Abstract
A-type cyclin-dependent kinases (CDKs), also known as cdc2, are central to the orderly progression of the cell cycle. We made a functional Green Fluorescent Protein (GFP) fusion with CDK-A (Cdc2-GFP) and followed its subcellular localization during the cell cycle in tobacco cells. During interphase, the Cdc2-GFP fusion protein was found in both the cytoplasm and the nucleus, where it was highly resistant to extraction. In premitotic cells, a bright and narrow equatorial band appeared on the cell surface, resembling the late preprophase band, which disintegrated within 10 min as followed by time-lapse images. Cdc2-GFP was not found on prophase spindles but left the chromatin soon after this stage and associated progressively with the metaphase spindle in a microtubule-dependent manner. Arresting cells in mitosis through the stabilization of microtubules by taxol further enhanced the spindle-localized pool of Cdc2-GFP. Toward the end of mitosis, Cdc2-GFP was found at the midzone of the anaphase spindle and phragmoplast; eventually, it became focused at the midline of these microtubule structures. In detergent-extracted cells, the Cdc2-GFP remained associated with mitotic structures. Retention on spindles was prevented by pretreatment with the CDK-specific inhibitor roscovitine and was enhanced by the protein phosphatase inhibitor okadaic acid. Furthermore, we demonstrate that both the endogenous CDK-A and Cdc2-GFP were cosedimented with taxol-stabilized plant microtubules from cell extracts and that Cdc2 activity was detected together with a fraction of polymerized tubulin. These data provide evidence that the A-type CDKs associate physically with mitotic structures in a microtubule-dependent manner and may be involved in regulating the behavior of specific microtubule arrays throughout mitosis.
INTRODUCTION
The spatial and temporal coordination of cellular events contributes to the precise reproduction of cells at each division. The cyclin-dependent kinases (CDKs), which are conserved from yeast to animals to plants (Mironov et al., 1999; Pines, 1999), play a central role in coordinating these events. The sequential activation of CDKs by different cyclins at different times in the cell cycle ensures orderly progression through the cycle.
In yeast, there is only a single CDK that performs multiple functions, whereas in animals and plants, there are multiple CDKs that are specialized. Based on structural similarities, plant CDKs have been classified as CDK-A, CDK-B, etc. (Joubes et al., 2000). Canonical plant CDK, or CDK-A, is highly conserved at the amino acid sequence level and is most similar to the yeast Cdc2/CDC28 or animal CDK1-3 proteins.
All CDK-A proteins contain the conserved cyclin interaction or PSTAIRE motif and have been shown to functionally complement yeast cdc2/CDC28 mutations (Colasanti et al., 1991; Ferreira et al., 1991; Hirt et al., 1991, 1993; Fobert et al., 1996). CDK-A mRNAs are expressed in dividing and division-competent cells throughout the cell cycle, and their protein kinase activity becomes activated at both the G1-to-S and G2-to-M transitions (Bögre et al., 1997; Magyar et al., 1997). The overexpression of a kinase-negative mutant form of the Arabidopsis CDK-A blocks or slows down cell cycle progression in both G1- and G2-phases, further indicating its multiple roles in the cell cycle (Hemerly et al., 1995). Similarly, roscovitine, a drug with high specificity to canonical CDKs, blocks both G1-to-S and G2-to-M transitions (Planchais et al., 1997; Binarova et al., 1998) and results in disturbed spindle organization, such as monopolar spindle formation during mitosis (Binarova et al., 1998). CDK-A members were found to bind some of the D-type cyclins (Healy et al., 2000), and in the yeast two-hybrid assay it interacts with the A2-type cyclin (Roudier et al., 2000), but it is not known if CDK-A associates with B-type cyclins. A second class of CDKs is found only in plants (Doonan and Fobert, 1997), with members containing the PPTALRE or PPTTLRE motifs; it is named the CDK-B class (Joubes et al., 2000). These CDKs appear to have specific functions in S-, G2-, and M-phases, as suggested by their phase-specific expression and activity (Fobert et al., 1996; Magyar et al., 1997). However, their exact roles are not well understood.
CDK1 location changes dramatically during cell cycle progression in both yeast (Alfa et al., 1990) and animals (Bailly et al., 1989). A proportion of CDK1 is located in the interphase nucleus, where it is bound tightly to chromatin (Riabowol et al., 1989). During mitosis, CDK1 localizes to the spindle pole body in yeast (Alfa et al., 1990) and to centrosomes in animal cells (Bailly et al., 1989). During mitosis, CDK1 is known to associate with spindle microtubules (Leiss et al., 1992), and it regulates their stability through the phosphorylation of microtubule-associating proteins such as MAP4 or MAP215 (Verde et al., 1990; Tournebize et al., 2000); it also affects microtubule movements through the phosphorylation of motor proteins (Walczak et al., 1998). A recent report implicates CDK1 as having an important role in regulating the orientation of asymmetric divisions in Drosophila melanogaster (Tio et al., 2001).
In plants, the orientation of cell division is thought to be important for normal development, and in many tissues it is stringently controlled (Doonan, 2000). In most higher plant cells, the division plane is predicted by an enigmatic microtubule array called the preprophase band (PPB). Immunolocalization of CDK-A proteins, using antibodies against the conserved PSTAIRE domain or against the C terminus (Mineyuki et al., 1991; Colasanti et al., 1993; Bögre et al., 1997; Mews et al., 1997; Stals et al., 1997), indicate the presence of CDK-A at multiple locations: in the nucleus, on the mitotic chromosomes, and associated with several microtubule arrays, including the PPB, the spindle, and the phragmoplast. Another CDK, Medsa;CDK-B2;1, also is found on mitotic structures (Ayaydin et al., 2000). However, the reported localization patterns are complex and sometimes contradictory, depending on the source of the antibody and even the fixation conditions used.
In this study, we used a Green Fluorescent Protein (GFP) fusion with a CDK-A protein, Medsa;CDK-A;2, to follow its localization in living cells. We give a detailed description of its dynamic association with microtubules during mitosis and provide evidence that recruitment to the spindle is microtubule dependent. Furthermore, Cdc2 activity was found to be required for microtubule recruitment. Finally, we show that Medsa;CDK-A;2 and tubulin copurify with microtubules from mitotic cell extracts. These results collectively suggest that, in plants, CDK-A proteins have a continuous role in regulating microtubule organization throughout mitosis.
RESULTS
The Cdc2-GFP Fusion Protein Remains Functional as Tested by Yeast Complementation and by Measuring Its Protein Kinase Activity in Plant Cells
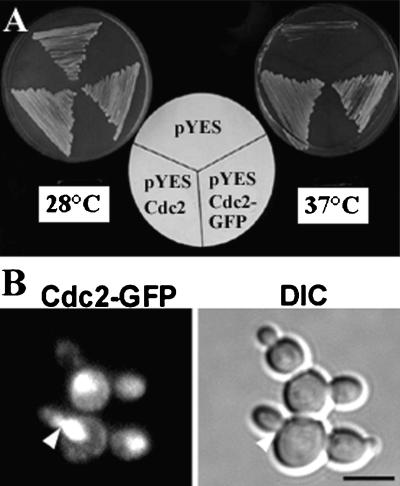
To investigate the dynamic localization of CDK-A proteins in living plant cells, we made a translational fusion between Medsa;CDK-A;2 and GFP, hereafter referred to as Cdc2-GFP. To establish that the Cdc2-GFP fusion product was a functional cyclin-dependent protein kinase, we tested its ability to complement temperature-sensitive strains of budding yeast carrying the CDC28-4 and CDC28-1N mutations (Surana et al., 1991). Figure 1A shows that expression of either Medsa;CDK-A;2 or the GFP fusion protein allowed the CDC28-1N temperature-sensitive mutant to grow at the restrictive temperature (37°C). Similar results were obtained for the CDC28-4 strain (data not shown). We observed yeast cells expressing Cdc2-GFP with an epifluorescence microscope and found that the signal was confined mostly to the nucleus and the bud neck (Figure 1B).
Figure 1.
Complementation Assay in Yeast.
(A) Complementation analysis of the Saccharomyces cerevisiae CDC28-1N temperature-sensitive mutation containing the empty pYES yeast shuttle vector, pYES with Medsa;CDK-A;2 (pYES Cdc2), or the fusion of Medsa;CDK-A;2 with GFP (pYES Cdc2-GFP) and grown at 28 and 37°C on induction medium containing galactose.
