinant enzymes were purified with a Ni-NTA Superflow (Qiagen). The specific activities of the recombinant enzymes were determined with 3H-histone or the 20mer peptides. The recombinant enzymes were incubated with 0.5 mM peptides, and the products were analyzed by HPLC (Wakosil II 5C18 HG, 4.6 × 250 mm, flow rate 1 ml/min) using a solvent system of 25% acetonitrile containing 0.1% TFA and a fluorescent detector.
Immunoprecipitation of the mHDAC6 complex and microtubule deacetylation assays
Mouse testes were isolated, sliced and homogenized in an ice-cold lysis buffer (500 µl/testis) containing 0.34 M sucrose, 60 mM KCl, 15 mM NaCl, 15 mM Tris–HCl pH 7.4, 0.65 mM spermidine, 2 mM EDTA, 0.5 mM EGTA, 0.05% Triton X-100, 1 mM DTT and 0.5 mM PMSF. The homogenate was incubated 30 min on ice and centrifuged at 16 000 g for 30 min at 4°C. The supernatant was recovered and centrifuged 1 h at 100 000 g at 4°C (cytoplasmic extract). An anti-mHDAC6 antibody (Verdel et al., 2000) was added to the extract at 4°C for 1 h. The immune complexes were precipitated using protein G–Sepharose, washed three times with lysis buffer and eluted with elution buffer (40 mM Tris–HCl pH 8, 10 mM MgCl2, 20% glycerol, 0.2% Tween-20, 0.3 M KCl and 2 mM PMSF) containing 1 mg/ml of the antigenic peptide at 4°C overnight.
Acetylated microtubules were prepared as described previously (Vallee and Collins, 1986). Tubulin deacetylation assay using mouse testis HDAC6 was as follows. Thirteen microliters of immunopurified mHDAC6 were incubated with 1.25 µg of tubulin isolated from TSA-treated cells in 20 µl for 3 h at 37°C. Reactions were stopped by the addition of SDS–PAGE loading buffer. Deacetylation of tubulin was visualized by immunoblotting using an anti-acetylated tubulin antibody.
Immunofluorescence and microscopy
Exponentially growing cells were plated on 18 mm coverslips and incubated overnight. After drug treatment, cells were washed in phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde/PBS for 10 min at 37°C. Coverslips were then rinsed three times with PBS, and cells were permeabilized with 1% Triton X-100/PBS for 5 min at room temperature. Coverslips were then incubated in blocking solution (5% calf serum/PBS) for 30 min at room temperature for reducing non-specific binding of the antibody. Incubation with the primary antibodies was carried out for 2 h at room temperature, and the coverslips were then washed three times with PBS. Alexa-Fluor anti-mouse, Alexa-Fluor anti-rabbit and Alexa-Fluor anti-rat immunoglobulin antibodies were used as secondary antibodies. After the coverslips had been washed three times with PBS and once with deionized water, they were observed under an epifluorescence microscope (Zeiss Axiophot).
Acknowledgments
Acknowledgements
We thank Dr E.Seto and Dr. E.Verdin for supplying the HDAC cDNAs, Dr H.Nakajima for the gift of FK228, Dr O.Nakanishi for MS-275, and S.Curtet for technical assistance. We also thank Dr T.-P.Yao, Ms C.Hubbert and Dr T.Usui for discussion and Dr H.Maruta and Dr S.Rousseaux for critical reading of the manuscript. This work was supported in part by the CREST Research Project, Japan Science and Technology Corporation, a special grant for Advanced Research on Cancer, and Special Coordination Funds for Promoting Science and Technology from the Ministry of Education, Culture Sports, Science and Technology, of the Japanese Government.
