Abstract
The activation of mitogen-activated protein kinases (MAPKs) is one of the earliest responses in plants challenged by avirulent pathogens or cells treated with pathogen-derived elicitors. Expression of a constitutively active MAPK kinase, NtMEK2DD, in tobacco induces the expression of defense genes and hypersensitive response–like cell death, which are preceded by the activation of two endogenous MAPKs, salicylic acid–induced protein kinase (SIPK) and wounding-induced protein kinase (WIPK). However, the roles that SIPK and WIPK each play in the process are unknown. Here we report that SIPK alone is sufficient to activate these defense responses. In tobacco leaves transiently transformed with SIPK under the control of a steroid-inducible promoter, the induction of SIPK expression after the application of dexamethasone, a steroid, leads to an increase of SIPK activity. The increase of SIPK activity is dependent on the phosphorylation of newly synthesized SIPK by its endogenous upstream kinase. In contrast, the expression of WIPK under the same conditions fails to increase its activity, even though the protein accumulates to a similar level. Studies using chimeras of SIPK and WIPK demonstrated that the C terminus of SIPK contains the molecular determinant for its activation, which is rather surprising because the N termini of SIPK and WIPK are more divergent. SIPK has been implicated previously in the regulation of both plant defense gene activation and hypersensitive response–like cell death based on evidence from pharmacological studies using kinase inhibitors. This gain-of-function study provided more direct evidence for its role in the signaling of multiple defense responses in tobacco.
INTRODUCTION
Plants have both preexisting and inducible defense mechanisms to ward off invading pathogens. The inducible defense of plants often includes rapid and localized cell death, known as the hypersensitive response (HR), the activation of a complex array of defense genes, and the production of antimicrobial phytoalexins (Yang et al., 1997; Scheel, 1998; Somssich and Hahlbrock, 1998; Martin, 1999). The activation of these defense responses is initiated by the plant recognition of pathogens, which is mediated either by a gene-for-gene interaction between a plant resistance (R) gene and a pathogen avirulence (Avr) gene or by the binding of a non-race-specific elicitor such as elicitin to a putative receptor (Keen, 1990; Staskawicz et al., 1995; Hammond-Kosack and Jones, 1996; Ricci, 1997; Martin, 1999). Signals generated by such interactions are transduced into cellular responses via several interlinked pathways (Hammond-Kosack and Jones, 1996; Scheel, 1998; Martin, 1999). Pharmacological studies using inhibitors of protein kinases and phosphatases implicated the involvement of protein phosphorylation and dephosphorylation in the induction of almost all defense responses, such as medium alkalization, reactive oxygen species generation, defense gene activation, and hypersensitive cell death (Grosskopf et al., 1990; Felix et al., 1991, 1994; Viard et al., 1994; Suzuki et al., 1995). An increasing body of evidence indicates that plant mitogen-activated protein kinases (MAPKs) are one of the key regulators in the signaling pathways (Ligterink et al., 1997; Stratmann and Ryan, 1997; Lebrun-Garcia et al., 1998; Zhang and Klessig, 1998b, 2000; Romeis et al., 1999; Kovtun et al., 2000).
MAPK cascades are major components downstream of receptors/sensors that transduce extracellular stimuli into intracellular responses in yeast and mammalian cells (Kyriakis and Avruch, 1996; Widmann et al., 1999; Davis, 2000). The basic assembly of a MAPK cascade is a three-kinase module conserved in all eukaryotes. MAPK, the last kinase in the three-kinase cascade, is activated by dual phosphorylation of a tripeptide motif (TXY) located in the activation loop (T-loop) between subdomains VII and VIII of the kinase catalytic domain. This phosphorylation is mediated by a MAPK kinase (MAPKK), which is activated, in turn, by MAPKK kinase. Multiple such modules exist in a cell to perform different functions. Kinases at different tiers in a module frequently are associated with each other or bond together by scaffold protein, which makes the cascade very efficient and able to respond to a stimulus within minutes (Widmann et al., 1999; Davis, 2000). The very steep stimulus/response curve enables MAPK modules to function as molecular switches to turn on the expression of a specific set of genes and cellular responses (Huang and Ferrell, 1996).
Salicylic acid–induced protein kinase (SIPK) and wounding-induced protein kinase (WIPK), two tobacco MAPKs, and their orthologs in other plant species have been implicated in plant defense signaling (Ligterink et al., 1997; Stratmann and Ryan, 1997; Lebrun-Garcia et al., 1998; Zhang and Klessig, 1998b, 2000; Romeis et al., 1999; Cardinale et al., 2000; Kovtun et al., 2000; Nühse et al., 2000). SIPK was first identified as salicylic acid (SA)-induced protein kinase and was later shown to be responsive to a number of biotic and abiotic stresses, including pathogen or pathogen-derived elicitors, ozone, wounding, salt, and osmolarity stresses (Romeis et al., 1999; Hoyos and Zhang, 2000; Mikolajczyk et al., 2000; Samuel et al., 2000; Zhang and Klessig, 2000). The activation of a second MAPK, WIPK, accompanies the activation of SIPK in resistant tobacco (NN) plants challenged with Tobacco mosaic virus or in tobacco suspension cells treated with fungal elicitins (Zhang and Klessig, 1998b; Zhang et al., 2000). The activation of WIPK is delayed and requires transcriptional activation and de novo synthesis of WIPK protein (Zhang et al., 2000).
The exact roles that these two MAPKs play in plant stress responses remain unknown. It is postulated that the amplitude and duration of SIPK activation, as well as whether only SIPK or both SIPK and WIPK are activated, might govern the outcomes based on precedent in mammalian systems. A number of stresses activate stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) and p38 kinase in mammals (Kyriakis and Avruch, 1996; Widmann et al., 1999; Davis, 2000). The amplitude and kinetics of their activation influence the fate of cells under stress. Transient activation of these MAPKs induces various defense responses and allows the cells to adapt to adverse environments. In contrast, persistent activation leads to apoptosis (Xia et al., 1995; Chen et al., 1996; Kyriakis and Avruch, 1996; Davis, 2000). In tobacco, transient activation of SIPK has been linked to l-phenylalanine ammonia lyase gene activation induced by fungal elicitors (Zhang et al., 1998), whereas long-lasting activation of SIPK and delayed activation of WIPK might be involved in regulating HR-like cell death in tobacco treated with fungal elicitins (Zhang et al., 2000). The induction of MAPK-like activities also is implicated in the death of tobacco cells treated with xylanase, a fungal elicitor from Trichoderma viride, and Arabidopsis cells treated with harpin from Pseudomonas syringae pv syringae (Desikan et al., 1999; Suzuki et al., 1999).
Using a conditional gain-of-function approach, we demonstrated recently that NtMEK2, a tobacco MAPKK, is upstream of SIPK and WIPK. Expression of a constitutively active mutant of NtMEK2 induces HR-like cell death in tobacco, which is preceded by the activation of endogenous SIPK and WIPK. In addition, the NtMEK2-SIPK/WIPK cascade appears to control the expression of 3-hydroxy-3-methylglutaryl CoA reductase (HMGR) and l-phenylalanine ammonia lyase, two defense genes encoding key enzymes in the phytoalexin and SA biosynthesis pathways (Yang et al., 2001). In this study, we attempted to address the roles that SIPK and WIPK each play in the process. Expression of SIPK under an inducible promoter in tobacco leaves resulted in an increase of SIPK activity that was sufficient to induce both defense gene expression and HR-like cell death. In contrast, expression of WIPK under the same conditions did not lead to an increase of its activity, although the protein was expressed at a similar level. Studies using chimeras of SIPK and WIPK demonstrated that the C termini of SIPK and WIPK contain the sequence information for their differential regulation. By demonstrating the roles of a second component in the NtMEK2-SIPK/WIPK cascade, our knowledge of the function of this MAPK pathway in plant defense signaling is greatly strengthened.
