Abstract
Hemodynamic shear stress is a fundamental determinant of vascular remodeling and atherogenesis. Changes in focal adhesions, cytoskeletal organization and gene expression are major responses of endothelial cells to shear stress. Here, we show that activation of the small GTPase Rac is essential for gene expression and for providing spatial information for shear stress-induced cell alignment. Fluorescence resonance energy transfer (FRET) localizes activated Rac1 in the direction of flow. This directional Rac1 activation is downstream of shear-induced new integrin binding to extracellular matrix. Additionally, Rac1 mediates flow-induced stimulation of nuclear factor κB (NF-κB) and the subsequent expression of intercellular cell adhesion molecule 1 (ICAM-1), an adhesion receptor involved in the recruitment of leukocytes to atherosclerotic plaque. These studies provide a unifying model linking three of the main responses to shear stress that mediate both normal adaptation to hemodynamic forces and inflammatory dysfunction of endothelial cells in atherosclerosis.
Keywords: cytoskeleton/fluid shear stress/mechanotransduction/NF-κB/Rac GTPase
Introduction
Endothelial cells (ECs) that line the vasculature are constantly subjected to hemodynamic forces imposed by blood flow, including fluid shear stress. Shear stress plays an active role in leukocyte/ECs interaction and subsequent leukocyte extravasation into inflamed tissue (Harlan, 1985; Osborn, 1990). These effects are mediated in part by modulating surface expression of adhesive proteins on the endothelium (Lawrence et al., 1987; Walpola et al., 1995). In this way, hemodynamic forces play a critical role in determining the distribution of atherosclerotic plaques that appear almost exclusively at regions of the vasculature where flow is disturbed due to curvature or at the proximal region of branching vessels.
Shear stress regulates expression of a variety of genes (Resnick and Gimbrone, 1995; Shyy et al., 1995a; Gimbrone et al., 1997; Khachigian and Collins, 1997; Resnick et al., 1997; Traub and Berk, 1998). Several trans-acting factors are activated by shear stress and subsequently induce the expression of target genes. Nuclear factor κB (NF-κB) was the first such factor to be described (Lan et al., 1994; Khachigian et al., 1995; Bhullar et al., 1998; Nagel et al., 1999). NF-κB is composed of protein dimers of the Rel/NF-κB family, with the p50/p65 dimer being the predominant form in vivo. In unstimulated cells, Rel/NF-κB dimers are sequestered in the cytoplasm by binding to members of the IκB family of inhibitor proteins. Upon activation, IκB is degraded and NF-κB translocates to the nucleus, where it regulates the transcription of multiple target genes by binding to promoter elements in many genes (Resnick and Gimbrone, 1995). An NF-κB consensus promoter was identified as the shear stress response element (SSRE) within the PDGF gene that mediated induction of PDGF by flow (Resnick and Gimbrone, 1995; Verma et al., 1995). Activated NF-κB was identified in smooth muscle cells, macrophages and ECs of human atherosclerotic tissue specimens (Brand et al., 1996) as well as in humans with unstable angina pectoris (Ritchie, 1998), suggesting a patho physiological role for NF-κB in atherosclerosis (Brand et al., 1997).
Rac1 contributes to reactive oxygen species (ROS) production in response to shear stress (Yeh et al., 1999), and ROS production in response to shear stress leads to increased expression of intercellular cell adhesion molecule 1 (ICAM-1) gene (Chiu et al., 1997). It has been shown previously that Rac mediates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation (Sulciner et al., 1996). Additionally, Rac, Rho and Cdc42 induce transcriptional activity of NF-κB by phosphorylation of IκB (Perona et al., 1997), and activation of Rac induces NF-κB binding and activity and enhances expression of cyclin D1 (Joyce et al., 1999). Toll-like receptor 2-mediated NF-κB activation also depends on Rac (Arbibe et al., 2000). More recently, Reyes-Reyes et al. (2001) reported that integrin signaling to NF-κB is mediated by Rac.
We showed previously that shear stress induces conformational activation of integrins followed by new binding of integrins to extracellular matrix (ECM) (Tzima, 2001). These newly occupied integrins subsequently initiate signals, including a transient inactivation of Rho. Here, we test the involvement of the related GTPase Rac1 in shear stress signaling. Our results show that Rac1 mediates both cytoskeletal alignment and the upregulation of ICAM-1, the latter through activation of NF-κB. These results show that many of the key shear stress responses are linked within a single, integrin-mediated pathway.
Results
Regulation of Rac1 activity by shear stress
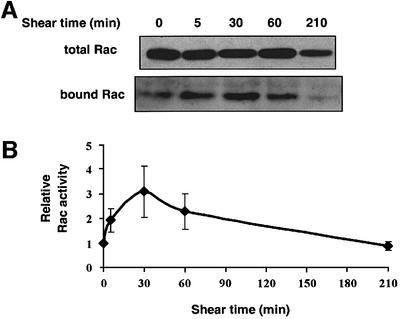
Binding of integrins to ECM proteins stimulates transient activation of Rac1 (Del Pozo et al., 2000). Since shear stress induces new integrin binding to ECM (Tzima, 2001), we examined whether shear stress could activate Rac1. Pull-down assays were performed in which Rac1 GTP loading was determined by specific binding of the active GTPase to the p21-binding domain of PAK1 (PBD) fused to glutathione S-transferase (GST) (Glaven et al., 1999; Del Pozo et al., 2000). Serum-starved bovine aortic ECs (BAECs) were subjected to shear stress for the indicated times, lysates were prepared and the amount of Rac1 precipitated with the GST–PBD determined by western blotting. The results showed that shear stress stimulated a transient increase in Rac1 activity that peaked at 30 min and then returned to basal levels (Figure 1).