(B) Localization of Cdc2-GFP expressed in yeast cells at different phases of the cell cycle as shown by a fluorescence image (left) and by a differential interference contrast (DIC) image to visualize the cells (right). The arrowhead indicates a bud neck, where a spindle is localized in yeast. Bar = 5 μm.
To express the Cdc2-GFP fusion protein in plants, we placed it under the control of a tetracycline-regulated promoter (Weinmann et al., 1994). This should repress Cdc2-GFP expression in tobacco lines containing the tetracycline repressor protein and thus avoid problems if the Cdc2-GFP expression was deleterious. However, we found that transformation efficiencies were similar in tobacco lines with and without the tetracycline repressor and concluded that the constitutive expression of Cdc2-GFP is not deleterious to plants. No phenotypic differences were observed in transformants from either line that were associated with the cdc2-GFP transgene, so we decided to continue these experiments only with plants constitutively expressing Cdc2-GFP.
Seed from five independent transgenic lines containing Cdc2-GFP as well as from five lines expressing GFP alone were germinated on hygromycin, and their root cells were observed by confocal laser scanning microscopy. Plants expressing the Cdc2-GFP fusion protein or GFP alone displayed a readily observable green fluorescence in the nucleus and the cytoplasm (data not shown). However, because of the strong autofluorescence of tobacco root tip cells, high resolution images could not be obtained. Therefore, we generated cell suspensions from selected GFP and Cdc2-GFP plants.
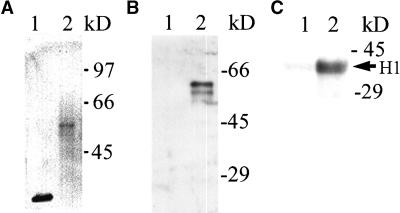
To establish that the fusion protein was being expressed and processed correctly, protein extracts from both GFP and Cdc2-GFP transgenic cell cultures were separated by SDS-PAGE, blotted, and probed with anti-PSTAIRE (data not shown) or anti-GFP (Figure 2A) antibodies. As predicted, the GFP transgenic lines contained a band of the expected 27 kD and the cdc2-GFP transgenic lines contained a band of 60 kD (Figure 2A). A weaker band with a reduced molecular mass also was detected in the Cdc2-GFP–expressing plants. The different forms might be attributable to post-translational modifications. Cdc2-GFP accumulates to a much lesser extent than GFP, and this was observed in several independent transgenic lines. The reduced levels of Cdc2-GFP may indicate either that the Cdc2-GFP fusion protein is not translated efficiently or that the protein is unstable compared with GFP. However, there is no evidence for extensive proteolysis, and no free GFP was detected in the cdc2-GFP lines.
Figure 2.
Cdc2-GFP Fusion Protein Is Expressed in a Correct Size, Binds to p13suc1, and Is Active in Tobacco Cells.
(A) Immunoblotting using extracts prepared from suspension cultured cells expressing GFP (lane 1) or Cdc2-GFP (lane 2) with a GFP-specific antibody.
(B) Binding of GFP (lane 1) or Cdc2-GFP (lane 2) to p13suc1. Extracts prepared as in (A) were mixed with p13suc1 beads, and the bound fractions were immunoblotted with a GFP-specific antibody.
(C) Histone H1 kinase activity of GFP (lane 1) and Cdc2-GFP (lane 2) immunopurified from extracts using a GFP-specific antibody. The phosphorylated histone H1 is indicated by an arrow.
Molecular mass markers are labeled at right.
To confirm that the Cdc2-GFP fusion protein is functional in plant cells, we tested its binding to a conserved regulator, p13suc1, and measured its protein kinase activity. The Cdc2-GFP fusion protein, but not GFP alone, could bind to p13suc1 (Figure 2B). When immunopurified from cell extracts using a GFP-specific antibody, the Cdc2-GFP fusion protein had protein kinase activity and readily phosphorylated histone H1 (Figure 2C). These biochemical experiments indicate that Cdc2-GFP is functional in plant cells.
Cdc2-GFP Is Bound Tightly to Chromatin in Interphase
Several independent GFP and Cdc2-GFP transgenic lines were observed using epifluorescence microscopy, but because all lines for a given construct were essentially similar, we chose a single representative GFP line and Cdc2-GFP line for detailed analyses.
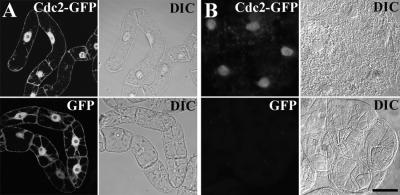
During interphase, Cdc2-GFP–derived fluorescence was weaker than, but broadly similar to, that of GFP. Both were localized to the nucleus and the cytoplasmic strands (Figure 3A). The size of the free GFP molecule is somewhat smaller than the reported size exclusion limit of the nuclear pores (Chytilova et al., 2000), and, consistent with this fact, GFP was distributed freely in both the cytoplasm and the nucleus. However, the size of the Cdc2-GFP fusion protein is above the size exclusion limit of the nuclear pores, suggesting that Cdc2-GFP is transported to the nucleus by an active mechanism.
Figure 3.
Cdc2-GFP Is Specifically Localized and Retained in the Nucleus.
(A) Fluorescent microscopic images of Cdc2-GFP and GFP (left) and the corresponding DIC images (right) in live tobacco cells.
(B) Fluorescent microscopic images of Cdc2-GFP and GFP cells extracted with 0.1% Triton X-100 (left) and the corresponding DIC images (right). Bar = 20 μm.
To further characterize the association of Cdc2-GFP with nuclear structures, we extracted both GFP– and Cdc2-GFP–expressing cells with a mild nonionic detergent. Under these conditions, membranes and the nuclear envelope were dissolved and soluble proteins were extracted. As expected, GFP was extracted completely by this treatment. However, most of the Cdc2-GFP fusion protein was retained in the nucleus (Figure 3B). A fuzzy halo around the nucleus within the extracted cell also was evident (Figure 3B), indicating that Cdc2-GFP is tightly bound. We further extracted these cells with increased salt concentrations (up to 0.5 M NaCl) and increased levels of detergent (up to 1% Triton X-100). None of these treatments completely eliminated nucleus-bound Cdc2-GFP (data not shown). We conclude that Cdc2-GFP is bound strongly and retained specifically in the nucleus.
Cdc2-GFP Accumulates in an Equatorial Band on the Cell Cortex during Late Preprophase
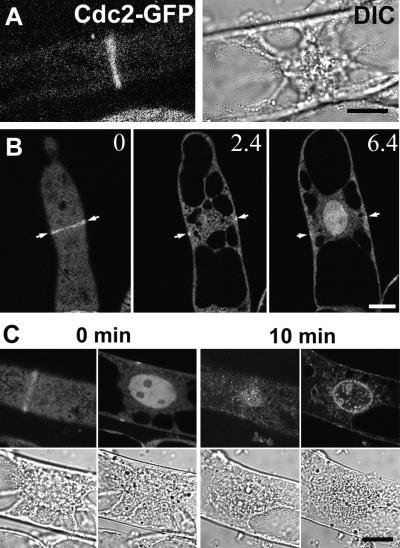
Previous reports indicated that CDK-A variably locates to the PPB, but there is some debate regarding the basis of this variation. It was reported that fixation conditions had a dramatic effect on the proportion of PPBs that stained with antibodies against CDK-A (Mineyuki et al., 1996). Using the cell lines described above, we observed a narrow fluorescent band resembling a PPB (Figure 4A). Differential interference contrast (DIC) images of the same cell showed that the nucleus was anchored in the center of the cell by transversally oriented cytoplasmic strands. All of these features are characteristics of cells in late G2-phase of the cell cycle. Confocal imaging revealed that these bands were located close to the plasma membrane and circumnavigated the cell (Figure 4B). In an optical section running across the cell surface, a full line of Cdc2-GFP fluorescence was apparent. In medial sections through the cytoplasm or through the nucleus, the signal resided at the cell surface on opposite sides of the nucleus. Cdc2-GFP also was present in the nuclei at this stage.
Figure 4.
Cdc2-GFP Is Localized to a PPB-Like Structure.
(A) Fluorescent microscopic image of a cell with a band-like structure (left) and the corresponding DIC image showing characteristic features of a cell in late G2-phase, such as the centrally positioned nucleus with condensing chromatin (right). Bar = 10 μm.
(B) Optical sections of a cell having a PPB-like structure circumnavigating the cell. Three focal planes of 0, 2.4, and 6.4 μm are shown from images taken with a confocal laser scanning microscope. Bar = 10 μm.