RESULTS
Induction of SIPK Expression Leads to an Increase of SIPK Activity in Tobacco Leaves
The activities of MAPKs in a cell are controlled by the opposing activities of MAPKKs, which phosphorylate and activate them, and MAPK phosphatases, which dephosphorylate and inactivate them (Widmann et al., 1999). In an unstimulated cell, the actions of a MAPKK and a MAPK phosphatase on a MAPK reach equilibrium to maintain the low basal activity of the MAPK. It is possible that one can shift this balance by abruptly changing the level of MAPK protein, which will aid in the identification of processes controlled by a specific MAPK pathway. Thus, we attempted to express SIPK under the control of a steroid-inducible promoter in an Agrobacterium tumefaciens–mediated transient transformation system (Scofield et al., 1996; Tang et al., 1996; Aoyama and Chua, 1997; Yang et al., 2000).
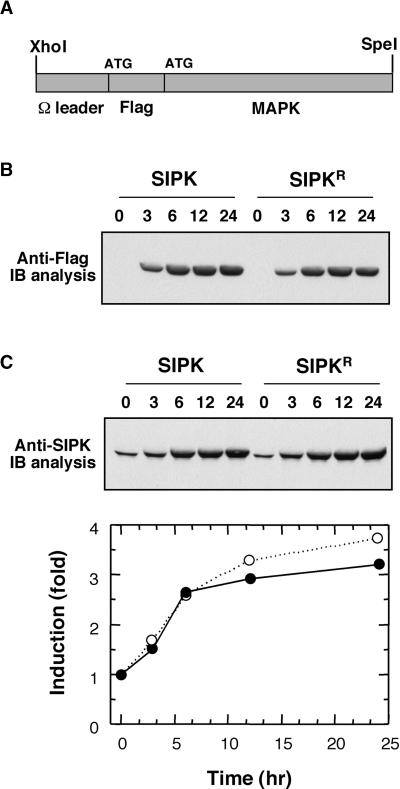
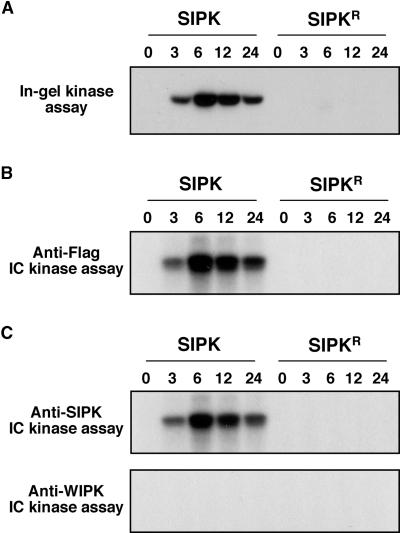
To facilitate the detection of transgene expression, a Flag tag was added to the N terminus of SIPK (Figure 1A). Agrobacterium cells carrying the pTA7002 construct were infiltrated into newly fully expanded leaves of 4- to 5-week-old tobacco plants. Transgene expression was induced by the application of 30 μM dexamethasone (DEX) 40 to 48 hr later. Immunoblot analysis with anti-Flag antibody revealed that the Flag-tagged SIPK from transgene expression started to accumulate within 3 hr (Figure 1B). Between 6 and 12 hr, the level of SIPK protein peaked and stayed at a similar level up to 24 hr. To determine the relative amount of transgene expression, immunoblot analysis using SIPK-specific antibody that recognizes both endogenous and Flag-tagged SIPK was performed (Figure 1C). Quantitation of the relative amount of SIPK indicated an almost threefold increase in SIPK protein within 6 to 12 hr (Figure 1C). The increase of Flag-tagged SIPK protein was accompanied by an increase of SIPK activity, as demonstrated by in-gel kinase activity assay (Figure 2A) and immune complex kinase assay with anti-Flag antibody (Figure 2B). A single kinase activity of 48 kD was revealed by the in-gel kinase assay, suggesting that WIPK is not activated during the process (Figure 2A). Immune complex kinase assays using SIPK- and WIPK-specific antibodies confirmed that the MAPK activity detected by in-gel kinase activity assay was SIPK and that no WIPK activity was involved (Figure 2C).
Figure 1.
Expression of SIPK and Its Inactive Mutant under the Control of a Steroid-Inducible Promoter.
(A) A simplified map of MAPK constructs in pTA7002 vector. SIPK or its mutant was inserted into the XhoI–SpeI sites of the steroid-inducible pTA7002 binary vector. The 5′ untranslated region of SIPK was replaced with the Ω sequence from Tobacco mosaic virus. To facilitate the detection of transgene expression, a Flag tag was added to the N terminus of SIPK.
(B) Induction of transgene expression. Tobacco leaves were infiltrated with Agrobacterium carrying SIPK or its inactive mutant with K90 replaced by R (SIPKR). DEX (30 μM) was infiltrated 48 hr later, and samples were taken at the times indicated. The expression of transgenes was monitored by immunoblot (IB) analysis using anti-Flag antibody.
(C) Relative levels of transgene expression in leaves under induced conditions. The levels of SIPK protein in leaves were determined by immunoblot analysis using anti-SIPK antibody. The relative amount of Flag-tagged SIPK (closed circles) or Flag-tagged SIPKR (open circles) versus endogenous SIPK was quantified using NIH Image software (National Institutes of Health, Bethesda, MD). The endogenous SIPK level before induction was normalized to 1.
Figure 2.
Expression of SIPK under the Inducible Promoter Increases SIPK Activity in Tobacco Leaves.
(A) The increase of SIPK activity in leaves under induced conditions. Kinase activities in the same protein extracts shown in Figure 1 were determined by in-gel kinase assay using MBP as a substrate.
(B) Newly synthesized Flag-tagged SIPK is active, as demonstrated by immune complex (IC) kinase assay. Protein extracts (50 μg) were immunoprecipitated with anti-Flag antibody (2 μg). Kinase activity of the immune complex was assayed subsequently as described in Methods, and the phosphorylated MBP was visualized by autoradiography.
(C) Only SIPK is activated after the application of DEX. Protein extracts (50 μg) from leaves transformed with Flag-tagged SIPK were immunoprecipitated with either SIPK-specific (anti-p48N) or WIPK-specific (anti-p44N) antibody (2.5 μg). Kinase activity of the immune complex was assayed subsequently as described in Methods, and the phosphorylated MBP was visualized by autoradiography.
Newly Synthesized SIPK Requires Phosphorylation to Become Active
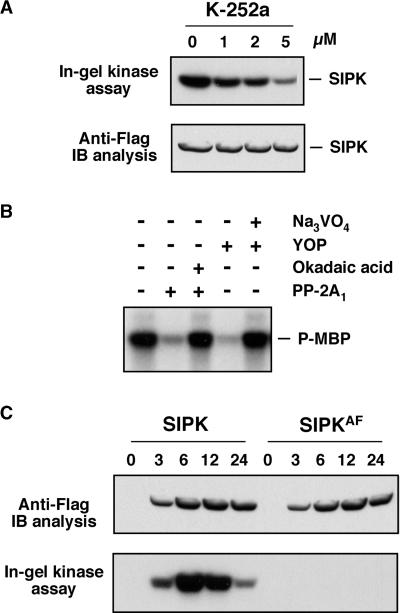
Expression of SIPKR, an inactive mutant of SIPK with the catalytic essential lysine (K90) substituted with arginine (R), did not lead to an increase in SIPK activity, although the protein was expressed at a similar level (Figures 1B and 2). This result indicates that the endogenous SIPK was not activated during the process. In other words, the newly synthesized SIPK from transgene expression was responsible for the increase of SIPK activity. To determine if the activation of newly synthesized SIPK requires phosphorylation by its upstream MAPKK, experiments were performed with kinase inhibitor. The kinase inhibitor K-252a was infiltrated at various concentrations together with DEX. DMSO, the solvent for the K-252a stock solution, was used as a control. Samples were collected 4 hr after induction, and SIPK activity was determined by in-gel kinase activity assay. As shown in Figure 3A, the activation of SIPK was reduced greatly with increasing concentrations of K-252a, demonstrating that the phosphorylation of SIPK is required for its activation. Immunoblot analysis with anti-Flag antibody showed that SIPK protein was induced to similar levels with or without kinase inhibitor (Figure 3A). Therefore, the possibility that the expression of SIPK was suppressed by the kinase inhibitor could be ruled out.
Figure 3.
The Increase of SIPK Activity Requires the Phosphorylation of Flag-Tagged SIPK by Its Endogenous Upstream MAPKK.