Fig. 1. Regulation of Rac activity by shear stress. (A) BAECs were subjected to shear stress for the indicated times. Rac activity is indicated by the amount of PBD-bound Rac, normalized to total Rac in whole cell lysates. (B) Quantitation of Rac activity relative to cells at time 0. Values are means ± SEM from four independent experiments, each of which was performed in duplicate.
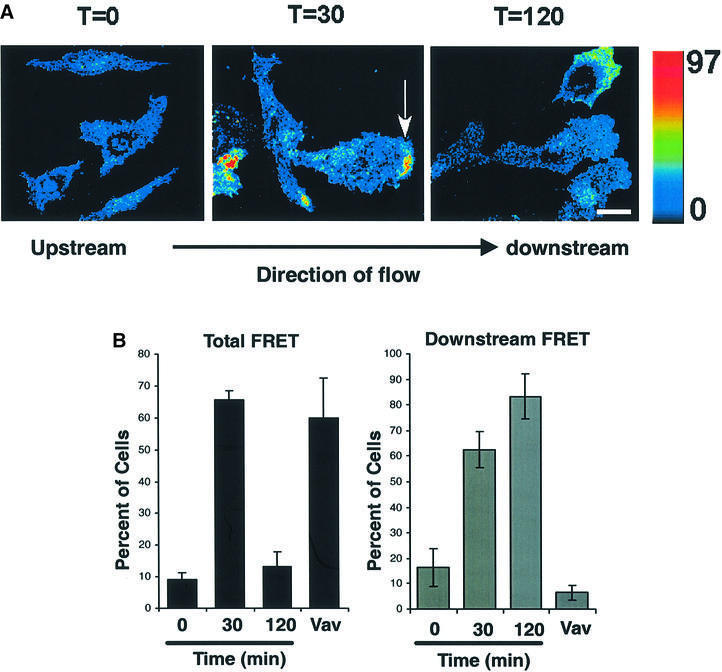
To visualize local Rac1 activation during shear stress, we used FLAIR (fluorescence activation indicator for Rho proteins) (Kraynov et al., 2000). We introduced GFP–Rac into cells together with the biosensor Alexa568-PBD. Rac1 activation results in binding of these two polypeptides leading to increased fluorescence resonance energy transfer (FRET) from GFP to Alexa568. FRET images showed high activity at the edges of cells subjected to shear stress (Figure 2A). Quantitation revealed a substantial increase in the fraction of cells that scored positive for FRET at 30 min of shear stress. While the number of positive cells decreased substantially at 120 min, the signal intensity in those cells was similar to the 30 min time point (Figure 2B). Importantly, the FRET signals were localized primarily at the downstream edges of the cells. To quantitate this effect, those cells where Rac1 activity was above the baseline were scored for spatial distribution. We found that the low level of Rac1 activity in static cells was randomly oriented relative to flow (Figure 2B). In contrast, in the majority of cells with elevated Rac1 activity after 30 min of shear stress, activity was strongly localized to the downstream edge. After 120 min, the number of cells showing elevated Rac1 activity was much lower, but in those cells where the intensity was above baseline the remaining signal was still highly oriented. Expression of the Rac1 nucleotide exchange factor Vav activated Rac1, but few cells showed polarization.
Fig. 2. Spatio-temporal localization of shear stress-induced Rac activity. (A) Cells transiently transfected with expression vectors for GFP fused to wild-type Rac1 (GFP–WTRac) were shear loaded with Alexa-PBD, plated on fibronectin for 2 h and subjected to shear stress or kept under static conditions for 30 min. Rac activation (FRET) is shown. In the color intensity scale, red represents high and blue low. Scale bar, 20 µm. Arrow points to area of high FRET. (B) Cells were first scored for the presence of a FRET signal that was above background (left graph). Those cells showing a positive signal were then scored for whether the signal was predominantly localized to the downstream edge, i.e. flow was from left to right and the strongest FRET signals were present on the right (right graph). As a positive control, cells were transfected with the Rac1 nucleotide exchange factor Vav and examined without shear. Values are means ± SEM, n = 3 and >100 cells were scored per condition.
New ECM binding mediates shear stress-induced Rac1 activation
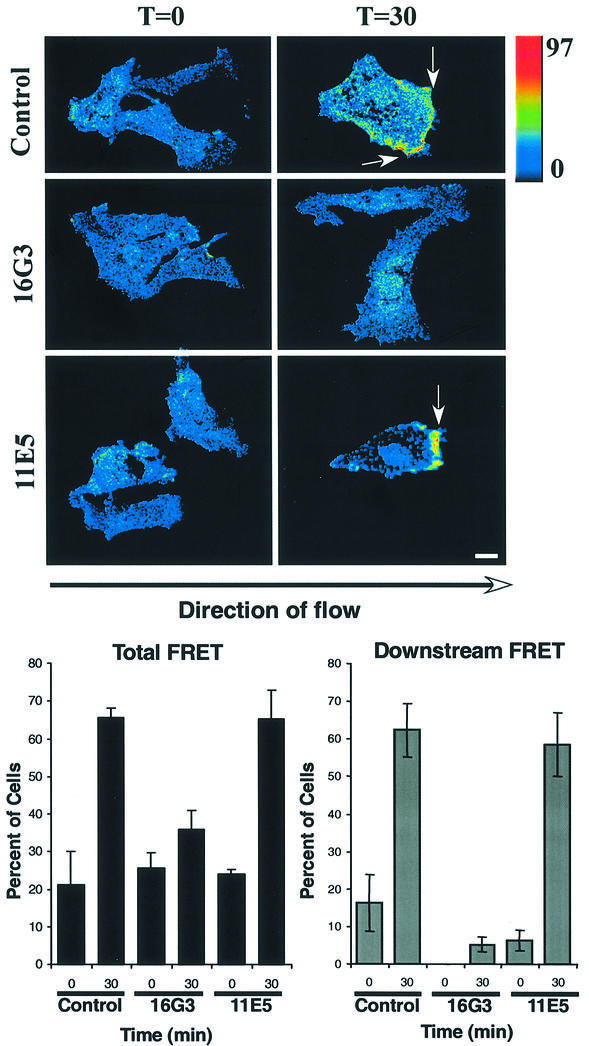
Integrins regulate Rac1 (Clark et al., 1998; Price et al., 1998; Del Pozo et al., 2000) and shear stress causes new integrin binding to ECM (Jalali et al., 2001; Tzima, 2001). Moreover, the time course of Rac1 activation (Figure 1) is consistent with it being downstream of integrin binding to ECM, which is detectable at 2 min (Tzima, 2001). To test whether Rac1 activation requires new integrin binding, ECs fully spread on fibronectin (FN) were treated with anti-FN antibodies. This treatment prevents formation of new integrin–ligand connections without disrupting existing adhesions (Jalali et al., 2001; Tzima et al., 2001). Shear stress was then applied and FRET assayed. The results showed that the blocking antibody 16G3 strongly inhibited the shear stress-induced increase in Rac1 activity, whereas the non-blocking antibody 11E5 had no effect (Figure 3). Interestingly, the residual FRET signal in the presence of blocking antibody showed no preferred direction. Taken together, these data demonstrate that new integrin binding to ECM determines the localized activation of Rac1 in response to shear stress.
