Abstract
Depletion of CD4+ T cells is the hallmark of HIV infection and AIDS progression. In addition to the direct killing of the viral-infected cells, HIV infection also leads to increased apoptosis of predominantly uninfected bystander cells. This is mediated in part through the HIV-1 Tat protein, which is secreted by the infected cells and taken up by uninfected cells. Using an affinity-purification approach, a specific and direct interaction of Tat with tubulin and polymerized microtubules has been detected. This interaction does not affect the secretion and uptake of Tat, but is critical for Tat to induce apoptosis. Tat binds tubulin/microtubules through a four-amino-acid subdomain of its conserved core region, leading to the alteration of microtubule dynamics and activation of a mitochondria-dependent apoptotic pathway. Bim, a pro-apoptotic Bcl-2 relative and a transducer of death signals initiated by perturbation of microtubule dynamics, facilitates the Tat-induced apoptosis. Our findings reveal a strategy by which Tat induces apoptosis by targeting the microtubule network. Thus HIV-1 Tat joins a growing list of pathogen-derived proteins that target the cytoskeleton of host cells.
Keywords: apoptosis/Bim/HIV-1/microtubule/Tat
Introduction
Human immunodeficiency virus type 1 (HIV-1) is the etiological agent for the acquired immunodeficiency syndrome (AIDS). HIV-1 encodes a small trans-acting regulatory protein, Tat, which is absolutely essential for viral replication and is conserved in the genomes of all primate lentiviruses (Jones and Peterlin, 1994). A primary role of Tat is in regulating productive and processive transcription from the HIV-1 long terminal repeat (LTR). The past decade has been a watershed for the biochemical analysis of the mechanism of Tat stimulation of HIV-1 transcription. In addition to this HIV-1-specific activity, Tat has also been shown to impinge upon many cellular functions, some of which are consistent with the fact that Tat can be secreted by the HIV-infected cells and act upon the neighboring bystander cells (Frankel and Pabo, 1988; Ensoli et al., 1990; Ensoli et al., 1993). Although the mechanisms for the secretion and uptake of Tat are mostly unclear, it is these unique properties that enable Tat to regulate cytokine gene expression and immune cell hyperactivation (Ott et al., 1997), stimulate the growth of Kaposi’s sarcoma cells (Ensoli et al., 1990) and induce apoptosis of uninfected T cells (Li et al., 1995; Westendorp et al., 1995; Bartz and Emerman, 1999). Apoptosis contributes to the massive depletion of CD4+ T cells and consequently to the loss of immune competence during HIV-1 infection (Meyaard et al., 1992; Fauci, 1993). Although the mechanisms controlling apoptosis are likely to be multifactorial, Tat and a few other HIV-1 gene products appear to contribute in part to the increased apoptosis associated with AIDS (Roshal et al., 2001).
The mechanisms by which Tat affects the diverse cellular functions are largely unknown. Here, we used an epitope-tagging and affinity-purification approach to identify Tat-associated cellular factors that may regulate or mediate the diverse activities of Tat. This has led to the identification of a specific and direct interaction between Tat and the αβ-tubulin dimer and polymerized microtubules in the cytoplasm of the cell. The Tat–tubulin interaction does not contribute to the secretion and uptake of Tat but appears to be critical for Tat to induce apoptosis in Jurkat T cells. Tat binds tubulin/microtubules through a four-amino-acid subdomain of its evolutionarily conserved core region, and this interaction alters microtubule dynamics, leading to the activation of a mitochondria-dependent apoptotic pathway.
The Bcl-2 family plays a key role in apoptosis by regulating the release of cytochrome c from the mitochondria (Budihardjo et al., 1999). Bim, a pro-apoptotic Bcl-2 family member with only the BH3 domain, induces apoptosis by antagonizing the activity of the anti-apoptotic Bcl-2 family members (O’Connor et al., 1998). In lymphocytes, Bim has been shown to be a dominant transducer of certain apoptotic signals including microtubule perturbation (Bouillet et al., 1999) and activated T-cell death (Hildeman et al., 2002). Here, we showed that Bim facilitates the Tat-induced apoptosis, as over-expression of BimL, one of the differentially spliced isoforms of Bim, sensitizes Tat-induced apoptosis. Furthermore, the Bim-deficient lymphocytes are more resistant than the wild-type cells to the killing effect of Tat. Together, our findings reveal a novel strategy by which Tat promotes the killing of the host cells by targeting the microtubule network in a process facilitated by Bim.
Results
Direct and specific interaction of the HIV-1 Tat protein with the αβ-tubulin dimer and polymerized microtubules in the cytoplasm
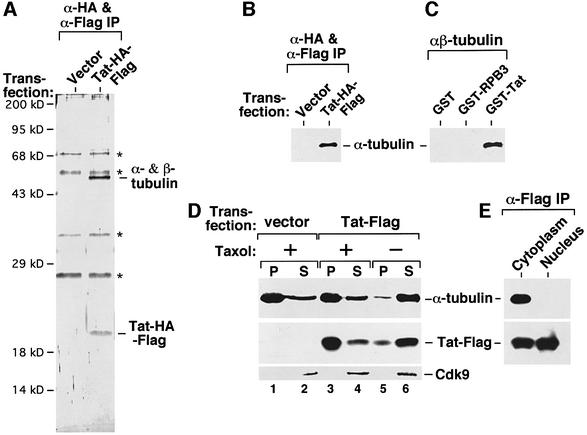
To identify Tat-associated cellular factors that may regulate or mediate the diverse activities of Tat (Jeang et al., 1999), a construct expressing HA and Flag double-tagged Tat (Tat–HA–Flag) was transiently transfected into human 293T cells. Tat and its associated proteins were isolated from the cell lysate by two rounds of affinity purification (O’Keeffe et al., 2000) and visualized by silver staining. A 50 kDa protein associated specifically with Tat–HA–Flag (Figure 1A). Microsequencing analysis identified it as a mixture of α- and β-tubulins, which are similar in molecular weight, known to form heterodimers and function as the building blocks of microtubules. The association of the tubulin dimer with Tat–HA–Flag was confirmed by western blotting with specific anti-α-tubulin antibodies (Figure 1B). In control experiments, anti-Flag immunoprecipitates from cells expressing several unrelated Flag-tagged proteins did not show any bound tubulin (data not shown), revealing the specificity of the Tat–tubulin interaction. A direct interaction between Tat and purified αβ-tubulin dimer (Sigma) was further demonstrated in a pull-down assay using immobilized GST–Tat (Figure 1C).