(A) K252a, a kinase inhibitor known to inhibit SIPK activation (Zhang et al., 1998, 2000), prevents the increase of SIPK activity after the application of DEX. K-252a was infiltrated at various concentrations into leaves together with DEX. Samples were taken 4 hr later. SIPK activities were determined by in-gel kinase assay with MBP as a substrate (top), and transgene expression was monitored by immunoblot (IB) analysis with anti-Flag antibody (bottom).
(B) Flag-tagged SIPK activation requires phosphorylation on both Thr and Tyr residues. SIPK protein from transgene expression was immunoprecipitated with anti-Flag antibody. Flag-tagged SIPK in the immune complex was then treated with either a Thr/Ser-specific protein phosphatase, PP-2A1 (0.25 units in 30 μL), or a Tyr-specific protein phosphatase, YOP (2 units in 30 μL), for 20 min at 30°C in the presence or absence of a phosphatase inhibitor. The PP-2A1 inhibitor, okadaic acid, and the YOP inhibitor, Na3VO4, were used at concentrations of 5 μM and 5 mM, respectively. After the phosphatase treatment, kinase activity was detected using MBP as a substrate. P-MBP, phosphorylated MBP.
(C) Activation of Flag-tagged SIPK requires the TEY motif. The Thr-218 and Tyr-220 residues in the TEY motif of SIPK were substituted with Ala (A) and Phe (F), respectively. SIPKAF, the SIPK(T218A/Y220F) mutant, was transformed into tobacco leaves as SIPK. Transgene expression was monitored by immunoblot analysis with anti-Flag antibody (top), and kinase activity was determined by in-gel kinase assay with MBP as a substrate (bottom).
To determine if the normal dual phosphorylation of the TEY activation motif was involved, Flag-tagged SIPK was immunoprecipitated out with anti-Flag antibody and treated with either PP-2A1, a Thr/Ser-specific protein phosphatase, or YOP, a Tyr-specific protein phosphatase. As shown in Figure 3B, treatment with either phosphatase abolished the kinase activity of Flag-tagged SIPK, suggesting that the phosphorylation on both residues is required for its activation. The MAPKK involved is likely NtMEK2, which we identified recently (Yang et al., 2001). In addition, mutation of the Thr-218 and Tyr-220 residues in the TEY motif of SIPK to Ala (A) and Phe (F) eliminated the activation of SIPK (Figure 3C). The SIPKAF mutant protein was expressed at a level similar to SIPK. These results demonstrated that the dual phosphorylation of the TEY motif in SIPK is required for its activation.
The C Terminus of SIPK Determines Its Activation
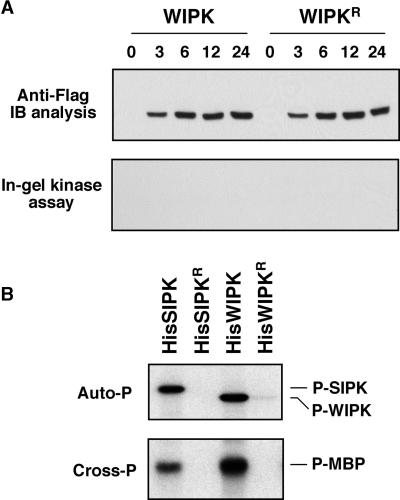
Expression of WIPK under the same conditions did not lead to an increase of its activity, even though WIPK protein accumulated to a similar level (Figure 4A). In in vitro phosphorylation assays using recombinant SIPK and WIPK proteins, WIPK was slightly more active in both autophosphorylation and cross-phosphorylation of myelin basic protein (MBP) (Figure 4B). These results suggest that WIPK and SIPK are regulated differentially at the post-translational level, which is another difference in the regulation of SIPK and WIPK activities. Previously, we demonstrated that WIPK differs from SIPK in its transcriptional activation by stress stimuli (Zhang and Klessig, 1998a; Zhang et al., 2000). One possible mechanism for such differential regulation at the post-translational level is the presence of high WIPK phosphatase activity in the cells. As a result, the newly expressed WIPK was kept in an inactive state. In animal systems, there are ample examples of such regulation of MAPK activity (Camps et al., 1999). Alternatively, a different MAPKK is involved in the activation of SIPK, which is unable to phosphorylate and activate WIPK. Previously, we identified NtMEK2 as the upstream kinase that can activate both SIPK and WIPK (Yang et al., 2001). However, we cannot exclude the presence of other MAPKKs that can activate only SIPK or WIPK. There are precedents in both yeast and animals for the involvement of more than one MAPKK in the activation of a MAPK under different conditions (Widmann et al., 1999; Davis, 2000).
Figure 4.
Expression of WIPK under the Inducible Promoter Does Not Result in an Increase of Its Activity.
(A) Expression of WIPK under the same conditions used for SIPK fails to result in an increase of its activity. Tobacco leaves were infiltrated with Agrobacterium carrying WIPK or its inactive K73R mutant (WIPKR). DEX (30 μM) was infiltrated 48 hr later, and samples were taken at the times indicated. Expression of transgenes was monitored by immunoblot (IB) analysis using anti-Flag antibody (top), and kinase activities in the leaf tissues were determined by in-gel kinase assay using MBP as a substrate (bottom).
(B) Recombinant WIPK is active in both autophosphorylation and cross-phosphorylation of MBP. Autophosphorylation activities of 0.5 μg of His-tagged wild-type SIPK and WIPK and their mutants were determined as described in Methods. Phosphorylated SIPK (P-SIPK) and WIPK (P-WIPK) were visualized by autoradiography after SDS-PAGE (top). Cross-phosphorylation (Cross-P) activities of SIPK and WIPK (0.1 μg) were determined using MBP as a substrate. Phosphorylated MBP (P-MBP) was visualized by autoradiography after SDS-PAGE (bottom).
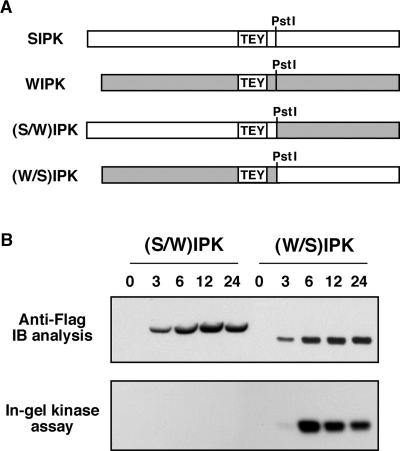
SIPK and WIPK share 71.5% identity in their amino acid sequences. One unique characteristic of SIPK is its N-terminal extension (Zhang and Klessig, 1997). To determine if this N-terminal extension is related to SIPK activation, we constructed two chimeras (Figure 5A), one with the SIPK N terminus fused to the WIPK C terminus [(S/W)IPK] and the other with the WIPK N terminus fused to the SIPK C terminus [(W/S)IPK]. Both proteins were expressed in tobacco (Figure 5B, top). The (W/S)IPK protein is similar to WIPK in size, which is smaller than SIPK or (S/W)IPK. Unexpectedly, the newly synthesized (W/S)IPK was active, like SIPK, whereas (S/W)IPK was inactive, like WIPK (Figure 5B, bottom). This result suggests that the C terminus of SIPK contains the molecular determinant for its activity. This could be a result of its role in either promoting the phosphorylation/activation of Flag-tagged SIPK by an upstream kinase or preventing its dephosphorylation/inactivation by a MAPK phosphatase.
Figure 5.
The C Terminus of SIPK Plays an Important Role in Its Activation.
(A) Construction of SIPK and WIPK chimeras. A PstI site was introduced into the WIPK gene at the position corresponding to that of SIPK by site-directed mutagenesis. Two chimeric proteins were constructed by swapping the C termini of SIPK and WIPK.
(B) The (W/S)IPK, but not the (S/W)IPK, chimera is activated when expressed in tobacco leaves. Tobacco leaves were infiltrated with Agrobacterium carrying (S/W)IPK or (W/S)IPK in pTA7002 vector. DEX (30 μM) was infiltrated 48 hr later, and samples were taken at the times indicated. Expression of transgenes was monitored by immunoblot (IB) analysis using anti-Flag antibody (top), and kinase activities in the leaf tissues were determined by in-gel kinase assay using MBP as a substrate (bottom).