Fig. 3. Shear stress-induced Rac activation requires new ECM connections. Cells were transiently transfected with GFP–WTRac, shear loaded with Alexa-PBD and plated on FN for 2 h. Cells were incubated with anti-FN Fab fragments for 15 min and subjected to shear stress for the indicated times or kept under static conditions for an additional 30 min. Rac activation (FRET) is shown. Cells were scored for FRET-positive zones. Values are means ± SEM, n = 3 and >100 cells were scored per condition. Scale bar, 20 µm.
Rac1 activation is required for shear stress-induced cell alignment
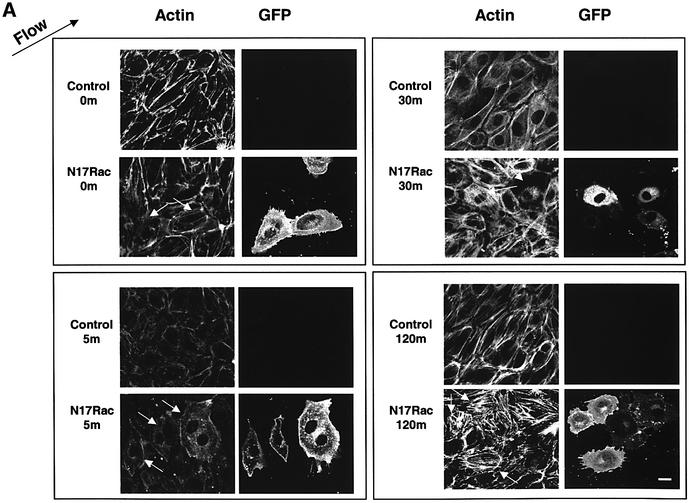
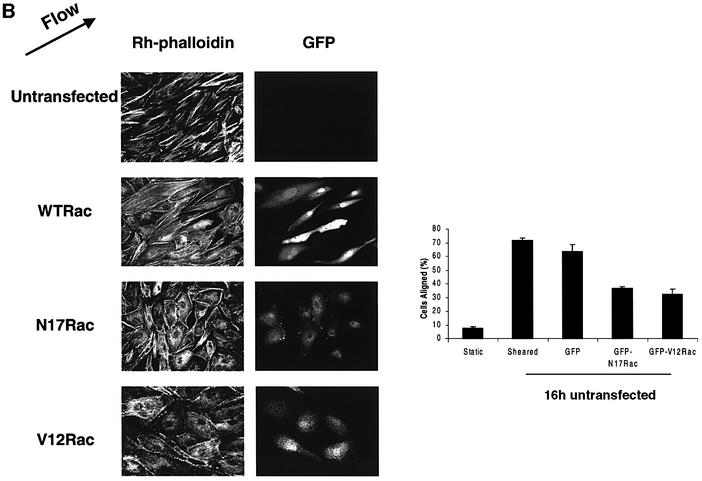
Cells exposed to shear stress for longer periods increase their stress fibers, elongate and align in the direction of flow (Galbraith et al., 1998). To address the relevance of Rac1 to this process, the effect of Rac1 inhibition on stress fiber alignment in the direction of flow was examined. Cells were transiently transfected with expression vectors for GFP fused to dominant-negative (N17) or wild-type (WT) Rac1. N17Rac did not cause detectable apoptosis in these cells, as determined by caspase 3 assays (data not shown). ECs exposed to shear stress initially showed a decrease in stress fibers at 5 min, which is associated with a decrease in Rho activity (Tzima, 2001). Stress fibers returned within 1–2 h in a partially aligned state, although maximal alignment requires ∼16 h. Transfected cells were subjected to shear stress for various times and F-actin visualized using rhodamine-phalloidin. Control cells initially showed a decrease in actin stress fibers followed by a recovery (Figure 4A). The restored stress fibers were noticeably aligned in the direction of flow at times as early as 1 h. N17Rac-expressing cells showed a similar decrease in actin staining followed by recovery; however, F-actin orientation was largely random. Even after 16 h, the orientation of actin stress fibers in cells expressing N17Rac was significantly inhibited (Figure 4B). Note that sheared cells expressing N17Rac showed increased linear stress fibers compared with unsheared cells; thus, the fraction that scores as aligned was higher than without shear, even though this fraction is only moderately above random (17%). To further test the idea that polarized Rac activity may be important for cell alignment, we expressed V12Rac, which we reasoned should induce uniform high levels of Rac activity throughout the cells. FRET assays confirmed that, in cells expressing V12Rac, activity was high throughout the cell without detectable polarization toward the downstream edge (data not shown). When alignment was scored after 16 h of shear, cells transfected with V12Rac showed a significant decrease in alignment (Figure 4B). Taken together, these data show that Rac1 is not required for global changes in actin polymerization but is essential for providing the spatial information needed to align in the direction of flow.