Fig. 1. A direct and specific interaction of Tat with the αβ-tubulin dimer and polymerized microtubules in the cytoplasm. (A) Affinity-purified Tat–HA–Flag associates with the αβ-tubulin dimer. Whole-cell lysates from 293T cells transfected with either an empty vector or a vector expressing Tat–HA–Flag were subjected to two rounds of immunoprecipitation with anti-Flag and anti-HA affinity-beads. Upon final elution with the HA peptide, Tat–HA–Flag and its associated factors (α-HA & α-Flag IP) were analyzed by SDS–PAGE and silver staining. Non-specific bands are denoted by asterisks. A specific 50 kDa Tat-associated band was recovered from the gel and digested with trypsin. Microsequencing analysis of the tryptic peptides identified it as a mixture of α- and β-tubulin. (B) The association of the tubulin dimer with the affinity-purified Tat–HA–Flag was confirmed by western blotting with antibodies specific for α-tubulin. (C) A GST pull-down assay reveals a direct interaction between immobilized GST–Tat and purified αβ-tubulin dimer. GST–RBP3 and GST alone were used as controls. Tubulin dimers associated with the beads were analyzed by anti-α-tubulin western blotting. (D) Tat also interacts with the polymerized microtubules. Cleared lysate of 293T cells transfected with either an empty vector or a Tat–Flag-expressing construct was incubated with (+) or without (–) taxol and then subjected to ultracentrifugation as described (Puthalakath et al., 1999). The levels of microtubule/tubulin, Tat–Flag and transcription factor Cdk9 in the pellet (P) and supernatant (S) were determined by western blotting. Cdk9 served as an internal control and remained in the supernatant under both (+) and (–) taxol conditions as expected. (E) The Tat–tubulin interaction occurs in the cytoplasm. Cytoplasmic and nuclear extracts were prepared from 293T cells transfected with a Tat–Flag-expression construct and subjected to anti-Flag immunoprecipitation. The precipitates were analyzed for the presence of α-tubulin and Tat–Flag by western blotting.
To determine whether Tat also binds to polymerized microtubules, cleared lysate from Tat–Flag-transfected 293T cells was treated with the microtubule-stabilizing drug taxol. Microtubules and microtubule-associated proteins were then separated from the soluble fraction by ultracentrifugation (Puthalakath et al., 1999). While ∼85% of Tat remained with free tubulin in the supernatant of an untreated lysate, about the same percentage of Tat was precipitated with the polymerized microtubules in the taxol-treated lysate (Figure 1D), indicating an interaction of Tat with the assembled microtubules.
Tat is best known for its nuclear transcriptional activity, whereas tubulin and microtubules reside in the cytoplasm in non-mitotic cells. To determine the location of the Tat–tubulin interaction, cytoplasmic and nuclear extracts were prepared from 293T cells transfected with a Tat–Flag-expression construct (Dignam et al., 1983). Tat was detected in the anti-Flag immunoprecipitates derived from both extracts. However, only the cytoplasmic Tat interacted with the tubulin dimer (Figure 1E), suggesting the cytoplasm as a likely location for this interaction.
Tat binds tubulin through a four-amino-acid segment within its conserved core region
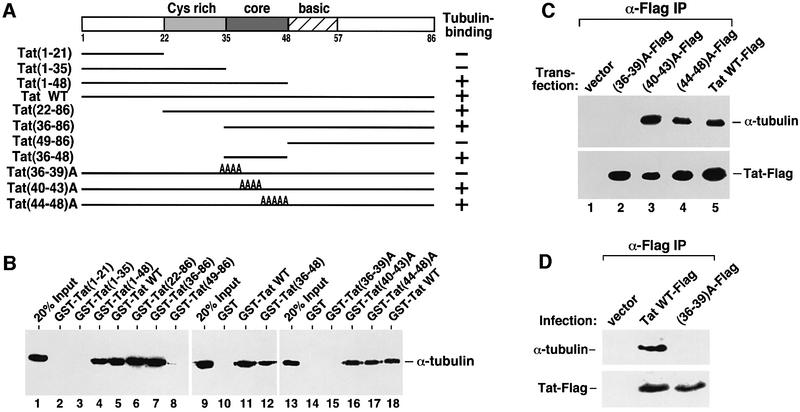
To map the tubulin-binding domain in Tat, a panel of GST–Tat fusion proteins containing various N- and C-terminally truncated Tat were adjusted to the same concentration and examined for their abilities to interact with the purified αβ-tubulin dimer (Figure 2A and B). A 13-amino-acid ‘core’ region (amino acids 36–48 of HIV-1 Tat) which is highly conserved among the various Tat proteins encoded by the lentiviruses HIV-1, HIV-2, SIV and EIAV (Jones and Peterlin, 1994) was found to be both necessary and sufficient for the Tat–tubulin interaction (Figure 2B, lanes 1–12). While substitutions of amino acids 40–43 or 44–48 with alanines in the Tat core region had virtually no effect on the Tat–tubulin interaction, substitutions of the first four residues (36–39) in a mutant GST–Tat termed (36–39)A significantly impaired this interaction (lanes 13–18). These four residues (Val– Cys–Phe–Ile) appeared to be collectively required for the Tat–tubulin interaction, since individual alanine substitutions of the four residues showed no significant impact (data not shown). The same four-amino-acid subdomain of Tat is also critical for the Tat–tubulin binding in 293T cells, as evidenced by the lack of any detectable interaction between the transfected Tat(36–39)A–Flag and the endogenous tubulin despite the stable expression of the Tat mutant (Figure 2C).
Fig. 2. Tat binds tubulin through a four-amino-acid subdomain within its conserved core region. (A) Domain structures of wild-type Tat, N- or C-terminally truncated Tat and alanine (A) substitution Tat mutants. The abilities of these Tat proteins to bind to αβ-tubulin dimers in vitro are summarized in the column on the right. The core region (amino acids 36–48) highly conserved among the various lentiviral Tat proteins is indicated as a dark-shaded box in the middle of Tat. (B) A GST pull-down assay identifies a four-amino-acid tubulin-binding subdomain in Tat. A panel of immobilized GST–Tat fusion proteins containing wild-type and various Tat mutants were adjusted to the same level (1 µg) and examined for their interactions with the purified αβ-tubulin dimer (1 µg) under conditions described previously (Chen et al., 1999). After extensive washes, the Tat-associated tubulin was detected by western blotting. (C) The four-amino-acid subdomain of Tat is also critical for the Tat–tubulin binding in vivo. Whole-cell lysates from 293T cells transfected with the indicated Tat constructs were subjected to anti-Flag immunoprecipitation and the precipitates were analyzed by western blotting for the presence of α-tubulin and the various Tat–Flag proteins. (D) The Tat–tubulin interaction occurs in retroviral-infected Jurkat T cells. Driven by the MuLV LTR, WT Tat–Flag and Tat(36–39)A-Flag were expressed from the pBabe-puro retroviral vector under infection conditions. The empty vector (pBabe-puro) provided a negative control. Cleared lysates from the infected cells were subjected to anti-Flag immunoprecipitation and the precipitates were analyzed for the presence of Tat–Flag and its associated tubulin by western blotting.