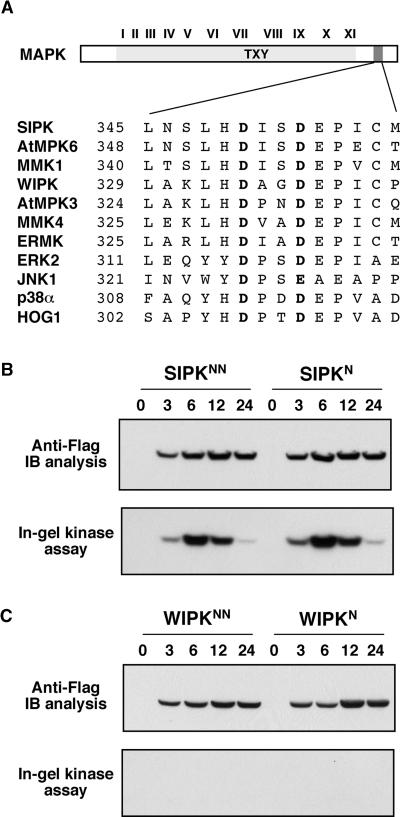
The common docking (CD) domain, which was first identified in MAPKs from yeast and mammals, also is conserved in plant MAPKs (Figure 6A). This domain contains two invariable acidic amino acid residues and is involved in the interaction of MAPKs with their upstream kinases and substrates as well as their negative regulators, MAPK phosphatases (Tanoue et al., 2000). A hyperactive allele of mammalian ERK2 with a substitution of Asn (N) for Asp (D) at position 319 (D319N) has significantly reduced sensitivity to MAPK phosphatase in vivo, which is analogous to the Drosophila melanogaster sevenmaker gain-of-function mutation (Brunner et al., 1994; Chu et al., 1996). This Asp is the second acidic residue in the CD domain. To determine if these two residues have similar functions in SIPK or WIPK, we mutated either the second or both Asp (D) residues to Asn (N). The SIPKN, SIPKNN, WIPKN, and WIPKNN mutants were then expressed in tobacco leaves under the control of the inducible promoter in the same conditions. As shown in Figures 6B and 6C, the proteins were expressed at levels similar to wild-type SIPK and WIPK. However, there was no enhancement of either SIPK or WIPK activity. In contrast, there was a slight decrease of kinase activity in the SIPK mutants, suggesting that the conserved Asp residues play a different role in plants and might be involved in SIPK activation. The role of these residues in WIPK regulation is likely to be the same, but this cannot be proven because no WIPK activity could be detected.
Figure 6.
Mutation of the Two Asp (D) Residues in the MAPK CD Domain Does Not Enhance the Activation of SIPK or WIPK.
(A) The CD domain is evolutionarily conserved in plants, animals, and yeast.
(B) Mutation of the conserved Asp residues in the CD domain to Asn does not enhance SIPK activation. D353 alone, or both D350 and D353, in the CD domain of SIPK were replaced with Asn (N). SIPKN, the SIPK(D353N) mutant, and SIPKNN, the SIPK(D350N/D353N) double mutant, were transformed into tobacco leaves. Transgene expression was monitored by immunoblot (IB) analysis with anti-Flag antibody (top), and MAPK activity was determined by in-gel kinase assay with MBP as a substrate (bottom).
(C) Substitution of the conserved Asp residues in the CD domain of WIPK fails to result in its activation. D337 alone, or both D334 and D337, in the CD domain of WIPK were replaced with Asn (N). WIPKN, the WIPK(D337N) mutant, and WIPKNN, the WIPK(D334N/D337N) double mutant, were transformed into tobacco leaves. Transgene expression was monitored by immunoblot analysis with anti-Flag antibody (top), and MAPK activity was determined by in-gel kinase assay with MBP as a substrate (bottom).
The Increase of SIPK Activity Is Sufficient to Induce HR-Like Cell Death
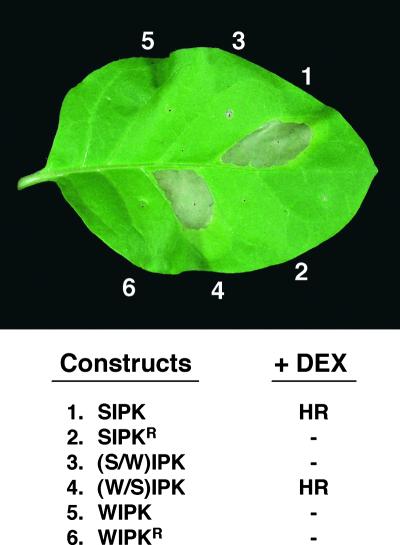
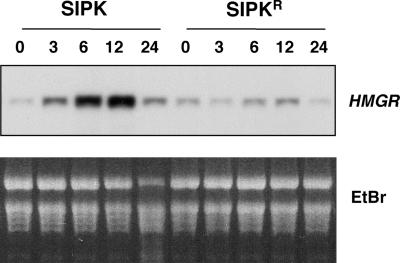
Increased MAPK activity by the expression of either SIPK or (W/S)IPK led to the activation of several defense responses. Within 12 to 16 hr after the induction of SIPK expression, cell death could be seen in small areas. By 30 hr, the whole area infiltrated with Agrobacterium carrying SIPK or (W/S)IPK collapsed, which was identical to the HR elicited by various pathogen or pathogen-derived elicitors (Figure 7). Besides the similarity at the macroscopic level, cell death was associated with electrolyte leakage and shrinkage of cytoplasm (data not shown), which are diagnostic for HR-like cell death. In addition to the HR-like cell death, the increase of SIPK and (W/S)IPK activity also induced the expression of HMGR (Figure 8 and data not shown), whose expression is induced in leaves after the activation of SIPK and WIPK by NtMEK2DD, an active mutant of NtMEK2 (Yang et al., 2001). The activation of HMGR was prolonged and correlated with SIPK activity in the leaves (Figure 8). The expression of inactive SIPKR or (S/W)IPK did not result in any of these phenotypes, suggesting that SIPK activity is required (Figure 7). The phenotype induced by SIPKN or SIPKNN was weaker and delayed, which correlates with its reduced activation (Figure 6B and data not shown). This evidence confirmed the previous hypothesis based on inhibitor studies that long-lasting activation of SIPK is involved in regulating HR-like cell death (Suzuki et al., 1999; Zhang et al., 2000).
Figure 7.
Induction of the Expression of SIPK and the (W/S)IPK Chimera Leads to HR-Like Cell Death.
Different sections of a tobacco leaf were infiltrated with Agrobacterium carrying the constructs indicated, and DEX (30 μM) was applied 48 hr later. The photograph was taken 30 hr after the application of DEX. HR indicates the development of HR-like necrosis in the leaf section, and dashes indicate no visible phenotype.
Figure 8.
The Increase of SIPK Activity Induces the Expression of the HMGR Gene.
Tobacco leaves were transformed transiently with SIPK. SIPKR, an inactive mutant of SIPK, was used as a control. Total RNA was isolated from samples collected at the times indicated after the application of DEX. Equal amounts of RNA (10 μg) were electrophoresed on a 1.2% formaldehyde-agarose gel and transferred to a Zeta-Probe membrane (Bio-Rad). The transcript levels of HMGR were determined by probing with an α-32P-CTP random primer–labeled cDNA insert, as described in Methods. An ethidium bromide (EtBr)-stained gel was used to show equal loading of samples.
DISCUSSION
SIPK is activated by a number of biotic and abiotic stresses in a SA-independent manner, even though it was identified initially as an SA-induced protein kinase (Zhang and Klessig, 1997, 2000; Romeis et al., 1999; Hoyos and Zhang, 2000; Mikolajczyk et al., 2000; Samuel et al., 2000). As a result, it may be more appropriate to call SIPK a stress-induced protein kinase. The activation of SIPK by pathogens and elicitors that cause cell death is long lasting, whereas fungal cell wall elicitors and abiotic stresses such as wounding, high salinity, and high/low osmolarity activate SIPK only transiently (Zhang and Klessig, 1998a, 1998b; Hoyos and Zhang, 2000; Mikolajczyk et al., 2000; Zhang et al., 2000). It is postulated that SIPK may regulate a shared pathway in response to these different stimuli. In addition, depending on the duration of SIPK activation, as well as whether WIPK is activated, different responses could be elicited. Using general kinase inhibitors, we demonstrated that the long-lasting activation of SIPK and/or the delayed activation of WIPK might be required for tobacco cell death induced by fungal elicitins (Zhang et al., 2000).