(C) Time-lapse images showing Cdc2-GFP during preprophase progression for a 10-min interval. The corresponding DIC images are shown underneath. Bar = 10 μm.
Time-lapse images were taken to follow cells with PPB-like Cdc2-GFP structure. The narrow band disintegrated within 10 min, and the green fluorescence became concentrated around the nucleus, presumably close to the nuclear envelope (Figure 4C). However, when time-lapse observations were prolonged, we never found these cells entering into mitosis and they always showed features of apoptosis (irregular shape of the nuclei, spotty pattern of chromatin condensation, etc.). Because the PPB-like Cdc2-GFP signal was a rare event and preprophase cells are not readily recognizable with DIC microscopy, the extended exposure of cells to light damage was unavoidable in these experiments.
To determine if the Cdc2-GFP band was formed during a particular phase of the cell cycle, we synchronized cells by releasing them from an aphidicolin block and monitored the appearance of bands. No bands were found in cells blocked in S-phase by aphidicolin. Bands first appeared ∼8 hr after release and reached a peak ∼10 to 12 hr after release, just before mitotic entry. However, at the peak, the number of the Cdc2-GFP bands was considerably lower (∼1%) compared with the percentage of PPBs determined from immunofluorescence staining for microtubules with anti–α-tubulin antibodies (∼10%). The whole range of microtubular PPB stages was visualized by immunofluorescence: from broad PPBs at the beginning of its formation to narrow bands at the latest stage of their existence, not long before nuclear envelope breakdown (data not shown). The extremely narrow Cdc2-GFP–derived bands, and its low percentage compared with PPBs, further suggest that Cdc2-GFP associates only with a portion of the PPBs, probably only for a brief period during late G2-phase.
The broad spectrum kinase inhibitor K252-a accumulates cells with PPBs when added to synchronized cells in early G2-phase (Katsuta and Shibaoka, 1992). Using this drug, we could increase the number of PPBs in our culture (up to 30%), but Cdc2-GFP bands were completely absent. This may indicate that the Cdc2-GFP band forms just after the K252-a arrest point or that protein kinase activity is required for Cdc2 association with the PPB.
Cdc2-GFP Relocates from Chromatin to Microtubules in the Metaphase Spindle
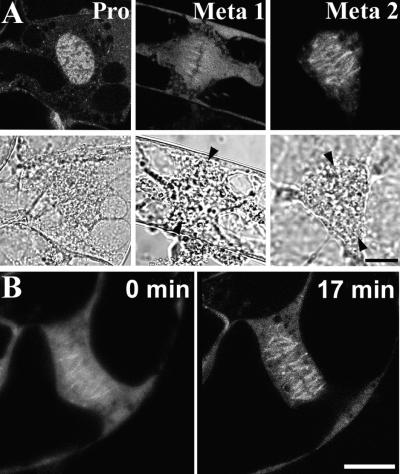
During prophase, which is indicated by the lack of visible nuclear envelope on the DIC image, Cdc2-GFP fluorescence remained in tight association with condensing chromatin (Figure 5A, Pro). Cdc2-GFP did not associate with any microtubule structures characteristic for this stage, such as the prophase spindle that forms at the two opposite poles around chromatin. The recruitment of Cdc2-GFP to microtubules began only in prometaphase, just before chromosome alignment at the metaphase plate, as judged by the DIC image (Figure 5A, Meta 1). At this stage, the Cdc2-GFP–chromatin association decreased and chromosomes were observed as negatively stained worm-like structures within a diffusely but brightly stained region that corresponded approximately to the spindle. At metaphase, much of the Cdc2-GFP–derived fluorescence took on a fibrillar appearance, probably as a result of association with the metaphase spindle (Figure 5A, Meta 2). In such cells, the chromosomes were aligned fully on the metaphase plate, as shown in the DIC image.
Figure 5.
Cdc2-GFP Relocates from Chromatin to the Metaphase Spindle.
(A) Fluorescent microscopic images of Cdc2-GFP in a prophase cell (Pro) and two metaphase cells (Meta 1 and Meta 2). The corresponding DIC images are shown underneath. Bar = 10 μm.
(B) Time-lapse images showing Cdc2-GFP during metaphase progression for a 17-min interval. Bar = 10 μm.
The dynamic recruitment of Cdc2-GFP to the metaphase spindle was demonstrated more clearly using time-lapse imaging. As shown in Figure 5B, the spindle staining was diffuse initially, but within 17 min, Cdc2-GFP accumulated progressively onto microtubule-like structures within the spindle.
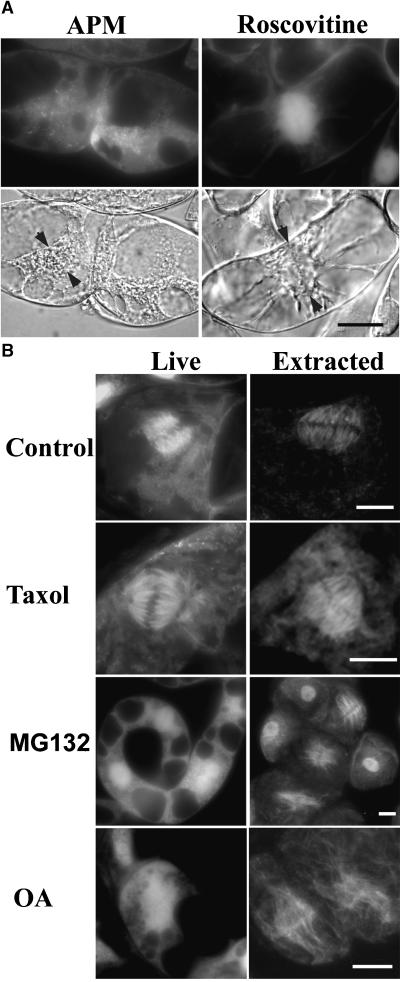
The spindle is a very complex structure, with a variety of components besides microtubules. To determine if the bright, microtubule-like Cdc2-GFP fluorescence was in fact microtubule dependent, we treated cells with the microtubule-depolymerizing drug amiprophos methyl. This treatment eliminated spindle-like Cdc2-GFP fluorescence and revealed that the signal was distributed diffusely in the whole cell (Figure 6A).
Figure 6.
Pharmacological Study of Cdc2-GFP Association with the Mitotic Spindle.
(A) Cdc2-GFP fluorescence in metaphase cells treated with 10 μM of the microtubule-depolymerizing drug amiprophos methyl (APM) or with 100 μM of the CDK inhibitor roscovitine. The corresponding DIC images are shown underneath. Arrowheads indicate the metaphase plates. Bar = 10 μm.
(B) Cdc2-GFP fluorescence in control metaphase cells and cells treated with 50 μM taxol, 100 μM MG132, and 0.2 μM okadaic acid (OA). Living cells are shown at left and cells extracted with 0.1% Triton X-100 are shown at right. Bars = 10 μm.
The accumulation of Cdc2-GFP on metaphase spindles may be related to cell cycle progression directly or indirectly as a result of changes in microtubule dynamics that occur at the metaphase/anaphase transition. To distinguish between these possibilities, we used two drugs that arrest cells in metaphase by different mechanisms. Microtubule turnover is required for chromosome separation, and taxol delays anaphase progression by stabilizing microtubules. MG132 inhibits the metaphase/anaphase transition by inhibiting the proteosome (Genschik et al., 1998). Both treatments resulted in the accumulation of arrested cells containing metaphase-like spindles. However, taxol dramatically intensified the fibrillar nature of Cdc2-GFP, whereas MG132 led to bright, diffuse staining in the spindle region.
Free GFP also accumulates in the region of the spindle, probably because of the increased concentration of all proteins in this region. To prove that Cdc2-GFP associates specifically with the mitotic spindle, we extracted nonfixed mitotic cells with low concentrations of nonionic detergent. Cdc2-GFP, but not GFP, remained associated with metaphase spindles in both taxol- and MG123-treated cells (Figure 6B, right), which demonstrates a strong and highly specific association with mitotic spindles. The different appearance of the spindle association of Cdc2-GFP in live and detergent-extracted cells might reflect the accumulation of Cdc2-GFP when proteolysis is blocked by MG132, or it might be the result of a high microtubule turnover in these cells.