Fig. 4. Dominant-negative Rac blocks shear stress-induced cell alignment. (A) Untransfected cells or those transiently transfected with GFP–N17Rac were subjected to shear stress for the indicated times (min) or kept under static conditions. Cells were fixed and stained with rhodamine-phalloidin. Direction of flow is indicated by an arrow. Scale bar, 20 µm. White arrows indicate transfected cells. (B) Untransfected or cells transiently transfected with GFP–WTRac, GFP–N17Rac or GFP–V12Rac were subjected to shear stress for 16 h. The cells were fixed and stained with rhodamine-phalloidin. Direction of flow is indicated by an arrow. The graph shows percentage of cells aligned in the direction of flow (± 30°). Values are means ± SEM, n = 3 and >100 cells were scored per condition.
Shear stress induces Rac1-dependent NF-κB translocation
Shear stress leads to activation of NF-κB (Bhullar et al., 1998; Chien et al., 1998; Nagel et al., 1999), and there are numerous systems where NF-κB activation is mediated by Rac (Joyce et al., 1999, 2001). To test whether shear stress-dependent NF-κB activation requires Rac1, we investigated the localization of the p65 subunit after shear stress. Monolayers maintained under static conditions in low serum showed minimal NF-κB activation, as documented by NF-κB (p65) staining in the cytoplasm (Figure 5A). After exposure of cells to shear stress for 30 min, most cells exhibited nuclear localization of p65, consistent with NF-κB activation noted in previous studies (Bhullar et al., 1998). However, in cells transfected with N17Rac, flow induced minimal translocation of NF-κB from the cytosol to the nucleus. In control experiments, expression of GFP–WTRac or GFP alone did not affect the nuclear translocation of NF-κB. These results show that activation of NF-κB in response to shear stress requires Rac1 activity.
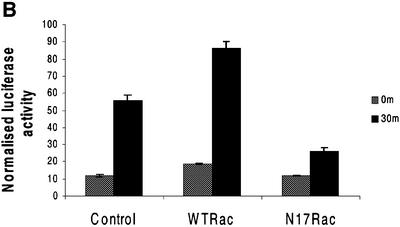
Fig. 5. Rac mediates shear stress-dependent NF-κB activation. (A) BAECs transfected with GFP–WTRac or GFP–N17Rac were kept under static conditions or subjected to shear stress for 30 min, then fixed and stained for p65. Arrows point to transfected cells lacking nuclear p65. Nuclear translocation was scored (n = 4, >100 cells per condition). Values are means ± SEM. Scale bar, 20 µm. (B) BAECs transfected with HIV(LTR)-Luc and pSVRenilla plus WTRac or N17Rac were kept as static controls or subjected to shear stress for 30 min. Normalized luciferase activities represent means ± SEM (n = 3).
To determine whether shear stress-induced NF-κB transcriptional activity is regulated by Rac1, cells were co-transfected with a luciferase reporter for NF-κB, HIV(LTR)-Luc, together with WTRac or N17Rac. In the presence of WTRac, shear stress increased luciferase activity 5-fold, which was somewhat higher than in vector-transfected cells. In contrast, much less luciferase was induced in cells co-transfected with N17Rac (Figure 5B). The increased luciferase activity due to WTRac overexpression in this assay contrasts with NF-κB translocation where WTRac had no effect; this difference may be due to the quantitative nature of the luciferase assay, whereas scoring cells for translocation is done on an all-or-none basis.
Rac1 activation by shear stress mediates NF-κB-dependent expression of ICAM-1
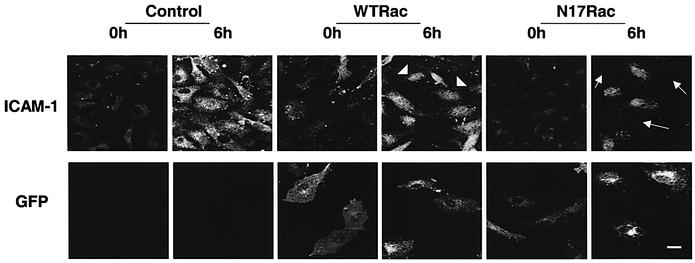
NF-κB is responsible for the expression of several shear-responsive genes, including ICAM-1, VCAM-1 and the macrophage chemoattractant protein 1 (MCP-1) (Landry et al., 1997). To relate Rac1-dependent NF-κB activation to these events, we addressed whether the flow-induced Rac1 activation is instrumental to the upregulation of ICAM-1 on vascular endothelium. Cells were transfected with GFP–WTRac or GFP–N17Rac and exposed to shear stress or kept as static controls. ICAM-1 was detected by immunofluorescence microscopy. Untransfected ECs or cells transfected with GFP–WTRac showed increased surface expression of ICAM-1 after 6 h of shear stress. In contrast, transfection with GFP–N17Rac clearly inhibited the shear-induced expression of ICAM-1 on the luminal surface of BAECs (Figure 6). Interleukin-1-induced upregulation of ICAM-1 was not inhibited by N17Rac (data not shown). These results demonstrate that Rac1 is required for cell surface expression of ICAM-1 in response to flow.
Fig. 6. Shear stress-induced Rac activation mediates NF-κB-dependent expression of ICAM-1. Untransfected and BAECs transfected with GFP–WTRac or GFP–N17Rac were kept under static conditions or subjected to shear stress for 6 h. Cells were stained with polyclonal anti-ICAM-1 antibody followed by CY5-conjugated goat anti-rabbit IgG. Transfected cells were identified by GFP. Arrows point to transfected cells. Scale bar, 20 µm.