In this article, we demonstrate that the long-lasting increase of SIPK activity alone induces defense gene activation and HR-like cell death. The increase of SIPK activity in tobacco leaves after the induction of its expression requires the phosphorylation of newly synthesized SIPK by its upstream kinase, possibly NtMEK2 (Yang et al., 2001). In contrast, the expression of WIPK under the same conditions did not result in an increase of its activity, although the protein was expressed at a similar level. Studies using chimeras of SIPK and WIPK demonstrated that the C terminus of SIPK determines its activation, which is surprising because SIPK and WIPK differ more in their N termini. The increase of SIPK activity precedes the activation of HMGR expression and, within 30 hr, HR-like necrosis on leaves of 4- to 5-week-old tobacco plants. These results indicate that the activation of SIPK alone is sufficient to induce HR-like cell death. WIPK activation also is associated with cell death during plant disease resistance (Zhang and Klessig, 1998b; Zhang et al., 2000). However, its role in the process is unclear at present. Cell death induced by SIPK is a little delayed compared with cell death induced by active NtMEK2DD, in which both SIPK and WIPK are activated (Yang et al., 2001). In addition, SIPK alone is insufficient to induce cell death in older leaves, in which transgene expression is much lower because of reduced transcriptional and translational activities (data not shown). However, the expression of NtMEK2DD is capable of inducing cell death in such leaves. These results suggest that WIPK activation might play a role in accelerating the cell death process. The lack of MAPK activation and defense responses in leaves transformed with SIPKR, WIPK, WIPKR, or (S/W)IPK rules out the possibility that the defense responses in leaves transformed with SIPK are caused by nonspecific effects of the DEX-inducible system.
The activity of a MAPK in cells is controlled through phosphorylation activation by its upstream kinase, MAPKK, and dephosphorylation inactivation by its negative regulator, MAPK phosphatase. In unstimulated cells, there is a low basal level of SIPK activity, suggesting that a small portion of its upstream kinase is active. It appears that this basal level activity was responsible for Flag-tagged SIPK activation in our experiments, because kinase inhibitor prevented SIPK activation (Figure 3A). In addition, when SIPKR, an inactive mutant of SIPK, was expressed, no kinase activation was detected (Figure 2). This evidence suggests that no MAPKK activation is involved; otherwise, one would expect the activation of endogenous SIPK in leaves transformed with SIPKR. The fact that only newly synthesized SIPK was activated also suggests that the endogenous SIPK might be kept inactive by association with its phosphatase. It was reported that the autophosphorylation of recombinant AtMPK4 on the Tyr residue in the TEY motif leads to partial activation of the protein (Huang et al., 2000). Both SIPK and WIPK recombinant proteins are capable of autophosphorylation on the Tyr in the presence of ATP. However, autophosphorylation resulted in little increase of their MBP-phosphorylating activity (data not shown). In contrast, phosphorylation of both proteins by their upstream kinases generated highly active MAPKs (Yang et al., 2001). As a result, it was concluded that the increase of SIPK activity is caused by the phosphorylation of Flag-tagged SIPK by basal level MAPKK activity rather than by autophosphorylation.
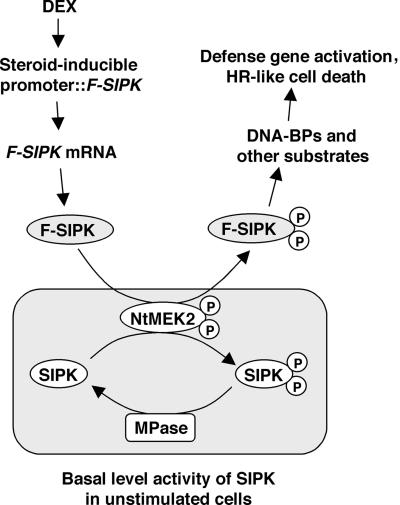
Figure 9 depicts our model showing why the expression of SIPK under an inducible promoter results in an increase of its activity. A sudden increase of SIPK protein disrupts the balance in the cells. After the newly synthesized SIPK becomes activated by the basal level MAPKK activity, there is not enough MAPK phosphatase activity to inactivate SIPK, which leads to an increase of net SIPK activity in the cells. It is interesting that even though WIPK and SIPK share extensive homology and an upstream kinase, NtMEK2, their activities still could be regulated differentially at the post-translational level, as revealed in this study. One possible mechanism is that SIPK and WIPK have different negative regulators, that is, each has its own inactivating phosphatases, and WIPK phosphatase activity is much higher in cells. As a result, newly expressed WIPK is kept in an inactive state. Results from studies with chimeras of SIPK and WIPK suggest that the sequence information that determines this differential regulation resides in the C termini of SIPK and WIPK. SIPK and WIPK both have the conserved DXXD/E motif in their CD domains, which has been shown to be involved in the inactivation of MAPK by MAPK phosphatase in animal systems (Brunner et al., 1994; Chu et al., 1996; Tanoue et al., 2000). However, substitution of the second Asp (D) or both Asp residues with Asn (N) did not enhance the activity of SIPK or WIPK (Figure 6).
Figure 9.
Model of the Possible Mechanism of SIPK Activation in Cells under Induced Conditions.
In unstimulated cells, the actions of the upstream kinase of SIPK, possibly NtMEK2, which activates SIPK, and the unknown MAPK phosphatase (MPase), which inactivates SIPK, reach equilibrium to give a low basal activity of SIPK. A sudden increase of Flag-tagged SIPK protein (F-SIPK) from transgene expression after DEX application leads to the increase of SIPK activity in the cells, and there is not enough MAPK phosphatase to inactivate them. The increase of total SIPK activity leads to the phosphorylation of its substrate(s), which in turn evokes tobacco defense responses such as defense gene activation and HR-like cell death. BPs, binding proteins; P, phosphorylated.
Besides the CD domain, the other mechanism that mediates the selective activation of mammalian MAPKs is the T-loop between kinase subdomains VII and VIII (Enslen et al., 2000). However, it is unlikely that the T-loop of SIPK is the molecular determinant for its differential activation, because (S/W)IPK, which is not active, contains the T-loop of SIPK, whereas (W/S)IPK, which is active, contains the T-loop of WIPK. This result suggests that plant MAPKs may have different mechanism(s) in determining the specificity of their activation. When Flag-tagged WIPK was coexpressed with NtMEK2DD, a constitutively active NtMEK2 mutant, high level WIPK activity resulting from transgene expression was detected, together with the activation of endogenous SIPK (data not shown). This result indicates that a higher level of MAPKK activity is needed to counteract the WIPK-inactivating phosphatase in cells, either through simple opposing enzymatic activities or through the displacement of the phosphatase from WIPK protein. Both upstream MAPKKs and MAPK phosphatases have been shown to interact with MAPKs in animal systems (Widmann et al., 1999). In agreement with this conclusion, we found that 35S::WIPK transgenic plants did not show higher WIPK activity, even though WIPK protein was overexpressed in more than half of the transgenic lines (S. Zhang and D.F. Klessig, unpublished data). This is in contrast to the results reported by Seo et al. (1995)(1999) that overexpression of WIPK leads to an increase of WIPK activity and an alteration of plant wounding responses. The cause of the difference between our observations is unclear at present.