The dependence on the four-amino-acid subdomain for the Tat–tubulin interaction was also examined in infected Jurkat T cells where Tat was produced from a retroviral vector to mimic the HIV infection conditions. Flag-tagged wild-type Tat and (36–39)A were cloned into the pBabe-puro vector (Morgenstern and Land, 1990) under the control of the MuLV LTR. The retroviral constructs were transfected into Phoenix retrovirus packaging cells and the viral supernatants used to infect Jurkat T cells. Again, wild-type Tat but not (36–39)A produced by the viruses associated with tubulin in anti-Flag immunoprecipitates (Figure 2D), revealing the dependence of Tat on the same four-amino-acid subdomain for binding tubulin in infected Jurkat cells. Furthermore, by using a retroviral infection system which produces Tat at close to physiological level, this result also ruled out Tat over-expression as a cause for the observed Tat–tubulin interaction in transfected 293T cells.
Mutation of the tubulin-binding domain in Tat does not affect the release and cytoplasmic uptake of Tat
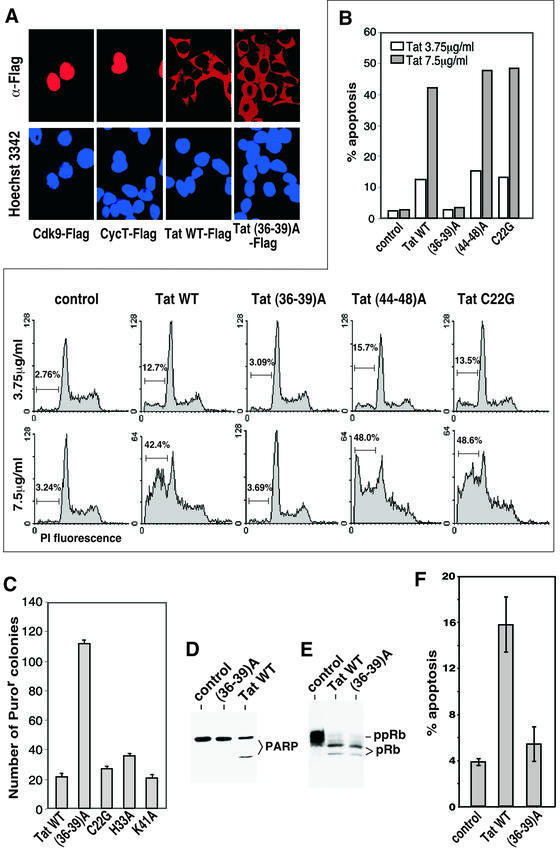
In acutely HIV-infected cells or cells transfected with a Tat-expressing construct, efficient expression of Tat results in its release into the medium in the absence of any cell lysis (Ensoli et al., 1990, 1993), and the secreted protein can then be taken up by bystander cells (Ensoli et al., 1993; Frankel and Pabo, 1988). Although the mechanisms remain to be elucidated, these properties have enabled Tat to exert a number of biological effects on bystander cells (Jeang et al., 1999). Because the microtubule network is intimately involved in intracellular protein trafficking and transport, an assay described previously (Ensoli et al., 1993) that measures the coupled release and uptake of Tat was used to examine the role of the Tat–tubulin interaction in this process. Human 293T cells transfected with constructs expressing Flag-tagged wild-type Tat, Tat(36–39)A, Cdk9 or cyclin T1 were mixed 1:1 with untransfected cells at 24 h post-transfection. The co-cultivated cells were analyzed 1 day later by anti-Flag immunofluorescence staining (Figure 3A). Using Hoechst 3342-generated nuclear DNA staining patterns as a reference, human transcription factors Cdk9 and cyclin T1 were expressed as nuclear proteins in ∼20% of transfected cells and neither protein was released into the medium and taken up by untransfected cells. In contrast, wild-type Tat was found predominantly in the cytoplasmic region of every cell within the entire population (Figure 3A), indicating a coupled release and cytoplasmic uptake of Tat by the co-cultivated cells. These data agree with the previous observations that bystander cells take up the secreted Tat in mostly the cytoplasm and only a small portion can reach the nucleus to activate HIV-1 transcription (Ensoli et al., 1993). Strong whole-cell staining of Tat was also observed in a small number of cells (data not shown), which were probably the transfected Tat-producing cells. Importantly, both wild-type Tat and Tat(36–39)A defective in tubulin binding demonstrated a similar efficiency in their coupled release and cytoplasmic uptake (Figure 3A), which was observed over a broad range of Tat concentrations (data not shown). Thus the Tat–tubulin interaction did not appear to contribute to the coupled release and uptake of Tat.
Fig. 3. The tubulin-binding domain of Tat is required for Tat-induced apoptosis but not for G1 delay. (A) Mutation of the tubulin-binding domain in Tat does not affect the release and cytoplasmic uptake of Tat. Transfected 293T cells expressing Flag-tagged wild-type Tat, Tat(36–39)A, Cdk9 or cyclin T1 were mixed 1:1 with untransfected cells at 24 h post-transfection. The co-cultivated cells were analyzed 1 day later by anti-Flag immunofluorescence staining. The Hoechst 3342-generated nuclear DNA staining pattern was used as a reference. (B) The tubulin-binding activity of Tat is required for Tat-induced apoptosis. Recombinant wild-type Tat and the indicated Tat mutants were added at two different concentrations into the cultures of Jurkat cells and the percentage of apoptotic cells with hypodiploid nuclei was analyzed by flow cytometry and summarized in a column chart. (C) Sustained expression of Tat active in tubulin-binding suppresses colony formation of 293T cells. Constructs expressing the indicated Tat proteins were co-transfected with pBabe-Puro conferring puromycin resistance at a 30:1 ratio into 293T cells. The expression levels of the transfected Tat proteins were similar (data not shown). The number of puromycin-resistant colonies was counted after 2 weeks of incubation and plotted. (D) Tat treatment of Jurkat cells causes the cleavage of PARP. Recombinant wild-type Tat and mutant Tat(36–39)A were added at 3.75 µg/ml into the cultures of Jurkat cells. Whole-cell lysates were prepared 18 h later and analyzed by anti-PARP western blotting. (E) Tat prevents phosphorylation of the retinoblastoma protein (pRb) independent of its tubulin-binding activity. Whole-cell lysates from Jurkat cells treated with either wild-type Tat or Tat(36–39)A were analyzed for the presence of the phosphorylated (ppRb) and under-phosphorylated (pRb) retinoblastoma proteins by western blotting as described (Kundu et al., 1998). (F) Expression of wild-type Tat but not Tat(36–39)A under retroviral infection conditions causes apoptosis. Wild-type Tat or Tat(36–39)A was co-produced with GFP from an IRES-containing retroviral vector. The infected GFP-positive Jurkat cells were gated by flow cytometry and the percentage of cell death determined by propidium iodide uptake. A similar level of expression of the wild-type and mutant Tat was detected in the infected cells (data not shown). Viruses that expressed GFP alone were used as a control.