To determine whether Cdc2 activity is required for its localization to spindles, we treated cells synchronized for mitosis with 100 μM roscovitine. This level of roscovitine is known to inhibit CDK-A kinase activity in vivo (Binarova et al., 1998). In roscovitine-treated cells, we never observed fibrillar Cdc2-GFP signals. In metaphase cells (as judged by DIC), only diffuse Cdc2-GFP fluorescence was observed in the spindle region (Figure 6A). Moreover, detergent extraction of roscovitine-treated cells completely removed all GFP signal from the spindle region, indicating that cdc2 kinase activity is required for both the premetaphase and postmetaphase association of CDK-A with the spindle. When roscovitine-treated cells were stained for microtubules, many abnormal spindles were observed, indicating that Cdc2 activity also is required for normal spindle structure. After prolonged treatments with roscovitine, a high proportion of binucleate cells appeared in the cultures. These observations are consistent with our previous conclusion that CDK-A kinase activity is required for normal mitotic progression in Vicia faba root tip cells (Binarova et al., 1998).
On the other hand, okadaic acid, which inhibits serine-threonine protein phosphatases (Wolniak and Larsen, 1992), resulted in the accumulation of fibrillar spindle–localized Cdc2-GFP. This accumulation was resistant to detergent extraction (Figure 6B). In most of these okadaic acid–treated cells, abnormally elongated anaphase spindles were apparent, suggesting that phosphorylation regulates not only the recruitment of Cdc2 to spindles but also microtubule turnover and stability.
Cdc2-GFP Accumulates at the Midzone of the Anaphase Spindle and Phragmoplast and Reassociates with Chromatin during Cytokinesis
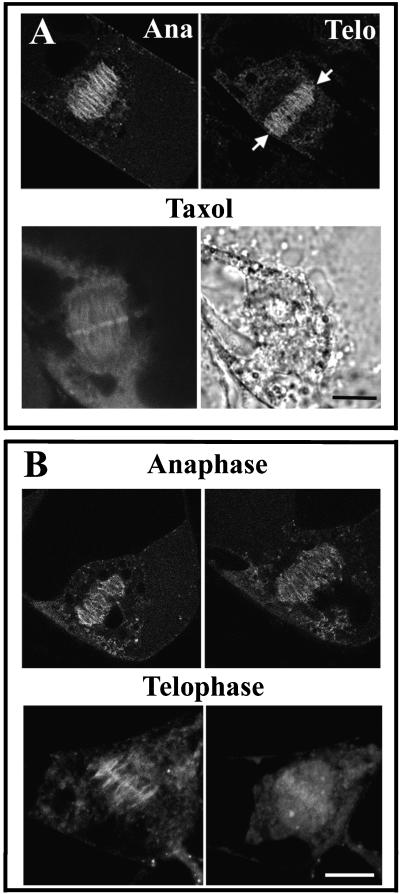
As chromosomes are aligned on the metaphase plate and become attached to the spindle, anaphase is initiated and sister chromatids are pulled apart and segregated into daughter cells. These events coincide with the proteolysis of mitotic cyclins in animal cells (Clute and Pines, 1999). Because in animal cells Cdc2 localization to microtubules is mediated by B-type cyclins, it was somewhat surprising to find that Cdc2-GFP remained associated with the spindle throughout anaphase in tobacco cells (Figure 7A, Ana). This association was restricted to the midzone of the anaphase spindle and was missing at the poles, although the pole microtubules were present and could be detected by immunofluorescence staining for tubulin in these cells (data not shown). This indicates that Cdc2-GFP associates only with a subset of microtubules during anaphase. Furthermore, when anaphase cells were treated with taxol to stabilize microtubules, the Cdc2-GFP–derived fluorescence accumulated at the midzone and became focused in a sharp line (Figure 7A, Taxol). Time-lapse studies performed on anaphase cells showed that Cdc2-GFP remained associated with the midzone anaphase spindle as the spindle elongated from anaphase A to anaphase B (Figure 7B, Anaphase).
Figure 7.
Cdc2-GFP Localization during Anaphase and Telophase.
(A) Cdc2-GFP fluorescence in anaphase (Ana) and telophase (Telo) cells is shown at top. Arrows indicate the accumulation of Cdc2-GFP signal on the midline of the phragmoplast in a telophase cell. The bottom images show an anaphase cell treated with taxol (left, fluorescent image; right, corresponding DIC image). The accumulation of Cdc2-GFP along the midline of the anaphase spindle was pronounced in taxol-treated cells. Bar = 10 μm.
(B) Time-lapse images showing Cdc2-GFP relocalization during 30-min intervals in an anaphase cell (top) and a telophase cell (bottom). Bar = 10 μm.
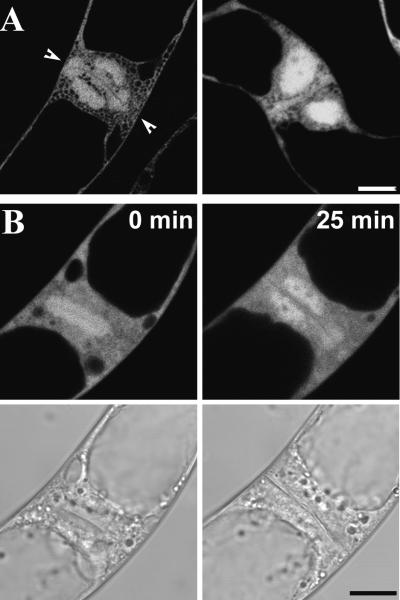
In late anaphase to telophase, a plant-specific cytokinetic apparatus, the phragmoplast, is built, and Cdc2-GFP–derived fluorescence also decorated this structure (Figure 7A, Telo). Again, this fluorescence did not distribute evenly with phragmoplast microtubules but was more concentrated at the midzone of the phragmoplast. The phragmoplast localization of Cdc2-GFP also was transient; time-lapse images suggested that it lasts ∼30 min, after which the signal becomes diffuse (Figure 7B, Telophase). At later stages in telophase, Cdc2-GFP began to reassociate with chromatin as the phragmoplast grew toward the cell periphery (Figure 8A). Time-lapse images showed that this event takes ∼25 min (Figure 8B). The appearance of Cdc2-GFP fluorescence on chromatin concurred with the formation of the cell wall and the reappearance of the nuclear envelope, as shown in the DIC image (Figure 8B, bottom).
Figure 8.
Reassociation of Cdc2-GFP with Chromatin in Late Telophase.
(A) Cdc2-GFP fluorescence is shown in two late telophase cells. Arrowheads indicate the forming cell wall. Bar = 10 μm.
(B) Time-lapse fluorescent images of Cdc2-GFP in a late telophase cell during a 25-min interval. The corresponding DIC images are shown underneath. Bar = 10 μm.
CDK-A as Well as Cdc2-GFP Copurifies with Microtubules
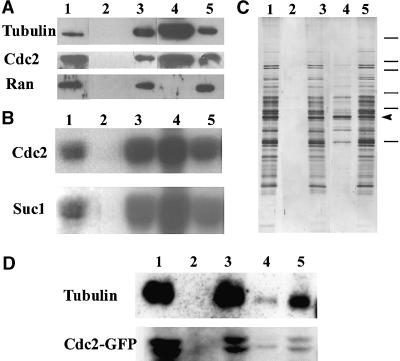
Our Cdc2-GFP and drug experiments indicated that CDK-A becomes associated progressively with subsets of microtubules during mitosis. To determine whether CDK-A actually binds to microtubules, we performed microtubule spin-down experiments. These depend on the use of taxol to polymerize endogenous tubulin from cell extracts. We used V. faba meristems for these experiments because they are rich in mitotic cells and because the taxol polymerization method has been optimized in this system. The microtubules and their associated proteins are then pelleted through a sucrose cushion. Proteins specifically cosedimenting with the polymerized microtubules were determined by silver staining and protein gel blotting with anti–α-tubulin and CDK-A–specific antibodies (Bögre et al., 1997). In the absence of taxol, no microtubule polymerization occurred; thus, both the α-tubulin and the CDK-A remained in the supernatant (Figure 9A). When taxol was added to the extracts to promote the formation of microtubules, a large amount of α-tubulin was found in the pellet together with an estimated 20 to 30% of the total CDK-A protein. The absence of any contamination from the abundant Ran protein (Moore and Blobel, 1993) in the microtubule pellet confirmed the specificity of the CDK-A–microtubule association (Figure 9A). Silver staining of a gel with these samples further indicated that specific proteins cosedimented with polymerized endogenous tubulin only when taxol was added to the extracts (Figure 9C).
Figure 9.