The duration of MAPK activation could be affected by either how long the upstream kinase stays active or if a MAPK phosphatase is induced, or both (Camps et al., 1999; Widmann et al., 1999). Compared with the activation of MAPK, its inactivation processes are less well understood. There is even less information about how MAPKK is inactivated, which certainly affects MAPK activation. In plants, both Tyr protein phosphatase and Thr/Ser protein phosphatase have been implicated in the inactivation of MAPKs based on in vitro evidence (Gupta et al., 1998; Meskiene et al., 1998). In animal systems, the inactivation of MAPKs by dual specificity phosphatases (DSPs), a subfamily of Tyr protein phosphatases, is well documented (Sun et al., 1993; Camps et al., 1999). Some DSPs appear highly selective for inactivating distinct MAPKs. This enzymatic specificity is attributable in part to the catalytic activation of the DSP after binding to the target MAPKs (Camps et al., 1998). In addition, the expression of DSP genes is induced strongly by a number of stimuli that also activate the MAPKs, thus providing a sophisticated transcriptional mechanism for the targeted inactivation of selected MAPK activities (Camps et al., 1999). There also is evidence that the transient activation of SIPK, or its orthologs in other species, by wounding or other stresses is a result of the de novo synthesis of a MAPK phosphatase. Treatment of cells with α-amanitin, a transcriptional inhibitor, or cycloheximide, a translational inhibitor, prolongs MAPK activation (Suzuki and Shinshi, 1995; Bögre et al., 1997). Potential candidates that may be involved in such negative regulation include a PP-2C type of phosphatase and a Tyr phosphatase (Meskiene et al., 1998; Xu et al., 1998). The transcripts of both genes are induced by stresses that could be involved actively in the inactivation of MAPK.
The experimental system we used in this study mimics the long-lasting activation of SIPK induced by pathogen infection. SIPK activity starts to increase 3 hr after the application of DEX, peaks at 6 hr, and stays at a relatively high level until 24 hr, when HR-like cell death becomes visible (Figure 2). Previous pharmacological studies have linked the long-lasting activation of SIPK with cell death in tobacco cells treated with fungal elicitins and xylanase (Suzuki et al., 1999; Zhang et al., 2000). In mammalian cells, the kinetics of MAPK activation has been shown to influence the fate of cells under stress. Transient activation of SAPK/JNK and p38 induces various defense responses and allows the cells to adapt to adverse environments; in contrast, persistent activation of these two MAPKs leads to apoptosis (Kyriakis and Avruch, 1996; Widmann et al., 1999). Recently, it was shown that SAPK/JNK is involved in regulating cytochrome c release in the mitochondrial death signaling pathway (Tournier et al., 2000).
How plant HR cell death, a form of programmed cell death, is regulated and how SIPK is integrated in the signaling pathway(s) remain to be discovered. Nevertheless, the finding that a plant MAPK may play a role similar to that of SAPK/JNK is intriguing, because SIPK has the TEY activation motif and likely is derived from the ancient ERK1/2 (Caffrey et al., 1999). Therefore, the analogous functions of SIPK in plants and SAPK/JNK in animals probably evolved independently and represent an example of parallel evolution. The expression of a number of proteins involved in animal apoptosis showed similar effects in plants (reviewed by Lam et al., 1999). However, no homolog of these animal cell death regulators could be identified in the fully sequenced Arabidopsis genome (The Arabidopsis Genome Initiative, 2000). This raises two possibilities: either plants have only functional, but not sequence, homologs of these animal cell death regulators, or plant cell death is regulated and executed by different mechanisms. The identification of a SIPK substrate(s) should shed light on the mechanism of cell death regulated by this MAPK pathway.
METHODS
Mutagenesis and Construction of SIPK and WIPK Chimeras
Various mutants of salicylic acid–induced protein kinase (SIPK) and wounding-induced protein kinase (WIPK) were generated by QuickChange site-directed mutagenesis (Stratagene) and confirmed by sequencing. To prepare (S/W)IPK and (W/S)IPK chimera constructs, a PstI site was introduced into the WIPK gene at the position corresponding to that of SIPK by site-directed mutagenesis. The change of the nucleotide sequence does not alter the amino acid sequence. Two chimeric genes were generated by swapping the C termini of SIPK and WIPK.
Agrobacterium-Mediated Transient Transformation
Tobacco plants (Nicotiana tabacum cv Xanthi nc [NN]) were grown at 25°C in a growth room programmed for a 14-hr light cycle. Four- to 5-week-old tobacco plants were used for experiments. Agrobacterium tumefaciens–mediated transient transformation experiments were performed as described previously (Yang et al., 2001). SIPK, WIPK, and their mutants or chimeras with a Flag epitope at their N termini were inserted into the XhoI–SpeI sites of the steroid-inducible pTA7002 binary vector (Aoyama and Chua, 1997). The 5′ untranslated region of SIPK or WIPK was replaced with the Ω sequence from Tobacco mosaic virus (Gallie et al., 1987). Agrobacterium LBA4404 carrying different constructs was grown overnight in YM medium (Life Technologies, Grand Island, NY) containing 100 μg/mL streptomycin, 50 μg/mL kanamycin, and 100 μM acetosyringone. Cells were collected by centrifugation (4000g), resuspended to OD600 = 0.8 in Murashige and Skoog (1962) medium (pH 5.9) with 100 μM acetosyringone, and infiltrated into the fully expanded leaves. The expression of transgene was induced by the infiltration of dexamethasone (DEX; 30 μM) 40 to 48 hr later. Samples for protein and RNA preparation were collected at the times indicated in each figure, quick frozen in liquid N2, and stored at −80°C until use.
Preparation of Protein Extracts
Protein was extracted from leaf tissue by grinding with small plastic pestles in extraction buffer (100 mM Hepes, pH 7.5, 5 mM EDTA, 5 mM EGTA, 10 mM DTT, 10 mM Na3VO4, 10 mM NaF, 50 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 5 μg/mL antipain, 5 μg/mL aprotinin, 5 μg/mL leupeptin, and 10% glycerol). After centrifugation at 18,000g for 30 min in a microcentrifuge, supernatants were transferred into clean tubes, quickly frozen in liquid nitrogen, and stored at −80°C. The concentration of protein extracts was determined using the Bio-Rad protein assay kit with BSA as the standard.
In-Gel Kinase Activity Assay
The in-gel kinase activity assay was performed as described previously (Zhang and Klessig, 1997). Briefly, extracts containing 10 μg of protein were electrophoresed on 10% SDS-polyacrylamide gels embedded with 0.25 mg/mL myelin basic protein (MBP) in the separating gel as a substrate for the kinases. After electrophoresis, SDS was removed by washing the gel with washing buffer (25 mM Tris, pH 7.5, 0.5 mM DTT, 0.1 mM Na3VO4, 5 mM NaF, 0.5 mg/mL BSA, and 0.1% [v/v] Triton X-100) three times each for 30 min at room temperature. The kinases were allowed to renature in 25 mM Tris, pH 7.5, 1 mM DTT, 0.1 mM Na3VO4, and 5 mM NaF at 4°C overnight with three changes of buffer. The gel was then incubated at room temperature in 30 mL of reaction buffer (25 mM Tris, pH 7.5, 2 mM EGTA, 12 mM MgCl2, 1 mM DTT, and 0.1 mM Na3VO4) with 200 nM ATP plus 50 μCi of γ-32P-ATP (3000 Ci/mmol) for 60 min. The reaction was stopped by transferring the gel into 5% (w/v) trichloroacetic acid and 1% (w/v) NaPPi. The unincorporated γ-32P-ATP was removed by washing in the same solution for at least 6 hr with five changes. The gel was dried on Whatman 3MM paper and exposed to Kodak XAR-5 film. Prestained size markers (Bio-Rad) were used to calculate the sizes of kinases.
Purification of Recombinant Protein and in Vitro Kinase Assay
A BamHI site was introduced in front of the ATG start codon of SIPK and WIPK by polymerase chain reaction and then ligated in frame into the pET-28a(+) vector (Novagen, Madison, WI). Mutations were introduced by QuickChange site-directed mutagenesis (Stratagene) and confirmed by sequencing. BL21(DE3) cells transformed with pET-28a(+) constructs were induced with 0.5 mM isopropylthio-β-galactoside for 3 hr. His-tagged proteins were purified using nickel columns (Amersham Pharmacia Biotech), concentrated, and desalted using Centricon-10 (Millipore, Bedford, MA).