The tubulin-binding domain in Tat is required for Tat-induced apoptosis but not for G1 delay
Extracellular Tat has been shown to induce apoptosis and increase sensitivity to apoptotic signals in both primary CD4+ T cells and T-cell lines, contributing in part to the progressive loss of T cells associated with AIDS (Li et al., 1995; Westendorp et al., 1995; Bartz and Emerman, 1999). Since the Tat–tubulin interaction is not required for the release and uptake of Tat, we investigated whether it is required for Tat-induced apoptosis. Recombinant Tat proteins were added to the cultures of Jurkat T cells and their apoptotic effects were analyzed by flow cytometry. Treatment of cells with wild-type Tat, an alanine-substitution mutant Tat(44–48)A or a transcriptionally inactive Tat mutant C22G, all three of which displayed a similar and strong tubulin-binding capability (Figure 2 and data not shown), caused significant and dose-dependent apoptosis as indicated by increases in the amount of hypodiploid nuclei (Figure 3B). In contrast, Tat(36–39)A was completely incapable of inducing apoptosis, suggesting that the tubulin binding but not the transcriptional activity of Tat is critical for Tat-induced apoptosis.
In support of the above data obtained in Jurkat cells with recombinant Tat proteins, sustained expression from stably transfected constructs of wild-type Tat and three transcriptionally inactive Tat mutants, C22G, H33A and K41A (Rice and Carlotti, 1990), which are active in tubulin binding (data not shown) suppressed colony formation of 293T cells more potently than did expression of Tat(36–39)A (Figure 3C). This result further underscores the importance of the tubulin-binding activity of Tat in induction of apoptosis.
The caspase family of cysteine proteases plays a pivotal role in mediating apoptosis through proteolysis of specific targets. In Jurkat cells treated with wild-type Tat, a cleaved 85 kDa fragment of PARP, a target of the downstream effector caspases such as caspase-3 and caspase-6 (Lazebnik et al., 1994), was observed (Figure 3D), indicating an activation of the caspase cascade. Although Tat(36–39)A failed in this regard, it is still active in preventing the phosphorylation of retinoblastoma protein pRb (Figure 3E), an activity thought to be responsible for the Tat-induced G1 delay (Kundu et al., 1998).
Because the tubulin-binding subdomain of Tat (amino acids 36–39) contains Phe38, a residue essential for Tat to transactivate the HIV-1 LTR (Rice and Carlotti, 1990), it is not feasible to examine the apoptotic activity of the mutant HIV-1 virus expressing Tat(36–39)A, which would express few if any HIV-1 proteins. To circumvent this limitation and analyze the apoptotic effect of Tat expressed at close to physiological level, we expressed wild-type Tat and Tat(36–39)A from recombinant retroviruses in Jurkat T cells under the infection conditions. Infected cells co-expressing GFP and Tat from two open reading frames separated by an IRES in the pMX-Tat-IRES-GFP retroviral vector (Liu et al., 1997) were analyzed by flow cytometry to detect apoptotic cells which were able to absorb propidium iodide. After subtracting the background level of apoptosis caused by the control virus expressing only GFP, the virus-produced wild-type Tat induced 7.6 times more apoptosis than did (36–39)A, revealing a key role of the tubulin-binding domain in this process (Figure 3F).
Tat induces apoptosis by preventing microtubule depolymerization
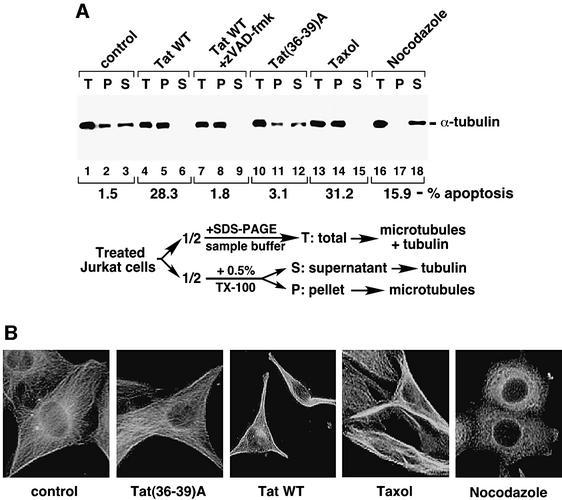
Microtubule dynamics, a fundamental property of microtubules, is critical for diverse cellular functions and regulated by many factors. Persistent perturbation of microtubule dynamics by microtubule-damaging drugs is known to cause apoptosis (Sorger et al., 1997; Wang et al., 2000). With the observation that the Tat–tubulin interaction may contribute to apoptosis, we investigated whether Tat may exert this effect by altering microtubule dynamics. Extracts containing free tubulin dimers (S), or polymerized microtubules (P) or a mixture of both (T) were each prepared from Jurkat cells treated with wild-type Tat, Tat(36–39)A or the microtubule-perturbation drugs taxol or nocodazole and were then analyzed by anti-α-tubulin western blotting (Figure 4A). As expected, the microtubule-stabilizing drug taxol and the destabilizing drug nocodazole altered the partitioning of microtubules and tubulin in opposite directions and both caused significant apoptosis (Figure 4A). Like taxol, wild-type Tat, but not Tat(36–39)A, also caused a significant stabilization of microtubules and a concurrent reduction of the level of unpolymerized tubulin in the cell. The broad-spectrum caspase inhibitor zVAD-fmk inhibited the Tat-induced apoptosis, but had no effect on Tat stabilization of microtubules, indicating that this latter event occurred during the initiation of apoptosis and was not simply a consequence of caspase activation.
Fig. 4. Tat induces apoptosis by preventing microtubule depolymerization. (A) Tat treatment of Jurkat cells stabilizes microtubules and this effect precedes the activation of caspases. Upon treatment of Jurkat cells with the indicated agents, half the cells were boiled in SDS–PAGE sample buffer to obtain a mixture of total tubulin and microtubules in the cell (T). The other half were extracted with a buffer containing 0.5% Triton X-100 and subjected to centrifugation to obtain the pellet (P) containing assembled microtubules and supernatant (S) containing free tubulin dimers as described (Nguyen et al., 1999). The amount of tubulin/microtubules present in each fraction was analyzed by anti-α-tubulin western blotting. In a parallel experiment, apoptosis induced by the various agents was determined by flow cytometry as in Figure 3A, and the percentage of apoptosis was indicated below the western blot. (B) Alterations of the cellular microtubule network upon Tat treatment. NIH 3T3 cells were treated with the indicated reagents and analyzed by immunofluorescence staining using α-tubulin antibodies to visualize the microtubule network.