CDK and Cdc2-GFP Are Associated with Polymerized Microtubules in Microtubule Spin-Down Experiments.
(A) Protein gel blot analysis of protein extracts from V. faba root tips probed with anti-tubulin, anti-Cdc2, and anti-Ran antibodies. Lane 1, total cell extract; lanes 2 and 3, the pellet and supernatant, respectively, of total extract after high speed centrifugation in the absence of taxol; lanes 4 and 5, as in lanes 2 and 3 but with taxol.
(B) Histone H1 kinase activities in the same samples as in (A). CDK was purified from these samples by immunoprecipitation with an anti-Cdc2 antibody or by binding to p13suc1 and loaded in the same order as in (A). Histone H1 was used as a substrate.
(C) Silver-stained SDS-polyacrylamide gel of samples as described in (A). Molecular mass markers from top to bottom are 212, 116, 97.4, 66.2, 57.5, and 40 kD. The position of tubulin is marked with an arrowhead.
(D) Cdc2-GFP from tobacco cells is precipitated in a taxol-dependent manner. Samples were prepared and loaded in a similar manner as in (A) from suspension-cultured tobacco cells expressing Cdc2-GFP. Results of immunoblot analysis with anti-tubulin and anti–Cdc2-GFP antibodies are shown.
To determine the activity of CDK-A in the microtubule cosedimentation experiments, we purified CDK from the fractions by its binding to p13suc1 or by immunoprecipitation with anti–CDK-A–specific antibodies and assayed for its kinase activity. As expected, in the control, when no taxol was added to the extracts, all CDK activity remained in the supernatant, but when microtubules were polymerized with taxol within the extracts, a large proportion of activity was detected in the pellet (Figure 9B). This finding shows that the microtubule-bound CDK-A complex is an active kinase.
We also determined whether the Cdc2-GFP fusion protein associated with microtubules in extracts of tobacco cells. Because tobacco cells in culture were more vacuolated and thus resulted in a more diluted extract, less microtubule could be polymerized and sedimented (Figure 9D). Nevertheless, the Cdc2-GFP fusion protein cosedimented specifically with microtubules, as shown by the GFP-specific antibody on protein blots (Figure 9D). Interestingly, the lower Cdc2-GFP band was enriched in the microtubule-bound fraction, which could represent the active form of the protein.
DISCUSSION
We used GFP fluorescence to follow the location of a functional Cdc2-GFP fusion protein during the cell cycle of tobacco cells, showing that CDK-A is located at the nucleus and cytoplasm during interphase but associates with parts of the PPB, spindle, and phragmoplast during mitosis and cytokinesis. Time-lapse studies revealed that the association between Cdc2-GFP and microtubule-containing structures varied depending on the stage of mitosis and seemed to be related to the stage of mitosis, microtubule dynamics, and its own kinase activity. Finally, we demonstrated by in vitro microtubule cosedimentation assay that a significant fraction of the cdc2 protein can associate physically with microtubules, including active kinase complexes. Together, these data support the idea that Cdc2 protein kinase associates specifically with microtubules and plays an important role in microtubule organization during plant mitosis.
Functionality of the Cdc2-GFP Fusion Protein
The use of GFP fusion proteins to provide an accurate representation of any given protein depends on some assumptions regarding the ability of that fusion protein to substitute for the endogenous protein. Because we cannot easily undertake gene replacement experiments in plants, these assumptions must be tested in other, indirect ways. The PSTAIRE-containing CDKs from several plant species were shown to complement temperature-sensitive cdc2 alleles in Schizosaccharomyces pombe and CDC28 alleles in Saccharomyces cerevisiae (Colasanti et al., 1991; Hirt et al., 1991, 1993; Fobert et al., 1996). We show that the alfalfa MedsaCDK-A;2–GFP fusion protein and the wild-type MedsaCDK-A;2 gene can complement yeast CDC28 temperature-sensitive alleles equally well, suggesting that the addition of GFP to MedsaCDK-A;2 does not impair its function in yeast. Furthermore, when expressed in yeast or tobacco, neither MedsaCDK-A;2 nor its GFP fusion protein had any deleterious effects. Mutant forms of Cdc2 can interfere with cell division in both yeast and plants (Hemerly et al., 1995). Notably, the alfalfa Cdc2-GFP fusion protein locates correctly to the same subcellular locations, the nucleus and spindle, as the endogenous yeast CDC28 protein (Alfa et al., 1990). Finally, the Cdc2-GFP fusion protein is very similar biochemically to the endogenous plant protein in that it binds to the yeast Cdc2 interactor, suc1, and is an active histone H1 kinase. Together, these data demonstrate that Cdc2-GFP is fully functional and likely is a good reporter for the endogenous Cdc2.
Cdc2 Localization during Interphase
Our data show that Cdc2-GFP accumulates at several subcellular locations, including the nucleus, the PPB, the spindle, and the phragmoplast. This confirms and clarifies the observations made using CDK-A antibodies on fixed cells (Colasanti et al., 1993; Bögre et al., 1997; Mews et al., 1997; Stals et al., 1997). Given the problems intrinsic to immunolocalization in plants, the complexity of cdc2 staining patterns has led to considerable debate regarding the true localization. We have been able to follow living cells during key transitions in the cell cycle, revealing the dynamic association between cdc2 and subsets of microtubules.
During interphase, the pattern of Cdc2-GFP localization is superficially similar to that of GFP. GFP is believed to enter the nucleus by passive diffusion, because its size is just at the discrimination limit of the nuclear pore. However, the Cdc2-GFP fusion protein is 60 kD and should be excluded effectively from the nucleus, suggesting active nuclear uptake. We also have presented evidence that Cdc2-GFP is retained tightly within the nucleus, probably by its association with chromatin, because detergent and salt extraction failed to release the nuclear Cdc2-GFP. Similar treatments completely released GFP from the nucleus. Strong Cdc2 binding to chromatin has been reported in animal cells (Bailly et al., 1989). In alfalfa interphase cells, we have shown that the nucleus-localized CDK-A (but not the cytoplasm-localized CDK-A) is an active kinase (Bögre et al., 1997). Sequestration of CDK-A to the cytoplasm therefore may prevent its activation, as is the case in checkpoint-arrested yeast and mammalian cells (Kumagai and Dunphy, 1999). The distribution of Cdc2-GFP responds to sucrose or phosphate starvation by becoming more cytoplasmic (M. Weingartner, unpublished results), further indicating that CDK-A localization is regulated actively.
The association of a minor part of the Cdc2-GFP pool with interphase microtubules cannot be excluded, but it appears to be under the detection limit of GFP-derived fluorescence and might be masked by the abundant cytoplasmic pool of Cdc2-GFP. However, when the cytoplasmic pool was extracted with detergent, we observed a fluorescent halo around the nucleus, and we have preliminary data that Cdc2 copurifies with microtubules polymerized from extracts of G1-arrested cells, although in much smaller quantities than from mitotic extracts (P. Binarova, unpublished data). In nondividing brain cells, CDK-related kinases, their regulators, and phosphatases are transported dynamically and assembled into multimeric complexes with cytoskeleton proteins at specific locations; thus, they regulate cell shape (Veeranna et al., 2000). Similar spatial and temporal control of cytoplasm- and membrane-associated microtubule nucleation and organization might operate in large acentrosomal plant cells in which various microtubular arrays change their positions during cell cycle progression.
Preprophase Band
The PPB, which is formed at the cell surface just before mitosis, predicts the plane of cell division but disassembles just before mitosis (Mineyuki, 1999). Recruitment of CDK-A to the PPB seems to be a transient event just before the disassembly of the PPB. Cdc2-GFP, like CDK-A, is found with only a small proportion of PPB (Colasanti et al., 1993; Mews et al., 1997). We determined when Cdc2-GFP localized to the PPB by cell cycle synchronization and arrest with the protein kinase inhibitor K252-a. K252-a arrests cells in G2-phase and accumulates cells with PPBs (Katsuta and Shibaoka, 1992). However, we never observed Cdc2-GFP associating with PPB in K252-a–arrested cells, indicating that the CDK-A association depends on the phosphorylation of some component in the PPB or that the association occurs after the arrest point of K252-a. The extremely narrow configuration of the Cdc2-GFP band and its disappearance within a short time interval also suggest that Cdc2-GFP is associated with a late PPB structure. Correspondingly, one of the maize mitotic cyclins, Zeama;CycB1;2, associates transiently with the late PPB, whereas Zeama;CycA1;1 is localized to the PPB over a longer period (Mews et al., 1997). Microinjection of active CDK-A kinase to plant cells before division selectively accelerated the disassembly of the PPB but not of other microtubule structures (Hush et al., 1996), whereas the inhibition of CDKs by the drug roscovitine delayed this process (Binarova et al., 1998). Together, these data suggest that CDK-A directly induces the disassembly of PPB in preprophase.