The autophosphorylation assay was performed by incubating equal amounts (0.5 μg) of purified recombinant SIPK, WIPK, and their mutants in reaction buffer (25 mM Tris, pH 7.5, 10 MnCl2, 1 mM EGTA, and 1 mM DTT) in the presence of 25 μM γ-32P-ATP (∼3000 dpm/pmol) at 30°C for 30 min. The reaction was stopped by the addition of SDS loading buffer, and kinase activities were detected by autoradiography after SDS-PAGE. Phosphorylation activities of mitogen-activated protein kinase (MAPK) and its mutants were determined using MBP (final concentration, 0.25 μg/μL) as a substrate under the same conditions as in the autophosphorylation assay, except that 0.1 μg of MAPK was used in each reaction.
Immunoblot Analysis
For immunoblot analysis, 10 μg of total protein per lane was separated on 10% SDS-polyacrylamide gels, and the proteins were transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH) by semidry electroblotting. After blocking for 1 hr in Tris-buffered saline (20 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) with 5% nonfat dried milk (Carnation) at room temperature, the membranes were incubated with either anti-Flag (1:10,000 dilution; Sigma) or Ab-p48N (0.15 μg/mL) (Zhang et al., 1998). After washing in Tris-buffered saline four times, the blots were incubated with a horseradish peroxidase–conjugated secondary antibody (1:10,000 dilution; Sigma), and the complexes were visualized using an enhanced chemiluminescence kit (DuPont–New England Nuclear) according to the manufacturer's instructions.
Immune Complex Kinase Activity Assay
For immune complex kinase activity assays, protein extract (50 μg) was incubated with anti-Flag antibody (2 μg), Ab-p48N (2.5 μg), or Ab-p44N (2.5 μg) in immunoprecipitation buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM NaF, 10 mM β-glycerophosphate, 2 μg/mL antipain, 2 μg/mL aprotinin, 2 μg/mL leupeptin, and 0.1% Tween 20) at 4°C for 2 hr on a rocker. Approximately 20 μL (packed volume) of protein G– or protein A–agarose washed in immunoprecipitation buffer was added, and the incubation was continued for another 4 hr. Agarose bead–protein complexes were pelleted by brief centrifugation and washed twice with 1.5 mL of immunoprecipitation buffer, once with immunoprecipitation buffer plus 1 M NaCl, and twice with 1 mL of kinase reaction buffer. Kinase activity in the complex (equivalent to 20 μg of starting protein) was assayed at room temperature for 20 min in a final volume of 25 μL containing 0.1 mg/mL MBP, 10 μM ATP, and 1 μCi of γ-32P-ATP. The reaction was stopped by the addition of SDS-PAGE sample loading buffer. After electrophoresis on a 15% SDS-polyacrylamide gel, the phosphorylated MBP was visualized by autoradiography.
Treatment of Flag-Tagged SIPK with Phosphatases
Flag-tagged SIPK was immunoprecipitated from 50 μg of protein extract with anti-Flag antibody (2 μg) in immunoprecipitation buffer at 4°C for 2 hr on a rocker. Approximately 20 μL (packed volume) of protein G–agarose was added, and the incubation was continued for another 4 hr. Agarose bead–protein complexes were pelleted by brief centrifugation and washed three times with 1.5 mL of immunoprecipitation buffer and once with reaction buffer. Immune complex with SIPK was then treated with either the Thr/Ser-specific phosphatase, PP-2A1 (0.25 units in 30 μL; Upstate Biotechnology, Lake Placid, NY), or the Tyr-specific protein phosphatase, YOP (2 units in 30 μL; New England Biolabs, Beverly, MA), for 20 min at 30°C in the presence or absence of a phosphatase inhibitor. The PP-2A1 inhibitor, okadaic acid, and the YOP inhibitor, Na3VO4, were used at concentrations of 5 μM and 5 mM, respectively. After phosphatase treatment, kinase activity was detected by the in-solution kinase assay using MBP as a substrate.
RNA Gel Blot Analysis
RNA was extracted using Trizol reagent (Life Technologies) according to the manufacturer's instructions. Ten micrograms of total RNA per lane was separated on 1.2% formaldehyde-agarose gels, transferred to a Zeta-Probe membrane (Bio-Rad), and hybridized with random primer–labeled 3-hydroxy-3-methylglutaryl CoA reductase cDNA insert as described previously (Yang et al., 2001).
GenBank Accession Numbers
The accession numbers for the MAPKs are as follows: SIPK, U94192; AtMPK6, D21842; MMK1, Q07176; WIPK, D61377; AtMPK3, D21839; MMK4, X82270; ERMK, Y12785; ERK2, A40033; JNK1, S71100; p38α, Q16539; and HOG1, AAA34680.
Acknowledgments
We thank Dr. N.-H. Chua for pTA7002 vector, Dr. D. Klessig for tobacco HMGR cDNA, and Drs. W. Folk and A. Kenzior for plasmid carrying the Ω leader sequence and the Flag epitope. S.Z. was supported by National Science Foundation Grant MCB-9974796 and the University of Missouri Research Board.
References
- Aoyama, T., and Chua, N.-H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11, 605–612. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 108, 796–815. [DOI] [PubMed] [Google Scholar]
- Bögre, L., Ligterink, W., Meskiene, I., Barker, P.J., Heberle-Bors, E., Huskisson, N.S., and Hirt, H. (1997). Wounding induces the rapid and transient activation of a specific MAP kinase pathway. Plant Cell 9, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner, D., Oellers, N., Szabad, J., Biggs, W.H., III, Zipursky, S.L., and Hafen, E. (1994). A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76, 875–888. [DOI] [PubMed] [Google Scholar]
- Caffrey, D.R., O'Neill, L.A.J., and Shields, D.C. (1999). The evolution of the MAP kinase pathways: Coduplication of interacting proteins leads to new signaling cascades. J. Mol. Evol. 49, 567–582. [DOI] [PubMed] [Google Scholar]
- Camps, M., Nichols, A., Gillieron, C., Antonsson, B., Muda, M., Chabert, C., Boschert, U., and Arkinstall, S. (1998). Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Camps, M., Nichols, A., and Arkinstall, S. (1999). Dual specificity phosphatases: A gene family for control of MAP kinase function. FASEB J. 14, 6–16. [PubMed] [Google Scholar]
- Cardinale, F., Jonak, C., Ligterink, W., Niehaus, K., Boller, T., and Hirt, H. (2000). Differential activation of four specific MAPK pathways by distinct elicitors. J. Biol. Chem. 275, 36734–36740. [DOI] [PubMed] [Google Scholar]
- Chen, Y.-R., Meyer, C.F., and Tan, T.-H. (1996). Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in g radiation-induced apoptosis. J. Biol. Chem. 271, 631–634. [DOI] [PubMed] [Google Scholar]
- Chu, Y., Solski, P.A., Khosravi-Far, R., Der, C.J., and Kelly, K. (1996). The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem. 271, 6497–6501. [DOI] [PubMed] [Google Scholar]
- Davis, R. (2000). Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252. [DOI] [PubMed] [Google Scholar]
- Desikan, R., Clarke, A., Atherfold, P., Hancock, J.T., and Neill, S.J. (1999). Harpin induces mitogen-activated protein kinase activity during defense responses in Arabidopsis thaliana suspension cultures. Planta 210, 97–103. [DOI] [PubMed] [Google Scholar]
- Enslen, H., Brancho, D.M., and Davis, R.J. (2000). Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 19, 1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G., Grosskopf, D.G., Regenass, M., and Boller, T. (1991). Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc. Natl. Acad. Sci. USA 88, 8831–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix, G., Regenass, M., Spanu, P., and Boller, T. (1994). The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induced rapid hyperphosphorylation of specific protein as revealed by pulse labeling with [33P]phosphate. Proc. Natl. Acad. Sci. USA 91, 952–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie, D.R., Sleat, D.E., Watts, J.W., Turner, P.C., and Wilson, T.M.A. (1987). The 5-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 15, 3257–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskopf, D.G., Felix, G., and Boller, T. (1990). K-252a inhibits the response of tomato cells to fungal elicitors in vivo and their microsomal protein kinase in vitro. FEBS Lett. 275, 177–180. [DOI] [PubMed] [Google Scholar]