Immunofluorescence studies further supported the idea that Tat acts to prevent microtubule depolymerization (Figure 4B). In untreated cells, microtubule fibers appeared to fill the whole cytoplasm and extend virtually the entire distance to the edges of the cell. While microtubules were significantly fragmented in cells exposed to nocodazole, microtubule tangling and bundling, particularly in the cell periphery, were observed in taxol-treated cells as reported previously (Liao et al., 1995). Treatment of cells with wild-type Tat, but not with Tat(36–39)A, produced a pattern of microtubule network similar to that of taxol-treated cells. These observations, together with the above biochemical data, suggest that the Tat–tubulin interaction prevents microtubule depolymerization, which may initiate a signaling pathway leading to apoptosis.
Tat induces apoptosis through the mitochondria pathway
The taxol-perturbation of microtubules is known to induce apoptosis through a mitochondria-dependent pathway (André et al., 2000; Wang et al., 2000). One characteristic of this pathway is the cleavage and activation of caspase-9 (Budihardjo et al., 1999). Like taxol, treatment of Jurkat cells with wild-type Tat, but not with Tat(36–39)A, also caused efficient cleavage of caspase-9 (Figure 5A), suggesting that the death signals initiated by Tat perturbation of microtubules also transmit through the mitochondria pathway. In support of this model, Tat has previously been shown to cause changes in mitochondrial membrane permeability (Macho et al., 1999; Ferri et al., 2000).
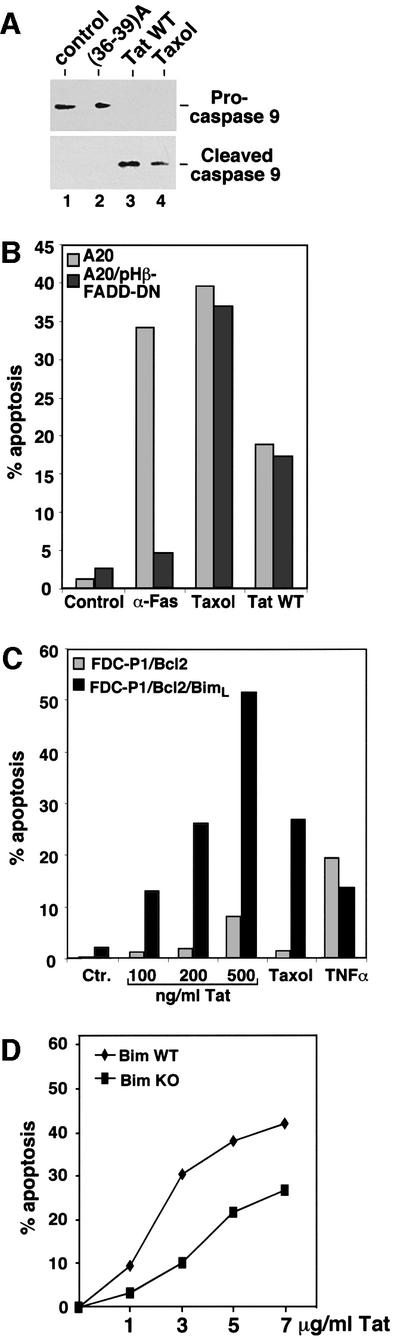
Fig. 5. Tat induces apoptosis through the mitochondria pathway. (A) Tat treatment of Jurkat cells causes efficient cleavage of procaspase-9. Anti-caspase 9 western blotting was performed to detect procaspase-9 and cleaved caspase-9 in whole-cell lysates of Jurkat cells treated with the indicated agents. (B) Inhibition of the death-receptor pathway by dominant negative FADD does not prevent Tat-induced apoptosis. Mouse A20 cells as well as an A20 derivative which stably expresses dominant negative FADD were treated with the indicated apoptosis-inducing agents. The percentage of apoptosis was determined by flow cytometry, and the data shown were representative of four independent experiments. (C) BimL sensitizes Tat-induced apoptosis. Mouse FDC-P1 cells which stably express Bcl-2 or Bcl-2 plus BimL were treated with the indicated apoptosis-inducing agents. The percentage of apoptosis was determined by flow cytometry, and the data shown were representative of three independent experiments. (D) Bim-deficient T lymphocytes are more resistant than wild-type cells to Tat-induced apoptosis. T cells were purified from spleens and lymph nodes of wild type and Bim–/– knockout mice and treated with different amounts of Tat as indicated in the figure. The T cells were stained with propidium iodide after 12 h of culture, and cell death was determined by flow cytometry.
Previous reports have also suggested that Tat may induce apoptosis by upregulating the expression of FasL (Westendorp et al., 1995) and caspase-8 (Bartz and Emerman, 1999), both of which presumably signal through the death-receptor pathway. To investigate the contribution of this pathway to Tat-induced apoptosis, the apoptotic activity of Tat was examined in mouse A20 cells stably expressing a dominant negative form of the Fas adaptor molecule FADD, which has been shown to block the death-receptor pathway completely (Zhang and Winoto, 1996; Newton et al., 1998). As expected, the dominant negative FADD efficiently inhibited apoptosis induced by the anti-Fas antibody but had no effect on the taxol-induced apoptosis (Figure 5B). Like taxol, Tat caused a similar level of apoptosis in both cells, suggesting that the Tat-induced apoptosis was unaffected by inhibition of the death-receptor pathway.
Bim promotes Tat-induced apoptosis
The pro-apoptotic Bcl-2 relative Bim induces apoptosis by antagonizing the activity of the anti-apoptotic Bcl-2 family members (O’Connor et al., 1998). In lymphocytes, Bim has been shown to be a major transducer of certain apoptotic signals including microtubule perturbation (Bouillet et al., 1999). Since both Tat and taxol may induce apoptosis by perturbing microtubule dynamics, the role of BimL, one of the differentially spliced isoforms of Bim, in Tat- and taxol-induced apoptosis was investigated (Figure 5C). Cells co-expressing BimL and Bcl-2 were significantly more sensitive to taxol- and Tat-induced apoptosis than cells expressing Bcl-2 alone. In contrast, a similar level of apoptosis was induced in both cell types by TNFα, which transduces apoptotic signals through the death-receptor pathway. This sensitizing effect of BimL on Tat- and taxol-induced apoptosis further underscores the importance of BimL in mediating apoptosis induced by agents that impinge upon microtubules.