Cdc2 Association with the Spindle
Cdc2-GFP did not accumulate on the prophase spindle but remained associated with condensing chromosomes. At this stage of the cell cycle, Cdc2 is involved in many important cellular processes, including nuclear envelope breakdown and chromatin condensation. The microinjection of active Cdc2 induces premature condensation of chromatin (Hush et al., 1996), whereas roscovitine inhibition of CDK activity delays it and prevents nuclear envelope breakdown (Binarova et al., 1998). As cells approach metaphase, Cdc2-GFP leaves the chromatin and is recruited progressively to the spindle, which seems to peak at metaphase. The interaction between CDK-A and plant spindle microtubules appeared to be strong, because it was resistant to detergent extraction and could be cosedimented with taxol-stabilized microtubules from cell extracts. This unusually stable interaction between cdc2 and microtubules might relate to plant-specific spindle formation without the spatial cue of centrosomes, where the bipolar spindle configuration from poles to kinetochores requires the selective stabilization and movement of microtubules.
Cytokinesis
The CDK complex is inactivated at the metaphase/anaphase transition by the degradation of the cyclin component by the mitosis-specific protein degradation machinery, the anaphase-promoting complex (Genschik et al., 1998). The degradation of cyclins containing the mitotic destruction box was confirmed using Cyclin B1–GFP, Cyclin B2–GFP, and Cyclin A2–GFP fusion proteins (M. Weingartner, unpublished results). However, a curious feature of some plant cyclins, such as Zeama;CycB1;1, Zeama;CycB2;1, and Zeama;CycA1;1, is their prolonged presence through anaphase and telophase in association with mitotic cytoskeleton structures, as revealed by immunolocalization studies (Mews et al., 1997). The cyclin degradation machinery is thought to associate with chromatin, and chromatin-bound cyclin is located appropriately for degradation (Clute and Pines, 1999). Thus, the recruitment of the CDK-A–cyclin complex to spindle and phragmoplast microtubules might protect a pool of cyclins from degradation by sequestering them away from the chromatin-located proteolysis machinery. Alternatively, CDK-A might associate with cyclins in anaphase and telophase, which do not contain a mitotic destruction box, and thus might not be targeted by the mitotic proteolysis machinery. Interestingly, it has been shown that the CycD2;1 and CycD3;1 transcripts accumulate during mitosis (Sorrell et al., 1999).
An important role for CDK-A in regulating cytoskeleton structures is prompted by its specific locations in anaphase and telophase. Cdc2-GFP is present at the midzone of the anaphase spindle, where new populations of microtubules assemble, but it is missing at the poles on the shortening kinetochore microtubules. Later, it accumulates at the midplane of cell division at the end of anaphase and along the midline of forming phragmoplast microtubules. Even though cytokinesis appears mechanistically different in plants and animals, recent studies indicate that the midplane of cell division might play a conserved role for signaling division sites during cytokinesis in plant, fungal, and animal cells, and a number of regulatory proteins accumulate at the spindle midzone during cytokinesis (Hales et al., 1999; Robinson and Spudich, 2000). In plant cells, two related mitogen-activated protein kinases (MAPKs), MMK3 from alfalfa and NTF6 from tobacco, were found transiently at the midplane of cell division by immunofluorescence (Calderini et al., 1998; Bögre et al., 1999). A potential upstream regulator of these MAPKs is NPK1, which also localizes to the midplane (Nishihama et al., 2001). NPK1 has a potential Cdc2 phosphorylation site and therefore is a putative Cdc2 kinase substrate. Another regulator of NPK1 is a mitosis-specific kinesin (Ito et al., 1998; Nishihama et al., 2001). The role and targets of this cytokinesis signaling pathway are not well understood, but it might regulate MAPs, such as MAP65 (Smertenko et al., 2000), or molecular motors, such as TPRK125 (Asada et al., 1997; Barroso et al., 2000) and AtPAKRP1 (Lee and Liu, 2000), which are located similarly at the midplane of cell division.
Modulation of Microtubule Dynamics and Movements by CDKs
Microtubules are intrinsically unstable structures because of a continuous flux of tubulin between “free” tubulin dimers and polymerized microtubules, and it is this dynamic instability that makes the microtubule cytoskeleton such an adaptable feature of cellular organization. There are growing numbers of microtubule-interacting proteins known in animal cells that modulate microtubule polymerization and dynamics and that are modified by cell cycle–dependent phosphorylation (Desai and Mitchison, 1997). Cyclin B1–CDK, when added to Xenopus laevis egg extracts, induced the dramatic shortening of microtubules, which was correlated with a severalfold increase in catastrophe frequency (Verde et al., 1992). Similarly, interphase but not mitotic plant extracts promoted the polymerization of long microtubules (Stoppin et al., 1996). In animal cells, the phosphorylation of MAP4 by Cdc2 results in its reduced affinity for microtubules during mitosis, which leads to greater microtubule instability (Ookata et al., 1997). Correspondingly, the elimination of cdc2 phosphorylation sites on MAP4 maintains its affinity for microtubules during mitosis and leads to defects in anaphase A chromosome movement (Shiina and Tsukita, 1999), similar to when plant Cdc2 kinase is inhibited with roscovitine (Binarova et al., 1998). XMAP215 is another MAP isolated from X. laevis eggs containing two conserved CDK1 phosphorylation sites. Its human homolog, pTOG, has been found to interact with Cyclin B1, thus targeting the Cdc2 kinase to microtubules during mitosis (Charrasse et al., 2000). However, unlike other MAPs isolated thus far, mitotic phosphorylation of XMAP215 by CDK1 does not reduce its microtubule binding but strongly decreases its activity to promote microtubule elongation (Vasquez et al., 1999). The stable interaction of Cdc2 with the spindle in our experiments could be attributable to a MAP with similar characteristics.
Plant microtubules are at least as dynamic as those in animals, are regulated similarly by MAPs, and are determined by their composition (plants contain multiple copies of functional α- and β-tubulin genes) and by various post-translational modifications (Lloyd and Hussey, 2001). Structural MAPs are just being molecularly identified in plants, and it is apparent that plant MAPs are highly specialized to selectively bind and regulate subsets of microtubules at specific locations. For example, NtMAP65-1 is bound selectively to a subset of interphase and certain mitotic microtubules (Smertenko et al., 2000). The apparent similarity in Cdc2 and NtMAP65-1 localizations (e.g., at the PPB, at the midzone of the anaphase spindle, and at the midline of the phragmoplast) might suggest a functional relationship.
Microtubule-associated motor proteins are essential in spindle assembly and function and localize to the kinetochores, the spindle poles, and the spindle midzone in animal and fungal cells. The minus end–directed motor, dynein, which is important for chromatin-mediated spindle assembly, was shown to be phosphorylated in mitosis by the Cdc2 kinase (Dell et al., 2000). HsEg5 from human cells and XLP2 from X. laevis are plus end–directed kinesin-related proteins (KRPs), which are required for centrosome separation, spindle assembly, and maintenance and which are controlled by cdc2/p34 phosphorylation (Blangy et al., 1995). Phosphorylation of HsEg5 increases during mitosis and promotes its spindle association. Depleting Eg5 results in monopolar spindles very similar to those found in roscovitine-treated plant cells (Binarova et al., 1998).
Three genes that encode KRPs have been identified in various plant species, and one of them, TKRP125, contains a conserved cdc2 phosphorylation site and belongs to the same bimC subfamily of KRPs, like Eg5 (Asada et al., 1997; Barroso et al., 2000). This plus end–directed motor protein localizes to the mitotic spindle at increasing levels toward the equatorial plane, to the interzone of the anaphase spindle, and to the phragmoplast. One of the suggested functions of TKRP125 is the sliding of interzone microtubules, resulting in the elongation of the anaphase spindle. Recording time-lapse images, we observed a similarly stable colocalization of Cdc2-GFP with interzone microtubules during the elongation of the anaphase spindle. Moreover, the stabilization of microtubules by taxol led to the focusing of Cdc2-GFP fluorescence to the midline, suggesting movement toward the plus end of the microtubules. Therefore, TKRP125 might be another potential binding partner and substrate that targets the Cdc2 kinase to spindle microtubules.