- Gupta, R., Huang, Y., Kieber, J., and Luan, S. (1998). Identification of a dual-specificity protein phosphatase that inactivates a MAP kinase from Arabidopsis. Plant J. 16, 581–589. [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack, K.E., and Jones, J.D.G. (1996). Resistance gene-dependent plant defense responses. Plant Cell 8, 1773–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos, M.E., and Zhang, S. (2000). Calcium-independent activation of salicylic acid–induced protein kinase and a 40-kilodalton protein kinase by hyperosmotic stress. Plant Physiol. 122, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C.-Y.F., and Ferrell, J.E.J. (1996). Ultrasensitivity in the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 93, 10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Li, H., Gupta, R., Morris, P.C., Luan, S., and Kieber, J.J. (2000). ATMPK4, an Arabidopsis homolog of mitogen-activated protein kinase, is activated in vitro by AtMEK1 through threonine phosphorylation. Plant Physiol. 122, 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen, N.T. (1990). Gene-for-gene complementarity in plant–pathogen interactions. Annu. Rev. Genet. 24, 447–463. [DOI] [PubMed] [Google Scholar]
- Kovtun, Y., Chiu, W.-L., Tena, G., and Sheen, J. (2000). Functional analysis of oxidative stress–activated mitogen-activated protein kinase cascade in plants. Proc. Natl. Acad. Sci. USA 97, 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis, J.M., and Avruch, J. (1996). Protein kinase cascades activated by stress and inflammatory cytokines. BioEssays 18, 567–577. [DOI] [PubMed] [Google Scholar]
- Lam, E., Pontier, D., and del Pozo, O. (1999). Die and let live: Programmed cell death in plants. Curr. Opin. Plant Biol. 2, 502–507. [DOI] [PubMed] [Google Scholar]
- Lebrun-Garcia, A., Ouaked, F., Chiltz, A., and Pugin, A. (1998). Activation of MAPK homologues by elicitors in tobacco cells. Plant J. 15, 773–781. [DOI] [PubMed] [Google Scholar]
- Ligterink, W., Kroj, T., zur Nieden, U., Hirt, H., and Scheel, D. (1997). Receptor-mediated activation of a MAP kinase in pathogen defense of plants. Science 276, 2054–2057. [DOI] [PubMed] [Google Scholar]
- Martin, G.B. (1999). Functional analysis of plant disease resistance genes and their downstream effectors. Curr. Opin. Plant Biol. 2, 273–279. [DOI] [PubMed] [Google Scholar]
- Meskiene, I., Bögre, L., Glaser, W., Balog, J., Brandstötter, M., Zwerger, K., Ammerer, G., and Hirt, H. (1998). MP2C, a plant protein phosphatase 2C, functions as a negative regulator of mitogen-activated protein kinase pathways in yeast and plants. Proc. Natl. Acad. Sci. USA 95, 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk, M., Awotunde, O.S., Muszynska, G., Klessig, D.F., and Dobrowolska, G. (2000). Osmotic stress induces rapid activation of a salicylic acid–induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell 12, 165–178. [PMC free article] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nühse, T., Peck, S.C., Hirt, H., and Boller, T. (2000). Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK6. J. Biol. Chem. 275, 7521–7526. [DOI] [PubMed] [Google Scholar]
- Ricci, P. (1997). Induction of the hypersensitive response and systemic acquired resistance by fungal proteins: The case of elicitins. In Plant–Microbe Interaction, G. Stacey and N.T. Keen, eds (New York: Chapman and Hall), pp. 53–75.
- Romeis, T., Piedras, P., Zhang, S., Klessig, D.F., Hirt, H., and Jones, J. (1999). Rapid Avr9 and Cf-9 dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound and salicylate responses. Plant Cell 11, 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, M.A., Miles, G.P., and Ellis, B.E. (2000). Ozone treatment rapidly activates MAP kinase signaling in plants. Plant J. 22, 367–376. [DOI] [PubMed] [Google Scholar]
- Scheel, D. (1998). Resistance response physiology and signal transduction. Curr. Opin. Plant Biol. 1, 305–310. [DOI] [PubMed] [Google Scholar]
- Scofield, S.R., Tobias, C.M., Rathjen, J.P., Chang, J.H., Lavelle, D.T., Michelmore, R.W., and Staskawicz, B.J. (1996). Molecular basis of gene-for-gene specificity in bacterial speck disease of tomato. Science 274, 2063–2065. [DOI] [PubMed] [Google Scholar]
- Seo, S., Okamoto, M., Seto, H., Ishizuka, K., Sano, H., and Ohashi, Y. (1995). Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science 270, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Seo, S., Sano, H., and Ohashi, Y. (1999). Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich, I.E., and Hahlbrock, K. (1998). Pathogen defence in plants: A paradigm of biological complexity. Trends Plant Sci. 3, 86–90. [Google Scholar]
- Staskawicz, B.J., Ausubel, F.M., Baker, B.J., Ellis, J.G., and Jones, J.D.G. (1995). Molecular genetics of plant disease resistance. Science 268, 661–667. [DOI] [PubMed] [Google Scholar]
- Stratmann, J.W., and Ryan, C.A. (1997). Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc. Natl. Acad. Sci. USA 94, 11085–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., Charles, C.H., Lau, L.F., and Tonks, N.K. (1993). MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75, 487–493. [DOI] [PubMed] [Google Scholar]
- Suzuki, K., and Shinshi, H. (1995). Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell 7, 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, K., Fukuda, Y., and Shinshi, H. (1995). Studies on elicitor-signal transduction leading to differential expression of defense genes in cultured tobacco cells. Plant Cell Physiol. 36, 281–289. [Google Scholar]
- Suzuki, K., Yano, A., and Shinshi, H. (1999). Slow and prolonged activation of the p47 protein kinase during hypersensitive cell death in a culture of tobacco cells. Plant Physiol. 119, 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Frederick, R.D., Zhou, J., Halterman, D.A., Jia, Y., and Martin, G.B. (1996). Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science 274, 2060–2063. [DOI] [PubMed] [Google Scholar]
- Tanoue, T., Adachi, M., Moriguchi, T., and Nishida, E. (2000). A conserved docking motif in MAP kinases common to substrates, activators and regulators. Natl. Cell Biol. 2, 110–116. [DOI] [PubMed] [Google Scholar]
- Tournier, C., Hess, P., Yang, D.D., Xu, J., Turner, T.K., Nimnual, A., Bar-Sagi, D., Jones, S.N., Flavell, R.A., and Davis, R.J. (2000). Requirement of JNK for stress-induced activation of the cytochrome c–mediated death pathway. Science 288, 870–874. [DOI] [PubMed] [Google Scholar]
- Viard, M.-P., Martin, F., Pugin, A., Ricci, P., and Blein, J.-P. (1994). Protein phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 104, 1245–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann, C., Gibson, S., Jarpe, M.B., and Johnson, G.L. (1999). Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180. [DOI] [PubMed] [Google Scholar]
- Xia, Z., Dickens, M., Raingeaud, J., Davis, R.J., and Greenberg, M.E. (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270, 1326–1331. [DOI] [PubMed] [Google Scholar]
- Xu, Q., Fu, H.-H., Gupta, R., and Luan, S. (1998). Molecular characterization of a tyrosine-specific protein phosphatase encoded by a stress-responsive gene in Arabidopsis. Plant Cell 10, 849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K.-Y., Liu, Y., and Zhang, S. (2001). Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 98, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Shah, J., and Klessig, D.F. (1997). Signal perception and transduction in plant defense responses. Genes Dev. 11, 1621–1639. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Li, R., and Qi, M. (2000). In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 22, 543–551. [DOI] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1997). Salicylic acid activates a 48 kD MAP kinase in tobacco. Plant Cell 9, 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. a). The tobacco wounding-activated MAP kinase is encoded by SIPK. Proc. Natl. Acad. Sci. USA 95, 7225–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (1998. b). N resistance gene–mediated de novo synthesis and activation of a tobacco MAP kinase by TMV infection. Proc. Natl. Acad. Sci. USA 95, 7433–7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., and Klessig, D.F. (2000). Pathogen-induced MAP kinases in tobacco. Results Probl. Cell Differ. 27, 65–84. [DOI] [PubMed] [Google Scholar]
- Zhang, S., Du, H., and Klessig, D.F. (1998). Activation of tobacco SIP kinase by both a cell wall–derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp. Plant Cell 10, 435–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Liu, Y., and Klessig, D.F. (2000). Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 23, 339–347. [DOI] [PubMed] [Google Scholar]