It has been shown that Bim-deficient lymphocytes are more resistant than wild-type cells to certain apoptotic stimuli such as cytokine deprivation, calcium ion flux and taxol-induced microtubule perturbation (Bouillet et al., 1999) and activated T-cell death (Hildeman et al., 2002). Since Tat may induce apoptosis by altering microtubule dynamics, the sensitivity of T cells derived from Bim–/– knockout mice to Tat-induced apoptosis was compared with that of wild-type cells (Figure 5D). Bim-deficient T cells have been shown to display a somewhat decreased basal level of apoptosis after overnight culturing (Bouillet et al., 1999; Hildeman et al., 2002; data not shown). After subtracting the respective basal levels to reveal specifically the Tat-induced apoptosis, the Bim–/– cells displayed an increased resistance to Tat compared with the wild-type cells, highlighting a critical role of Bim in Tat-induced apoptosis. It has been shown that the death-receptor-induced apoptosis and apoptosis signaling pathways regulated by members of the Bcl-2 family are distinct in their dependence on the Bim protein (Strasser et al., 1995; Bouillet et al., 1999). The stimulating effect of Bim on Tat-induced apoptosis further suggests that Tat induces apoptosis predominantly through the mitochondria-dependent pathway and not the death-receptor pathway.
Discussion
The data presented in this study are consistent with a model that Tat targets microtubules and transduces the death signal through a mitochondria-dependent pathway that is facilitated by the pro-apoptotic Bcl-2 family member Bim. Tat binds to tubulin/microtubules through part of its conserved core region both in vitro and under retroviral infection conditions in vivo. It will be of interest to determine whether microtubules are also targeted by the conserved regions of HIV-2 Tat and perhaps other lentiviral Tat proteins for apoptosis (Bartz and Emerman, 1999). The amino acid residues of Tat required for the Tat–tubulin interaction differ from those present in the tubulin-binding domains of conventional microtubule-associated proteins (MAPs), which typically contain positively charged residues (Hirokawa, 1994). Although not directly required for the Tat–tubulin binding (Figure 2), the arginine-rich domain of Tat adjacent to the tubulin-binding subdomain could conceivably provide the positive charge to neutralize the negatively charged tubulin C-termini in order to promote microtubule assembly.
The mechanistic details between Tat stabilization of microtubules and the initiation of the apoptotic-signaling pathway remain to be elucidated. The similarity between Tat and taxol in affecting microtubule dynamics suggests that their apoptotic pathways may share similar mechanisms or themes. BimL transduces the death signal initiated by microtubule perturbation, leading to apoptosis through the mitochondria pathway. It is normally sequestered to microtubules and can be released by apoptotic stimuli that impinge upon microtubules. The released BimL binds to and neutralizes anti-apoptotic activity of Bcl-2 (Puthalakath et al., 1999). The observation that BimL can synergize with Tat and taxol, but not with TNFα, in induction of apoptosis suggests that BimL may play a key role in Tat- and taxol-induced apoptosis. In fact, the higher expression level of BimL in T lymphocytes than in many other cell types (O’Reilly et al., 2000) may explain the higher sensitivity of the lymphocytes to Tat-induced apoptosis (Li et al., 1995; our unpublished data). A more direct demonstration of a tight link between the cellular level of Bim and the Tat-induced apoptosis is provided by the observation that the Bim-deficient T cells are more resistant than the wild-type cells to the killing effect of Tat. It is worth noting that the Bim–/– cells are not completely resistant to this Tat effect (Figure 5D), suggesting a possibility that other pro-apoptotic members of the Bcl-2 family may work in parallel with Bim to transduce the Tat-induced death signal. Future analyses are necessary to investigate whether Tat may play a direct and specific role in causing the release of BimL from microtubules and also to determine the possible involvement of other Bcl-2 family members in mediating Tat-induced apoptosis.
Enhanced apoptosis has been observed in lymph nodes of HIV-1-infected individuals (Fauci, 1993) and in lymphocytes isolated from AIDS patients (Meyaard et al., 1992). Studies using in situ labeling of lymph nodes from HIV-infected children and SIV-infected macaques have demonstrated that apoptosis occurs predominantly in bystander cells and not in the productively infected cells themselves (Finkel et al., 1995). Furthermore, in vitro co-culture experiments of HIV-1-infected and uninfected cells have indicated that, while uninfected CD4+ cells die by apoptosis, HIV-infected cells are resistant to HIV-induced cell death (Nardelli et al., 1995). These data suggest that the virus may have evolved an ability to induce apoptosis in bystander cells and prevent apoptosis in the infected cells. By targeting the very general microtubule cytoskeleton to induce apoptosis, it is hard to imagine that Tat itself could have accomplished such a high specificity by killing off only the uninfected cells. It is more likely that the death signals initiated by Tat perturbation of microtubule dynamics can be blocked in the HIV-infected cells.
Indeed, HIV has been reported to employ at least two different strategies to prevent the mitochondria-dependent apoptosis in infected cells. Two HIV-encoded proteins can regulate the activities of members of the Bcl-2 family, the main regulators of the mitochondria-dependent apoptotic pathway. HIV-1 Nef activates the phosphatidylinositol-3–PAK complex, which phosphorylates and inactivates the pro-apoptotic Bad protein. Consequently, wild-type Nef, but not a Nef mutant incapable of activating PAK, blocks apoptosis in T cells induced by HIV replication (Wolf et al., 2001). Furthermore, HIV-1 Vpr causes the up-regulation of the levels of the anti-apoptotic Bcl-2, and the down-modulation of the pro-apoptotic Bax (Conti et al., 1998).
In summary, the data presented in this study highlight the ability of Tat to target the microtubule cytoskeleton to induce apoptosis. The cytoskeleton is essential for diverse cellular functions. Many pathogens exploit this versatility and develop different strategies to target the cytoskeleton for a variety of purposes (Finlay and Cossart, 1997). The ability of HIV-1 Tat to induce apoptosis by targeting the microtubule network allows Tat to join a growing list of pathogen-encoded proteins which target the cytoskeleton of host cells.
Materials and methods
Affinity purification of Tat–HA–Flag and its associated proteins
The purification was performed essentially as described (O’Keeffe et al., 2000). 293T cells transiently transfected with a construct expressing Tat–HA–Flag were lysed in a buffer containing 50 mM HEPES–KOH pH 7.8, 500 mM NaCl, 5 mM EDTA, 1% NP-40, 3 mM dithiothreitol and 0.5 mM phenylmethylsulfonyl fluoride. Pre-cleared lysate was then incubated with anti-Flag antibody beads (Sigma) at 4°C for 2 h. After extensive washes in the same buffer, Tat–HA–Flag and its associated proteins were eluted with the Flag peptide. The eluted fraction was subjected to a second round of purification using anti-HA (mAb 12CA5) antibody beads followed by HA peptide elution. Peptide digestion and microsequencing analyses of the Tat-associated proteins were as described (O’Keeffe et al., 2000).