The localization of Cdc2-GFP in living cells, its physical interaction with microtubules, and the phenotypes of cells when Cdc2 is inhibited by roscovitine provide insights into the role of Cdc2 during mitosis. Our detailed pharmacological studies suggest that Cdc2 might regulate microtubule stability and movement. The Cdc2-GFP fusion protein and the assays we developed, together with the molecular probes becoming available, will allow further testing of Cdc2 functions in the regulation of MAPs and motor proteins.
METHODS
Plasmid Construction and Transformation Techniques
The S65T mutant of the plant codon–optimized synthetic Green Fluorescent Protein (GFP; Chiu et al., 1996) with a unique NcoI site at the initiation codon was obtained from Toni Schäffer (Ludwig-Maximilians-Universität, Munich, Germany). The GFP coding region was recloned as an EcoRI–XhoI fragment into the yeast shuttle vector pYES2 (Stratagene, La Jolla, CA) and as a KpnI–SalI fragment into the tetracycline-inducible binary plant expression vector pBinHygTx (Weinmann et al., 1994).
A chimeric Cdc2-GFP fusion was constructed by cloning the GFP gene as an NcoI–XhoI fragment into plasmid pBS-cdc2 carrying the Medsa;CDK-A;2 coding region with an inserted unique NcoI site at the 3′ terminus. The cdc2-GFP fusion gene was subcloned as a BstXI–XhoI fragment into the pYES2 yeast shuttle vector (pYES2-cdc2-GFP) and as a KpnI–SalI fragment into the tetracycline-inducible binary plant expression vector (pBinHygTx-cdc2-GFP). Yeast transformation and complementation assays were performed as described previously (Hirt et al., 1993). Transgenic plants were obtained by Agrobacterium tumefaciens–mediated leaf disc transformation with the tobacco (Nicotiana tabacum) lines Petit Havana SR1 and Samsung Tb-Tet (Weinmann et al., 1994). Five independent transformants for each construct were propagated, and seed were obtained. The hygromycin-resistant progeny were used in the experiments.
Cell Culture and Synchronization
Suspension cultures were initiated by placing stem segments of transgenic Cdc2-GFP and GFP lines of T2 generation in 1 mg·L−2 2,4-D containing Murashige and Skoog (1962) solid medium, and the resulting calli were transferred after 1 month into liquid Linsmaier and Skoog–modified medium (Sano et al., 1999) supplemented with 40 μg·mL−2 hygromycin B.
Synchronization started with a 1:5 dilution of 7-day-old culture. After 8 hr, 10 μg·L−2 aphidicoline was added to the medium. The aphidicoline was removed after 16 hr by five changes of medium in 1 hr. Drugs were added 7 hr after aphidicoline removal in G2-phase or at 10 hr in mitosis. The following drug concentrations were used in these experiments: 10 μM amiprophos methyl, 50 μM taxol, 100 μM roscovitine (a gift from M. Strnad, Olomouc, Czech Republic), 0.2 μM okadaic acid, 2 μM K252a, and 100 μM MG132. Samples were collected at various intervals and used immediately for observation. To follow cell cycle progression, flow cytometric analysis was performed as described (Bögre et al., 1997) using a PAS2 flow cytometer (Partec, Münster, Germany).
Microscopy Techniques for GFP Detection
A drop of cell suspension was transferred on a slide, carefully covered with a cover slip, and observed with an upright fluorescence microscope (Axioplan 2; Zeiss, Jena, Germany) equipped with a GFP filter (HQ480/20X, HQ510/20M; AF Analysentechnik, Jena, Germany). Images were taken using a cooled charge-coupled device black-and- white digital camera (SPOT-2; Diagnostic Instruments, Burroughs, MI) and Metaview imaging software (Diagnostic Instruments).
Confocal images were taken using a Leica (Wetzlar, Germany) TNT laser scanning confocal microscope with argon laser illumination at 488 nm and through a fluorescein isothiocyanate filter set. For transmission light images, differential interference contrast optics were used with both the Zeiss and Leica microscopes. To reduce photobleaching, the excitation light was turned on only during image capture. Images were contrast enhanced using image-processing software (Photoshop; Adobe Systems, Mountain View, CA).
Detergent Extraction of Cells
Cells were extracted in detergent essentially as described previously for the preparation of cytoskeletons (Chan et al., 1996). Cells were incubated in the enzyme solution (1% [w/v] cellulase R10, 0.2% [w/v] mazerozyme R10 [Yakult, Tokyo, Japan], and 0.45 M sorbitol) in PME (0.1 M 1,4-piperazinediethanesulfonic acid, 1 mM MgSO4, and 1 mM EGTA, pH 6.9) for 15 min. Cells with partially digested cell walls were washed twice in PME with 0.45 M sorbitol and then incubated in extraction buffer (10% [w/v] DMSO, 0.05% [w/v] Nonidet P-40, and 0.45 M sorbitol in PME) for 15 min. The resulting detergent-extracted cells were washed twice in wash 2 (10% DMSO in PME) and observed directly with the fluorescence microscope.
Protein Blotting, Immunoprecipitation, p13Suc1 Binding, and Histone H1 Kinase Assay
The samples were homogenized in 3 volumes of homogenization buffer containing 25 mM Tris-HCl, pH 7.5, 15 mM MgCl2, 15 mM EGTA, 75 mM NaCl, 1 mM DTT, 0.1% (w/v) Tween 20, 15 mM 4-nitrophenylphosphatebis, 15 mM β-glycerophosphate, 0.5 mM Na3VO3, 1 mM NaF, 0.5 mM phenylmethylsulfonyl fluoride, and 5 μg/mL leupeptin and aprotinin. After centrifugation at 100,000g, the supernatant was collected and the protein concentration was measured with the Bradford reagent and adjusted to 2 mg·mL−2. SDS sample buffer was added, and the samples were heated for 2 min and stored at −20°C. After electrophoresis, the gels were electroblotted onto polyvinylidene difluoride (Millipore, Bedford, MA) membranes in 50 mM Tris base and 50 mM boric acid buffer, pH 8.3, in a liquid electroblotting system (Hoefer, San Francisco, CA) overnight at 30 V with cooling. For immunodetection, an anti-GFP rabbit serum (Molecular Probes, Eugene, OR) was applied in blocking buffer at a dilution of 1:1000 and alkaline phosphatase–conjugated goat anti–rabbit IgG (Sigma) was used as a second antibody in a dilution of 1:5000. The reaction was visualized with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrates (CDP-Star; Amersham).
Cyclin-dependent kinase (CDK) activities were measured in the samples after immunoprecipitation with the anti-Cdc2 antibody or the anti-GFP antibody or after binding to p13suc1 as described previously (Bögre et al., 1997). For GFP immunoprecipitation, 15 μL of mouse anti-GFP hybridoma supernatant (a gift from Jan Elliott, Australian National University, Canberra) and 25 μL of anti-mouse Ig agarose beads (Sigma) were added to the cell extracts and the tubes were rotated for 3 to 4 hr. Washing conditions were the same as for Cdc2 (Bögre et al., 1997).
Microtubule Cosedimentation Assay
Extracts of Vicia faba root meristem or tobacco-cultured cells were homogenized in Hepes buffer containing 50 mM Hepes, pH 7.4, 1 mM EGTA, 1 mM MgCl2, 75 mM NaCl, 1 mM DTT, 0.1% (w/v) Tween 20, 15 mM 4-p-nitrophenylphosphate, 15 mM β-glycerophosphate, 0.5 mM Na3VO3, 1 mM NaF, 0.5 mM phenylmethylsulfonyl fluoride, and 5 μg·mL−2 leupeptin and aprotinin. The samples were centrifuged at 27,000g for 60 min. To polymerize microtubules in the extract, GTP was added to 1 mM and taxol was added to 20 μM. After 15 min of polymerization, the extracts were loaded on top of the cushion of Hepes buffer with 40% sucrose and centrifuged at 20,000g for 40 min. After three washes with Hepes buffer, the pellet was resuspended in 50 μL of SDS sample buffer and loaded for electrophoresis and protein gel blotting. CDK activity measurements were made by resuspending the pellets in the same volume as the original extracts and using equal volumes for immunoprecipitations and p13suc1 binding, followed by protein kinase assay as described previously (Bögre et al., 1997). Monoclonal mouse anti–α-tubulin, DMA1 (Sigma), polyclonal rabbit anti–CDK-A (Bögre et al., 1997), polyclonal anti-Ran (Babco, Richmond, CA), and monoclonal mouse anti-GFP (provided by Jan Elliott) antibodies were used for immunodetection.