References
- Bannister A.J., Miska,E.A., Görlich,D. and Kouzarides,T. (2000) Acetylation of importin-α nuclear import factors by CBP/p300. Curr. Biol., 10, 467–470. [DOI] [PubMed] [Google Scholar]
- Billger M., Stromberg,E. and Wallin,M. (1991) Microtubule-associated proteins-dependent colchicine stability of acetylated cold-labile brain microtubules from the Atlantic cod, Gadus morhua. J. Cell Biol., 113, 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M.M., Baas,P.W. and Humphries,S. (1989) Dynamics of α-tubulin deacetylation in intact neurons. J. Neurosci., 9, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P., Allis,C.D. and Sassone-Corsi,P. (2000) Signaling to chromatin through histone modifications. Cell, 103, 263–271. [DOI] [PubMed] [Google Scholar]
- Furumai R., Komatsu,Y., Nishino,N., Khochbin,S., Yoshida,M. and Horinouchi,S. (2001) Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. Proc. Natl Acad. Sci. USA, 98, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Cruz,M.A., Bowen,J., Gu,L., Pennock,D.G. and Gorovsky,M.A. (1995) Acetylation of lysine 40 in α-tubulin is not essential in Tetrahymena thermophila. J. Cell Biol., 129, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger C.M., Hassig,C.A. and Schreiber,S.L. (1999) Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl Acad. Sci. USA, 96, 4868–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W. and Roeder,R.G. (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell, 90, 595–606. [DOI] [PubMed] [Google Scholar]
- Guardiola A.R. and Yao,T.P. (2002) Molecular cloning and characterization of a novel histone deacetylase HDAC10. J. Biol. Chem., 277, 3350–3356. [DOI] [PubMed] [Google Scholar]
- Imai S., Armstrong,C.M., Kaeberlein,M. and Guarente,L. (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature, 403, 795–800. [DOI] [PubMed] [Google Scholar]
- Kao H.Y., Downes,M., Ordentlich,P. and Evans,R.M. (2000) Isolation of a novel histone deacetylase reveals that class I and class II deacetylases promote SMRT-mediated repression. Genes Dev., 14, 55–66. [PMC free article] [PubMed] [Google Scholar]
- Khochbin S., Verdel,A., Lemercier,C. and Seigneurin-Berny,D. (2001) Functional significance of histone deacetylase diversity. Curr. Opin. Genet. Dev., 11, 162–166. [DOI] [PubMed] [Google Scholar]
- Kijima M., Yoshida,M., Sugita,K., Horinouchi,S. and Beppu,T. (1993) Trapoxin, an antitumor cyclic tetrapeptide, is an irreversible inhibitor of mammalian histone deacetylase. J. Biol. Chem., 268, 22429–22435. [PubMed] [Google Scholar]
- Komatsu Y. et al. (2001) Cyclic hydroxamic-acid-containing peptide 31, a potent synthetic histone deacetylase inhibitor with antitumor activity. Cancer Res., 61, 4459–4466. [PubMed] [Google Scholar]
- Kouzarides T. (2000) Acetylation: a regulatory modification to rival phosphorylation? EMBO J., 19, 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M.H. and Allis,C.D. (1998) Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays, 20, 615–626. [DOI] [PubMed] [Google Scholar]
- LeDizet M. and Piperno,G. (1986) Cytoplasmic microtubules containing acetylated α-tubulin in Chlamydomonas reinhardtii: spatial arrangement and properties. J. Cell Biol., 103, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Hernault S.W. and Rosenbaum,J.L. (1985) Chlamydomonas α-tubulin is posttranslationally modified by acetylation on the ε-amino group of a lysine. Biochemistry, 24, 473–478. [DOI] [PubMed] [Google Scholar]
- MacRae T.H. (1997) Tubulin post-translational modifications—enzymes and their mechanisms of action. Eur. J. Biochem., 244, 265–278. [DOI] [PubMed] [Google Scholar]
- Maruta H., Greer,K. and Rosenbaum,J.L. (1986) The acetylation of α-tubulin and its relationship to the assembly and disassembly of microtubules. J. Cell Biol., 103, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska E.A., Karlsson,C., Langley,E., Nielsen,S.J., Pines,J. and Kouzarides,T. (1999) HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J., 18, 5099–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno R.D. and Schatten,G. (2000) Microtubule configurations and post-translational α-tubulin modifications during mammalian spermatogenesis. Cell Motil. Cytoskeleton, 46, 235–246. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Kim,Y.B., Terano,H., Yoshida,M. and Horinouchi,S. (1998) FR901228, a potent antitumor antibiotic, is a novel histone deacetylase inhibitor. Exp. Cell Res., 241, 126–133. [DOI] [PubMed] [Google Scholar]