Discussion
In this study, we investigated the role of Rac1 in shear stress-induced changes in cytoskeletal organization and gene expression. We report a transient upregulation of Rac1 activity by shear stress that is concentrated at the downstream edge of the cells. Similar to Rho (Tzima, 2001), regulation of Rac1 activity by shear stress was found to be due to integrin binding to ECM ligands. The prevalence of Rac1 activation in the direction of flow is of particular interest in light of the role of Rac1 in cell motility (Theriot and Mitchison, 1991; Michiels et al., 1995; Nobes and Hall, 1995) and directed cell movement (Chung et al., 2000). The localized rise in Rac1 activity and its requirement for new integrin–ligand connections suggest the attractive hypothesis that shear stress-induced new ECM connections determine the spatial localization of activated Rac1, which subsequently controls cell motility/morphology in a localized and directed fashion. Interestingly, Hahn and co-workers used FRET to show a gradient of Rac activity that is highest near the leading edge of migrating fibroblasts (Kraynov et al., 2000). The EC membrane resists imposed shear stress by increasing tension (Fung and Liu, 1993), which has been shown to be higher at the upstream edge (Liu et al., 1994). Spatial differences in shear stress (upstream versus downstream) have been detected within minutes after the application of shear stress by observing displacement of intermediate filaments (Helmke et al., 2000). Microtubule remodeling due to shear stress occurs preferentially in the upstream side of the cell (Galbraith et al., 1998). Likewise, shear stress increases membrane fluidity in the upstream side of cells (Butler et al., 2001). Importantly, shear stress results in the remodeling of focal adhesions, with new adhesions forming preferentially toward the downstream edge of the cell (Davies et al., 1994). Our data therefore suggest that the remodeling of focal adhesions originally observed by Davies and co-workers is responsible for the directional activation of Rac1.
In addition to Rac1 activity that is localized in the direction of flow, we observed that dominant-negative Rac1 inhibits cell alignment, in agreement with a recent report (Birukov et al., 2002). It should be noted that Rho is also required for cell alignment in this system (Li et al., 1999; Tzima, 2001), though Rho appears to be involved in the global dissolution and reformation of actin stress fibers. In contrast, Cdc42 was not found to influence actin stress fiber alignment (Li et al., 1999). It is well established that Rac mediates the formation of focal complexes and lamellipodia, whereas actin stress fiber formation is dependent on Rho (Ridley and Hall, 1992; Nobes and Hall, 1994). Cell motility in fibroblasts is likely to be controlled by Rho GTPases in a sequential and coordinate manner: Cdc42 can activate Rac which then activates Rho (Nobes and Hall, 1995). Small focal complexes initiated by Rac can mature into Rho-dependent focal adhesions; thus, activation of Rho by Rac may occur in a spatially specific manner (Rottner et al., 1999). Importantly, expression of N17Rac affected neither the initial dissolution nor the subsequent reformation of stress fibers during shear stress. Together with the ability of V12Rac to block alignment, these data suggest that correct spatial activation of Rac is required for the orientation of stress fibers with the direction of flow. Interestingly, although average Rac activity was much lower at later times, when evident it remained highly polarized; thus, Rac may continue to provide information about directionality at later times after initiation of flow.
Previous studies have indicated that shear stress can regulate the activities of NF-κB, Egr-1, c-Jun and c-Fos in cultured ECs (Hsieh et al., 1993; Lan et al., 1994; Nagel et al., 1994; Khachigian et al., 1995, 1997; Resnick and Gimbrone, 1995; Shyy et al., 1995b). NF-κB can in turn regulate genes encoding vasoactive or inflammatory substances and adhesive molecules (Davies and Tripathi, 1993; Morigi et al., 1995; Noris et al., 1995; De Martin et al., 2000). An SSRE has been identified within the promoter of ICAM-1, to which NF-κB binds (Resnick and Gimbrone, 1995) and which is responsible for the upregulation of ICAM-1 message by shear stress. Upregulation by shear stress of ICAM-1 is dependent on Rac1. ICAM-1 mediates leukocyte adhesion to ECs (Collins, 1993; Carlos and Harlan, 1994).
Our data define Rac1 as a major determinant of EC adaptation to shear stress and establish a pathway linking focal adhesion dynamics, cytoskeletal reorganization and gene expression in response to flow. We conclude that local modulation of integrin dynamics induces localized activation of Rac1. Rac1 subsequently provides directional information for cell alignment, a crucial adaptive process by which ECs reduce the local mechanical load and subsequent injury. In addition, Rac1, through its regulation of NF-κB, directs the expression of genes involved in inflammation. Thus, changes in focal adhesion dynamics triggered by flow appear to mediate several important downstream responses.
The results we obtained in response to the sudden onset of flow are likely to be relevant to locations of disturbed or turbulent shear in vivo, where ECs initiate inflammatory reactions that lead to atherosclerotic lesions. Regions of disturbed flow have elevated levels of NF-κB activity, increased uptake of lipoproteins, ICAM-1 expression and leukocyte recruitment as well as increased endothelial cell motility (Traub and Berk, 1998; Nagel et al., 1999). We therefore hypothesize that chronic Rac1 activation is a critical factor in atherogenesis.
Materials and methods
Cell culture and shear stress
BAECs were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS, 1% penicillin/streptomycin and 2 mM l-glutamine (Gibco-BRL, Gaithersberg, MD) in a humidified 5% CO2/95% air incubator at 37°C. BAECs on 38 × 76 mm slides at confluence were subjected to shear stress at 12 dynes/cm2 in a parallel plate flow chamber (Frangos et al., 1985; Tzima, 2001).