Cell death analyses
Apoptosis was induced in Jurkat T cells by 50 ng/ml TNFα, 10 µM nocodazole, 0.05 µM taxol or the indicated amounts of wild-type and mutant Tat proteins as specified in the figure legends. The broad-spectrum caspase inhibitor zVAD-fmk was used at 20 µM. Cell death was measured by flow cytometric analysis of hypodiploid nuclei stained with 20 µg/ml propidium iodide. For A20-derived cells, 0.5 µM taxol, 20 ng/ml Fas antibody or 15 µg/ml Tat were used to induce apoptosis. For colony formation assay, the Tat-expressing constructs were co-transfected with pBabe-Puro conferring puromycin resistance at a 30:1 ratio into 293T cells. The transfected cells were diluted 2 days later into fresh Dulbecco’s modified Eagle’s medium (DMEM) plus 10% fetal bovine serum (FBS) and 0.5 µg/ml of puromycin. The number of drug-resistant colonies was counted 2 weeks later.
Coupled release/uptake of Tat and immunofluorescence analysis
The procedure was essentially as described (Ensoli et al., 1993) with some modifications. 293T cells transfected with constructs expressing Tat–Flag or control proteins were mixed 1:1 with untransfected cells 24 h post-transfection. The localization of the transfected proteins in the co-cultivated cells was determined 1 day later by immunofluorescence staining. Cells were fixed in methanol at –20°C for 10 min, blocked with 10% BSA/0.1% Tween-20 in PBS and incubated with anti-Flag mouse monoclonal antibody (2 µg/ml; Sigma) followed by Texas Red-conjugated donkey anti-mouse IgG (7 µg/ml; Jackson ImmunoResearch).
Association of Tat with assembled microtubules
The procedure was essentially as described (Puthalakath et al., 1999) with some modifications. Tat–Flag-transfected 293T cells were lysed in a buffer containing 20 mM Tris–HCl pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1% Triton X-100, 10% glycerol, 2 mM dithiothreitol and 0.5 mM phenylmethylsulfonyl fluoride. Lysate was centrifuged at 10 000 r.p.m. for 10 min to remove debris. The cleared lysate was treated with 5 µg/ml of taxol at 37°C for 20 min to reassemble microtubules and the reaction mixture was loaded on top of a 20% sucrose cushion. Microtubules and their associated proteins were isolated by ultracentrifugation at 100 000 g for 1 h.
Mice
Female 15-week-old Bim wild-type mice and Bim-deficient littermate, described previously (Bouillet et al., 1999) and back-crossed 12 times to C57BL/6, were used for all experiments. All mice were kept under specific pathogen-free conditions in the Biological Resource Center at the National Jewish Medical and Research Center.
Bim–/– cell culture
To obtain T cells for cell culture, spleen and lymph nodes (axillary, brachial, inguinal, submandibular, mesenteric and peri-aortic) were homogenized through 100 µm nylon wool strainers. The single-cell suspensions were enriched for T cells by passage through nylon wool (Julius et al., 1973). After nylon wool enrichment, 1 × 106 cells were resuspended in complete tumor medium (s-MEM containing β-mercaptoethanol, penicillin, streptomycin, glutamine and 10% FBS) with the indicated amount of Tat. T cells were cultured for 12 h and then processed for flow cytometry.
Retroviral infection
pBabe-puro-Tat–Flag or pMX-Tat-IRES-GFP was transfected into Phoenix retrovirus packaging cells by Lipofectmin-Plus reagent (Invitrogen) according to the manufacturer’s instructions. Two days later, 70 ml of viral supernatant was mixed with an equal volume of 1 × 106/ml Jurkat cell culture, and polybrene was added to the final concentration of 4 µg/ml. Cells were harvested 2 days post-infection for immunoprecipitation or flow cytometry analyses as indicated in the text and figure legends.
Acknowledgments
Acknowledgements
We thank the A.Strasser, P.Marrack, R.Tjian, D.Koshland, A.Winoto, R.Heald laboratories, H.Nolla and the University of California at Berkeley cell-sorting facility for technical help and valuable reagents, and K.Luo, J.Mougous and S.Stroschein for comments on the manuscript. This work was supported by grants from the National Institutes of Health (AI-41757) and the American Cancer Society (RSG-01-171-01-MBC) to QZ, and by a US Army Breast Cancer Predoctoral Fellowship to DC (DAMD17-02-1-0321). M.W. is an NICHD Fellow of the Pediatric Scientist Development Program (NICHD K12-HD00850-17).
References
- André N., Braguer,D., Brasseur,G., Gonçalves,A., Lemesle-Meunier,D., Guise,S., Jordan,M.A. and Briand,C. (2000) Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells. Cancer Res., 60, 5349–5353. [PubMed] [Google Scholar]
- Bartz S.R. and Emerman,M. (1999). Human immunodeficiency virus type 1 Tat induces apoptosis and increases sensitivity to apoptotic signals by up-regulating FLICE/caspase-8. J. Virol., 73, 1956–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P., Metcalf,D., Huang,D.C., Tarlinton,D.M., Kay,T.W., Köntgen,F., Adams,J.M. and Strasser,A. (1999) Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis and to preclude autoimmunity. Science, 286, 1735–1738. [DOI] [PubMed] [Google Scholar]
- Budihardjo I., Oliver,H., Lutter,M., Luo,X. and Wang,X. (1999) Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell. Dev. Biol., 15, 269–290. [DOI] [PubMed] [Google Scholar]
- Chen D., Fong,Y. and Zhou,Q. (1999) Specific interaction of Tat with the human but not rodent P-TEFb complex mediates the species-specific Tat activation of HIV-1 transcription. Proc. Natl Acad. Sci. USA, 96, 2728–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti L. et al. (1998) The HIV-1 vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: possible implications for the pathogenesis of AIDS. J. Exp. Med., 187, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J.D., Lebovitz,R.M. and Roeder,R.G. (1983) Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res., 11, 1475–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Barillari,G., Salahuddin,S.Z., Gallo,R.C. and Wong-Staal,F. (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature, 345, 84–86. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Buonaguro,L., Barillari,G., Fiorelli,V., Gendelman,R., Morgan,R.A., Wingfield,P. and Gallo,R.C. (1993) Release, uptake and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol., 67, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S. (1993) Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science, 262, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Ferri K.F., Jacotot,E., Blanco,J., Esté,J.A. and Kroemer,G. (2000) Mitochondrial control of cell death induced by HIV-1-encoded proteins. Ann NY Acad. Sci., 926, 149–164. [DOI] [PubMed] [Google Scholar]