- Nogales E., Whittaker,M., Milligan,R.A. and Downing,K.H. (1999) High-resolution model of the microtubule. Cell, 96, 79–88. [DOI] [PubMed] [Google Scholar]
- Piperno G. and Fuller,M.T. (1985) Monoclonal antibodies specific for an acetylated form of α-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol., 101, 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., LeDizet,M. and Chang,X.J. (1987) Microtubules containing acetylated α-tubulin in mammalian cells in culture. J. Cell Biol., 104, 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V.M., Emiliani,S., Verdin,E., Webb,Y., Breslow,R., Rifkind,R.A. and Marks,P.A. (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc. Natl Acad. Sci. USA, 95, 3003–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. (2000) Cytoskeleton: functions for tubulin modifications at last. Curr. Biol., 10, R801–R803. [DOI] [PubMed] [Google Scholar]
- Saito A., Yamashita,T., Mariko,Y., Nosaka,Y., Tsuchiya,K., Ando,T., Suzuki,T., Tsuruo,T. and Nakanishi,O. (1999) A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc. Natl Acad. Sci. USA, 96, 4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Asai,D.J., Bulinski,J.C. and Kirshner,M. (1987) Posttranslational modification and microtubule stability. J. Cell Biol., 105, 2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin-Berny D., Verdel,A., Curtet,S., Lemercier,C., Garin,J., Rousseaux,S. and Khochbin,S. (2001) Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell. Biol., 21, 8035–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner D.E. and Berger,S.L. (2000) Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev., 64, 435–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl B.D. and Allis,C.D. (2000) The language of covalent histone modifications. Nature, 403, 41–45. [DOI] [PubMed] [Google Scholar]
- Turner B.M. (1993) Decoding the nucleosome. Cell, 75, 5–8. [PubMed] [Google Scholar]
- Turner B.M. (2000) Histone acetylation and an epigenetic code. BioEssays, 22, 836–845. [DOI] [PubMed] [Google Scholar]
- Vallee R.B. and Collins,C.A. (1986) Purification of microtubules and microtubule-associated proteins from sea urchin eggs and cultured mammalian cells using taxol, and use of exogenous taxol-stabilized brain microtubules for purifying microtubule-associated proteins. Methods Enzymol., 134, 116–127. [DOI] [PubMed] [Google Scholar]
- Verdel A. and Khochbin,S. (1999) Identification of a new family of higher eukaryotic histone deacetylases. Coordinate expression of differentiation-dependent chromatin modifiers. J. Biol. Chem., 274, 2440–2445. [DOI] [PubMed] [Google Scholar]
- Verdel A., Curtet,S., Brocard,M.P., Rousseaux,S., Lemercier,C., Yoshida,M. and Khochbin,S. (2000) Active maintenance of mHDA2/mHDAC6 histone-deacetylase in the cytoplasm. Curr. Biol., 10, 747–749. [DOI] [PubMed] [Google Scholar]
- Wang A.H., Bertos,N.R., Vezmar,M., Pelletier,N., Crosato,M., Heng,H.H., Th’ng,J., Han,J. and Yang,X.J. (1999) HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol., 19, 7816–7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L. (1975) Microtubules as drug receptors: pharmacological properties of microtubule protein. Ann. N. Y. Acad. Sci., 253, 213–231. [DOI] [PubMed] [Google Scholar]
- Wilson P.J. and Forer,A. (1997) Effects of nanomolar taxol on crane-fly spermatocyte spindles indicate that acetylation of kinetochore microtubules can be used as a marker of poleward tubulin flux. Cell Motil. Cytoskeleton, 37, 20–32. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Kijima,M., Akita,M. and Beppu,T. (1990) Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J. Biol. Chem., 265, 17174–17179. [PubMed] [Google Scholar]
- Yoshida M., Horinouchi,S. and Beppu,T. (1995) Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. BioEssays, 17, 423–430. [DOI] [PubMed] [Google Scholar]