Plasmids and transfections
HIV(LTR)-Luc is a luciferase reporter driven by the human immunodeficiency virus long terminal repeat that contains two binding sites for NF-κB (Nabel and Baltimore, 1987). For cytoskeletal alignment assays, BAECs at ∼50% confluency in 60 mm tissue culture dishes were transfected with 2.5 µg of GFP, GFP–N17Rac, GFP–V12Rac or GFP–WTRac (Del Pozo et al., 1999) using Effectene according to the manufacturer’s instructions (Qiagen, San Diego, CA). Transfections for FRET assays used 0.5 µg of GFP–WTRac. After 10 h in growth medium, cells were starved overnight in 0.5% serum prior to shear stress.
GTPase assays
BAECs were seeded on slides coated with FN (5 µg/cm2), grown to confluence and starved overnight in 0.5% serum. Pull-down assays for activated Rac1 were performed as described previously (Del Pozo et al., 2000). FLAIR assays were performed as described previously (Del Pozo et al., 2002), with slight modifications. Briefly, BAECs transiently transfected with GFP–Rac were serum starved overnight in 0.5% serum-containing media, loaded with Alexa-PBD as described previously (Clarke and McNeil, 1992), plated on FN-coated glass slides, allowed to adhere for 2 h and then subjected to shear. Images of fixed cells were acquired using a Bio-Rad 1024 Confocal Microscope. Calculations to account for bleedthrough and background were performed as described previously (Kraynov et al., 2000). The resultant corrected 8-bit FRET images typically had a fluorescence intensity range of 0–78 and were displayed using pseudocolor, where blue was closest to 0 and red closest to 78. For blocking of unoccupied FN sites, the following antibodies were used as described previously (Tzima, 2001): 16G3, which blocks both the αvβ3 and α5β1 binding sites for FN; and 11E5, which is a non-function blocking antibody (a generous gift from Dr K.M.Yamada).
Fluorescence microscopy
Cells were fixed for 30 min in 2% formaldehyde in PBS, permeabilized in 0.2% Triton X-100/PBS and blocked with 10% goat serum. Cells were stained with an antibody against the p65 subunit of NF-κB (Santa Cruz Biotechnology) followed by CY5-conjugated goat anti-rabbit IgG (Sigma). Where indicated, cells were stained with TRITC-phalloidin (Sigma) to visualize F-actin or an antibody against ICAM-1, used without permeabilization. Confocal serial sectioned images were acquired using a Bio-Rad 1024 MRC Confocal Microscope.
Luciferase activity assays
HIV(LTR)-Luc, GFP–WTRac or GFP–N17Rac (2.0 µg) were co-transfected into BAECs for the luciferase induction assay. The pSVRenilla plasmid was also co-transfected to monitor the transfection efficiency. Cells were lysed in buffer containing 1% Triton X-100. ATP and luciferin were then added to the lysate in a luminometer for measuring the total light output. The normalized luciferase activities represent the firefly luciferase activity corrected for renilla luciferase as a measure of transfection efficiency.
Acknowledgments
Acknowledgements
A special thanks to N.B.Alderson for preparing Alexa-PBD. This work was supported by PHS grants P01 HL48728 (to M.A.S.) and P01 HL64382 (to M.A.S. and S.C.). M.A.D.P. is a Leukemia & Lymphoma Society Special Fellow (3347-02).
References
- Arbibe L., Mira,J.P., Teusch,N., Kline,L., Guha,M., Mackman,N., Godowski,P.J., Ulevitch,R.J. and Knaus,U.G. (2000) Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol., 1, 533–540. [DOI] [PubMed] [Google Scholar]
- Bhullar I.S., Li,Y.S., Miao,H., Zandi,E., Kim,M., Shyy,J.Y. and Chien,S. (1998) Fluid shear stress activation of IκB kinase is integrin-dependent. J. Biol. Chem., 273, 30544–30549. [DOI] [PubMed] [Google Scholar]
- Birukov K.G., Birukova,A.A., Dudek,S.M., Verin,A.D., Crow,M.T., Zhan,X., DePaola,N. and Garcia,J.G. (2002) Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am. J. Respir. Cell Mol. Biol., 26, 453–464. [DOI] [PubMed] [Google Scholar]
- Brand K. et al. (1996) Activated transcription factor nuclear factor-κB is present in the atherosclerotic lesion. J. Clin. Invest., 97, 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand K., Page,S., Walli,A.K., Neumeier,D. and Baeuerle,P.A. (1997) Role of nuclear factor-κB in atherogenesis. Exp. Physiol., 82, 297–304. [DOI] [PubMed] [Google Scholar]
- Butler P.J., Norwich,G., Weinbaum,S. and Chien,S. (2001) Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am. J. Physiol. Cell. Physiol., 280, C962–C969. [DOI] [PubMed] [Google Scholar]
- Carlos T.M. and Harlan,J.M. (1994) Leukocyte–endothelial adhesion molecules. Blood, 84, 2068–2101. [PubMed] [Google Scholar]
- Chien S., Li,S. and Shyy,Y.J. (1998) Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension, 31, 162–169. [DOI] [PubMed] [Google Scholar]
- Chiu J.J., Wung,B.S., Shyy,J.Y., Hsieh,H.J. and Wang,D.L. (1997) Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler. Thromb. Vasc. Biol., 17, 3570–3577. [DOI] [PubMed] [Google Scholar]
- Chung C.Y., Lee,S., Briscoe,C., Ellsworth,C. and Firtel,R.A. (2000) Role of Rac in controlling the actin cytoskeleton and chemotaxis in motile cells. Proc. Natl Acad. Sci. USA, 97, 5225–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E.A., King,W.G., Brugge,J.S., Symons,M. and Hynes,R.O. (1998) Integrin-mediated signals regulated by members of the rho family of GTPases. J. Cell Biol., 142, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.S. and McNeil,P.L. (1992) Syringe loading introduces macromolecules into living mammalian cell cytosol. J. Cell Sci., 102, 533–541. [DOI] [PubMed] [Google Scholar]