- Finkel T.H., Tudor-Williams,G., Banda,N.K., Cotton,M.F., Curiel,T., Monks,C., Baba,T.W., Ruprecht,R.M. and Kupfer,A. (1995) Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nature Med., 1, 129–134. [DOI] [PubMed] [Google Scholar]
- Finlay B.B. and Cossart,P. (1997) Exploitation of mammalian host cell functions by bacterial pathogens. Science, 276, 718–725. [DOI] [PubMed] [Google Scholar]
- Frankel A.D. and Pabo,C.O. (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell, 55, 1189–1193. [DOI] [PubMed] [Google Scholar]
- Hildeman D.A., Zhu,Y., Mitchell,T.C., Bouillet,P., Strasser,A., Kappler,J. and Marrack,P. (2002) Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity, 16, 759–767. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. (1994) Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol., 6, 74–81. [DOI] [PubMed] [Google Scholar]
- Jeang K.T., Xiao,H. and Rich,E.A. (1999) Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem., 274, 28837–28840. [DOI] [PubMed] [Google Scholar]
- Jones K.A. and Peterlin,B.M. (1994) Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem., 63, 717–743. [DOI] [PubMed] [Google Scholar]
- Julius M.H., Simpson,E. and Herzenberg,L.A. (1973) A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur. J. Immunol., 3, 645–649. [DOI] [PubMed] [Google Scholar]
- Kundu M., Sharma,S., De Luca,A., Giordano,A., Rappaport,J., Khalili,K. and Amini,S. (1998) HIV-1 Tat elongates the G1 phase and indirectly promotes HIV-1 gene expression in cells of glial origin. J. Biol. Chem., 273, 8130–8136. [DOI] [PubMed] [Google Scholar]
- Lazebnik Y.A., Kaufmann,S.H., Desnoyers,S., Poirier,G.G. and Earnshaw,W.C. (1994) Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature, 371, 346–347. [DOI] [PubMed] [Google Scholar]
- Li C.J., Friedman,D.J., Wang,C., Metelev,V. and Pardee,A.B. (1995) Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science, 268, 429–431. [DOI] [PubMed] [Google Scholar]
- Liao G., Nagasaki,T. and Gundersen,G.G. (1995) Low concentrations of nocodazole interfere with fibroblast locomotion without significantly affecting microtubule level: implications for the role of dynamic microtubules in cell locomotion. J. Cell Sci., 108, 3473–3483. [DOI] [PubMed] [Google Scholar]
- Liu X., Sun,Y., Constantinescu,S.N., Karam,E., Weinberg,R.A. and Lodish,H.F. (1997) Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl Acad. Sci. USA, 94, 10669–10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macho A., Calzado,M.A., Jiménez-Reina,L., Ceballos,E., León,J. and Muñoz,E. (1999) Susceptibility of HIV-1-TAT transfected cells to undergo apoptosis. Biochemical mechanisms. Oncogene, 18, 7543–7551. [DOI] [PubMed] [Google Scholar]
- Meyaard L., Otto,S.A., Jonker,R.R., Mijnster,M.J., Keet,R.P. and Miedema,F. (1992) Programmed death of T cells in HIV-1 infection. Science, 257, 217–219. [DOI] [PubMed] [Google Scholar]
- Morgenstern J.P. and Land,H. (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res., 18, 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli B., Gonzalez,C.J., Schechter,M. and Valentine,F.T. (1995) CD4+ blood lymphocytes are rapidly killed in vitro by contact with autologous human immunodeficiency virus-infected cells. Proc. Natl Acad. Sci. USA, 92, 7312–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K., Harris,A.W., Bath,M.L., Smith,K.G. and Strasser,A. (1998) A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J., 17, 706–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H.L., Gruber,D. and Bulinski,J.C. (1999) Microtubule-associated protein 4 (MAP4) regulates assembly, protomer–polymer partitioning and synthesis of tubulin in cultured cells. J Cell Sci., 112, 1813–1824. [DOI] [PubMed] [Google Scholar]
- O’Connor L., Strasser,A., O’Reilly,L.A., Hausmann,G., Adams,J.M., Cory,S. and Huang,D.C. (1998) Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J., 17, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe B., Fong,Y., Chen,D., Zhou,S. and Zhou,Q. (2000) Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated Tat stimulation of HIV-1 transcription. J. Biol. Chem., 275, 279–287. [DOI] [PubMed] [Google Scholar]
- O’Reilly L.A., Cullen,L., Visvader,J., Lindeman,G.J., Print,C., Bath,M.L., Huang,D.C. and Strasser,A. (2000) The proapoptotic BH3-only protein bim is expressed in hematopoietic, epithelial, neuronal and germ cells. Am. J. Pathol., 157, 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M., Emiliani,S., Van Lint,C., Herbein,G., Lovett,J., Chirmule,N., McCloskey,T., Pahwa,S. and Verdin,E. (1997) Immune hyperactivation of HIV-1-infected T cells mediated by Tat and the CD28 pathway. Science, 275, 1481–1485. [DOI] [PubMed] [Google Scholar]
- Puthalakath H., Huang,D.C., O’Reilly,L.A., King,S.M. and Strasser,A. (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell, 3, 287–296. [DOI] [PubMed] [Google Scholar]
- Rice A.P. and Carlotti,F. (1990) Structural analysis of wild-type and mutant human immunodeficiency virus type 1 Tat proteins. J. Virol., 64, 6018–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshal M., Zhu,Y. and Planelles,V. (2001) Apoptosis in AIDS. Apoptosis, 6, 103–116. [DOI] [PubMed] [Google Scholar]
- Sorger P.K., Dobles,M., Tournebize,R. and Hyman,A.A. (1997) Coupling cell division and cell death to microtubule dynamics. Curr. Opin. Cell Biol., 9, 807–814. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris,A.W., Huang,D.C., Krammer,P.H. and Cory,S. (1995) Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J., 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.H., Wang,H.S. and Soong,Y.K. (2000) Paclitaxel-induced cell death: where the cell cycle and apoptosis come together. Cancer, 88, 2619–2628. [DOI] [PubMed] [Google Scholar]
- Westendorp M.O., Frank,R., Ochsenbauer,C., Stricker,K., Dhein,J., Walczak,H., Debatin,K.M. and Krammer,P.H. (1995) Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature, 375, 497–500. [DOI] [PubMed] [Google Scholar]
- Wolf D., Witte,V., Laffert,B., Blume,K., Stromer,E., Trapp,S., d’Aloja,P., Schurmann,A. and Baur,A.S. (2001) HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nature Med., 7, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Zhang J. and Winoto,A. (1996) A mouse Fas-associated protein with homology to the human Mort1/FADD protein is essential for Fas-induced apoptosis. Mol. Cell. Biol., 16, 2756–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]