- Collins T. (1993) Endothelial nuclear factor-κB and the initiation of the atherosclerotic lesion. Lab. Invest., 68, 499–508. [PubMed] [Google Scholar]
- Davies P.F. and Tripathi,S.C. (1993) Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ. Res., 72, 239–245. [DOI] [PubMed] [Google Scholar]
- Davies P.F., Robotewskyj,A. and Griem,M.L. (1994) Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J. Clin. Invest., 93, 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelPozo M.A., Vicente-Manzanares,M., Tejedor,R., Serrador,J.M. and Sanchez-Madrid,F. (1999) Rho GTPases control migration and polarization of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur. J. Immunol., 29, 3609–3620. [DOI] [PubMed] [Google Scholar]
- DelPozo M.A., Price,L.S., Alderson,N.B., Ren,X.D. and Schwartz,M.A. (2000) Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J., 19, 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelPozo M.A., Kiosses,W.B., Alderson,N.B., Meller,N., Hahn,K.M. and Schwartz,M.A. (2002) Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat. Cell Biol., 4, 232–239. [DOI] [PubMed] [Google Scholar]
- DeMartin R., Hoeth,M., Hofer-Warbinek,R. and Schmid,J.A. (2000) The transcription factor NF-κB and the regulation of vascular cell function. Arterioscler. Thromb. Vasc. Biol., 20, E83–E88. [DOI] [PubMed] [Google Scholar]
- Frangos J.A., Eskin,S.G., McIntire,L.V. and Ives,C.L. (1985) Flow effects on prostacyclin production by cultured human endothelial cells. Science, 227, 1477–1479. [DOI] [PubMed] [Google Scholar]
- Fung Y.C. and Liu,S.Q. (1993) Elementary mechanics of the endothelium of blood vessels. J. Biomech. Eng., 115, 1–12. [DOI] [PubMed] [Google Scholar]
- Galbraith C.G., Skalak,R. and Chien,S. (1998) Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil. Cytoskeleton, 40, 317–330. [DOI] [PubMed] [Google Scholar]
- Gimbrone M.A. Jr, Resnick,N., Nagel,T., Khachigian,L.M., Collins,T. and Topper,J.N. (1997) Hemodynamics, endothelial gene expression, and atherogenesis. Ann. N. Y. Acad. Sci., 811, 1–10. [DOI] [PubMed] [Google Scholar]
- Glaven J.A., Whitehead,I., Bagrodia,S., Kay,R. and Cerione,R.A. (1999) The Dbl-related protein, Lfc, localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem., 274, 2279–2285. [DOI] [PubMed] [Google Scholar]
- Harlan J.M. (1985) Leukocyte–endothelial interactions. Blood, 65, 513–525. [PubMed] [Google Scholar]
- Helmke B.P., Goldman,R.D. and Davies,P.F. (2000) Rapid displacement of vimentin intermediate filaments in living endothelial cells exposed to flow. Circ. Res., 86, 745–752. [DOI] [PubMed] [Google Scholar]
- Hsieh H.J., Li,N.Q. and Frangos,J.A. (1993) Pulsatile and steady flow induces c-fos expression in human endothelial cells. J. Cell. Physiol., 154, 143–151. [DOI] [PubMed] [Google Scholar]
- Jalali S., del Pozo,M.M., Chen,K.D., Miao,H., Li,Y.S., Schwartz,M.A., Shyy,J.Y. and Chien,S. (2001) Integrin-mediated mechano transduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc. Natl Acad. Sci. USA, 98, 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce D. et al. (1999) Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-κB-dependent pathway. J. Biol. Chem., 274, 25245–25249. [DOI] [PubMed] [Google Scholar]
- Joyce D., Albanese,C., Steer,J., Fu,M., Bouzahzah,B. and Pestell,R.G. (2001) NF-κB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev., 12, 73–90. [DOI] [PubMed] [Google Scholar]
- Khachigian L.M. and Collins,T. (1997) Inducible expression of Egr-1-dependent genes. A paradigm of transcriptional activation in vascular endothelium. Circ. Res., 81, 457–461. [DOI] [PubMed] [Google Scholar]
- Khachigian L.M., Resnick,N., Gimbrone,M.A.,Jr and Collins,T. (1995) Nuclear factor-κB interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J. Clin. Invest., 96, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian L.M., Anderson,K.R., Halnon,N.J., Gimbrone,M.A.,Jr., Resnick,N. and Collins,T. (1997) Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-chain promoter. Arterioscler. Thromb. Vasc. Biol., 17, 2280–2286. [DOI] [PubMed] [Google Scholar]
- Kraynov V.S., Chamberlain,C., Bokoch,G.M., Schwartz,M.A., Slabaugh,S. and Hahn,K.M. (2000) Localized Rac activation dynamics visualized in living cells. Science, 290, 333–337. [DOI] [PubMed] [Google Scholar]
- Lan Q., Mercurius,K.O. and Davies,P.F. (1994) Stimulation of transcription factors NF κB and AP1 in endothelial cells subjected to shear stress. Biochem. Biophys. Res. Commun., 201, 950–956. [DOI] [PubMed] [Google Scholar]
- Landry D.B., Couper,L.L., Bryant,S.R. and Lindner,V. (1997) Activation of the NF-κB and IκB system in smooth muscle cells after rat arterial injury. Induction of vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1. Am. J. Pathol., 151, 1085–1095. [PMC free article] [PubMed] [Google Scholar]
- Lawrence M.B., McIntire,L.V. and Eskin,S.G. (1987) Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood, 70, 1284–1290. [PubMed] [Google Scholar]
- Li S., Chen,B.P., Azuma,N., Hu,Y.L., Wu,S.Z., Sumpio,B.E., Shyy,J.Y. and Chien,S. (1999) Distinct roles for the small GTPases Cdc42 and Rho in endothelial responses to shear stress. J. Clin. Invest., 103, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Q., Yen,M. and Fung,Y.C. (1994) On measuring the third dimension of cultured endothelial cells in shear flow. Proc. Natl Acad. Sci. USA, 91, 8782–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F., Habets,G.G., Stam,J.C., van der Kammen,R.A. and Collard,J.G. (1995) A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature, 375, 338–340. [DOI] [PubMed] [Google Scholar]
- Morigi M., Zoja,C., Figliuzzi,M., Foppolo,M., Micheletti,G., Bontempelli,M., Saronni,M., Remuzzi,G. and Remuzzi,A. (1995) Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood, 85, 1696–1703. [PubMed] [Google Scholar]
- Nabel G. and Baltimore,D. (1987) An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature, 326, 711–713. [DOI] [PubMed] [Google Scholar]
- Nagel T., Resnick,N., Atkinson,W.J., Dewey,C.F.,Jr and Gimbrone,M.A.,Jr (1994) Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J. Clin. Invest., 94, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel T., Resnick,N., Dewey,C.F.,Jr and Gimbrone,M.A.,Jr (1999) Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler. Thromb. Vasc. Biol., 19, 1825–1834. [DOI] [PubMed] [Google Scholar]
- Nobes C. and Hall,A. (1994) Regulation and function of the Rho subfamily of small GTPases. Curr. Opin. Genet. Dev., 4, 77–81. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Noris M., Morigi,M., Donadelli,R., Aiello,S., Foppolo,M., Todeschini,M., Orisio,S., Remuzzi,G. and Remuzzi,A. (1995) Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions. Circ. Res., 76, 536–543. [DOI] [PubMed] [Google Scholar]
- Osborn L. (1990) Leukocyte adhesion to endothelium in inflammation. Cell, 62, 3–6. [DOI] [PubMed] [Google Scholar]
- Perona R., Montaner,S., Saniger,L., Sanchez-Perez,I., Bravo,R. and Lacal,J.C. (1997) Activation of the nuclear factor-κB by Rho, CDC42, and Rac-1 proteins. Genes Dev., 11, 463–475. [DOI] [PubMed] [Google Scholar]
- Price L.S., Leng,J., Schwartz,M.A. and Bokoch,G.M. (1998) Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell, 9, 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick N. and Gimbrone,M.A.,Jr (1995) Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J., 9, 874–882. [DOI] [PubMed] [Google Scholar]
- Resnick N., Yahav,H., Khachigian,L.M., Collins,T., Anderson,K.R., Dewey,F.C. and Gimbrone,M.A.,Jr (1997) Endothelial gene regulation by laminar shear stress. Adv. Exp. Med. Biol., 430, 155–164. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyes M., Mora,N., Zentella,A. and Rosales,C. (2001) Phosphatidylinositol 3-kinase mediates integrin-dependent NF-κB and MAPK activation through separate signaling pathways. J. Cell Sci., 114, 1579–1589. [DOI] [PubMed] [Google Scholar]
- Ridley A.J. and Hall,A. (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Ritchie M.E. (1998) Nuclear factor-κB is selectively and markedly activated in humans with unstable angina pectoris. Circulation, 98, 1707–1713. [DOI] [PubMed] [Google Scholar]
- Rottner K., Hall,A. and Small,J.V. (1999) Interplay between Rac and Rho in the control of substrate contact dynamics. Curr. Biol., 9, 640–648. [DOI] [PubMed] [Google Scholar]
- Shyy J.Y., Li,Y.S., Lin,M.C., Chen,W., Yuan,S., Usami,S. and Chien,S. (1995a) Multiple cis-elements mediate shear stress-induced gene expression. J. Biomech., 28, 1451–1457. [DOI] [PubMed] [Google Scholar]
- Shyy J.Y., Lin,M.C., Han,J., Lu,Y., Petrime,M. and Chien,S. (1995b) The cis-acting phorbol ester ‘12-O-tetradecanoylphorbol 13-acetate’-responsive element is involved in shear stress-induced monocyte chemotactic protein 1 gene expression. Proc. Natl Acad. Sci. USA, 92, 8069–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulciner D.J., Irani,K., Yu,Z.X., Ferrans,V.J., Goldschmidt-Clermont,P. and Finkel,T. (1996) rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-κB activation. Mol. Cell. Biol., 16, 7115–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot J.A. and Mitchison,T.J. (1991) Actin microfilament dynamics in locomoting cells. Nature, 352, 126–131. [DOI] [PubMed] [Google Scholar]
- Traub O. and Berk,B.C. (1998) Laminar shear stress: mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol., 18, 677–685. [DOI] [PubMed] [Google Scholar]
- Tzima E., del Pozo,M.A., Shattil,S.J., Chien,S. and Schwartz,M.A. (2001) Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J., 20, 4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I.M., Stevenson,J.K., Schwarz,E.M., Van Antwerp,D. and Miyamoto,S. (1995) Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev., 9, 2723–2735. [DOI] [PubMed] [Google Scholar]
- Walpola P.L., Gotlieb,A.I., Cybulsky,M.I. and Langille,B.L. (1995) Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler. Thromb. Vasc. Biol., 15, 2–10. [DOI] [PubMed] [Google Scholar]
- Yeh L.H., Park,Y.J., Hansalia,R.J., Ahmed,I.S., Deshpande,S.S., Goldschmidt-Clermont,P.J., Irani,K. and Alevriadou,B.R. (1999) Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am. J. Physiol., 276, C838–C847. [DOI] [PubMed] [Google Scholar]