Acknowledgments
Thanks to J. Elliott for providing the GFP antibody and to F. Klein (Botany Institute, Vienna University) for providing the epifluorescence microscopy facility at the initial phase of the project. Moreover, we thank Gireg Weingartner for his incredible patience while finishing this work. M.W. was supported by a Ph.D. fellowship from the Austrian Academy of Sciences. This work was supported by an Austrian Förderung der Wissenschaftlichen Forschung grant to E.H.-B., by European Union Framework V project ECCO Grant QLRT-1999-00454 to E.H.-B., L.B., and P.B., by Grant A5020803/1998 from the Grant Agency of the Czech Academy of Sciences to P.B., and by a Biotechnology and Biological Science Research Council grant to L.B. and J.-P.D.
References
- Alfa, C.E., Ducommun, B., Beach, D., and Hyams, J.S. (1990). Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature 347, 680–682. [DOI] [PubMed] [Google Scholar]
- Asada, T., Kuriyama, R., and Shibaoka, H. (1997). TKRP125, a kinesin-related protein involved in the centrosome-independent organization of the cytokinetic apparatus in tobacco BY-2 cells. J. Cell Sci. 110, 179–189. [DOI] [PubMed] [Google Scholar]
- Ayaydin, F., Vissi, E., Meszaros, T., Miskolczi, P., Kovacs, I., Feher, A., Dombradi, V., Erdodi, F., Gergely, P., and Dudits, D. (2000). Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M tran-sition and early mitotic microtubule organisation in alfalfa. Plant J. 23, 85–96. [DOI] [PubMed] [Google Scholar]
- Bailly, E., Doree, M., Nurse, P., and Bornens, M. (1989). p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 8, 3985–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso, C., Chan, J., Allan, V., Doonan, J., Hussey, P., and Lloyd, C. (2000). Two kinesin-related proteins associated with the cold-stable cytoskeleton of carrot cells: Characterization of a novel kinesin, DcKRP120–2. Plant J. 24, 859–868. [DOI] [PubMed] [Google Scholar]
- Binarova, P., Dolezel, J., Draber, P., Heberle-Bors, E., Strnad, M., and Bogre, L. (1998). Treatment of Vicia faba root tip cells with specific inhibitors to cyclin-dependent kinases leads to abnormal spindle formation. Plant J. 16, 697–707. [DOI] [PubMed] [Google Scholar]
- Blangy, A., Lane, H.A., d'Herin, P., Harper, M., Kress, M., and Nigg, E.A. (1995). Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Bögre, L., Zwerger, K., Meskiene, I., Binarova, P., Czizmadia, V., Planck, C., Wagner, E., Hirt, H., and Heberle-Bors, E. (1997). The cdc2Ms kinase is differently regulated in the cytoplasm and in the nucleus. Plant Physiol. 113, 841–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögre, L., Calderini, O., Binarova, P., Mattauch, M., Till, S., Kiegerl, S., Jonak, C., Pollaschek, C., Barker, P., Huskisson, N.S., Hirt, H., and Heberle-Bors, E. (1999). A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell 11, 101–114. [PMC free article] [PubMed] [Google Scholar]
- Calderini, O., Bogre, L., Vicente, O., Binarova, P., Heberle-Bors, E., and Wilson, C. (1998). A cell cycle regulated MAP kinase with a possible role in cytokinesis in tobacco cells. J. Cell Sci. 111, 3091–3100. [DOI] [PubMed] [Google Scholar]
- Chan, J., Rutten, T., and Lloyd, C. (1996). Isolation of microtubule-associated proteins from carrot cytoskeletons: A 120 kDa map decorates all four microtubule arrays and the nucleus. Plant J. 10, 251–259. [DOI] [PubMed] [Google Scholar]
- Charrasse, S., Lorca, T., Doree, M., and Larroque, C. (2000). The Xenopus XMAP215 and its human homologue TOG proteins interact with cyclin B1 to target p34cdc2 to microtubules during mitosis. Exp. Cell Res. 254, 249–256. [DOI] [PubMed] [Google Scholar]
- Chiu, W., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., and Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6, 325–330. [DOI] [PubMed] [Google Scholar]
- Chytilova, E., Macas, J., Sliwinska, E., Rafelski, S.M., Lambert, G.M., and Galbraith, D.W. (2000). Nuclear dynamics in Arabidopsis thaliana. Mol. Biol. Cell 11, 2733–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute, P., and Pines, J. (1999). Temporal and spatial control of cyclin B1 destruction in metaphase. Natl. Cell Biol. 1, 82–87. [DOI] [PubMed] [Google Scholar]
- Colasanti, J., Tyers, M., and Sundaresan, V. (1991). Isolation and characterization of cDNA clones encoding a functional p34cdc2 homologue from Zea mays. Proc. Natl. Acad. Sci. USA 88, 3377–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti, J., Cho, S.-O., Wick, S., and Sundaresan, V. (1993). Localisation of the functional p34cdc2 homolog of maize in the root tip and stomatal complex cells: Association with predicted division sites. Plant Cell 5, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell, K.R., Turck, C.W., and Vale, R.D. (2000). Mitotic phosphorylation of the dynein light intermediate chain is mediated by cdc2 kinase. Traffic 1, 38–44. [DOI] [PubMed] [Google Scholar]
- Desai, A., and Mitchison, T.J. (1997). Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117. [DOI] [PubMed] [Google Scholar]
- Doonan, J. (2000). Social controls on cell proliferation in plants. Curr. Opin. Plant Biol. 3, 482–487. [DOI] [PubMed] [Google Scholar]
- Doonan, J., and Fobert, P. (1997). Conserved and novel regulators of the plant cell cycle. Curr. Opin. Cell Biol. 9, 824–830. [DOI] [PubMed] [Google Scholar]
- Ferreira, P.C., Hemerly, A.S., Villarroel, R., Van Montagu, M., and Inze, D. (1991). The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell 3, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fobert, P.R., Gaudin, V., Lunness, P., Coen, E.S., and Doonan, J.H. (1996). Distinct classes of cdc2-related genes are differentially expressed during the cell division cycle in plants. Plant Cell 8, 1465–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschik, P., Criqui, M.C., Parmentier, Y., Derevier, A., and Fleck, J. (1998). Cell cycle-dependent proteolysis in plants: Identification of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor mg132. Plant Cell 10, 2063–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales, K.G., Bi, E., Wu, J.Q., Adam, J.C., Yu, I.C., and Pringle, J.R. (1999). Cytokinesis: An emerging unified theory for eukaryotes? Curr. Opin. Cell Biol. 11, 717–725. [DOI] [PubMed] [Google Scholar]
- Healy, J.M., Menges, M., Doonan, J.H., and Murray, J.A. (2000). The Arabidopsis D-type cyclins CycD2 and CycD3 both interact in vivo with the PSTAIRE cyclin-dependent kinase Cdc2a but are differentially controlled. J. Biol. Chem. 276, 7041–7047. [DOI] [PubMed] [Google Scholar]
- Hemerly, A., Engler, J.A., Bergounioux, C., Van Montagu, M., Engler, G., Inze, D., and Ferreira, P. (1995). Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J. 14, 3925–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt, H., Pay, A., Gyorgyey, J., Bako, L., Nemeth, K., Bogre, L., Schweyen, R.J., Heberle-Bors, E., and Dudits, D. (1991). Complementation of a yeast cell cycle mutant by an alfalfa cDNA encoding a protein kinase homologous to p34cdc2. Proc. Natl. Acad. Sci. USA 88, 1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt, H., Pay, A., Bogre, L., Meskiene, I., and Heberle-Bors, E. (1993). cdc2MsB, a cognate cdc2 gene from alfalfa, complements the G1/S but not the G2/M transition of budding yeast cdc28 mutants. Plant J. 4, 61–69. [DOI] [PubMed] [Google Scholar]
- Hush, J., Wu, L., John, P.C., Hepler, L.H., and Hepler, P.K. (1996). Plant mitosis promoting factor disassembles the microtubule preprophase band and accelerates prophase progression in Tradescantia. Cell Biol. Int. 20, 275–287. [DOI] [PubMed] [Google Scholar]
- Ito, M., Iwase, M., Kodama, H., Lavisse, P., Komamine, A., Nishihama, R., Machida, Y., and Watanabe, A. (1998). A novel cis-acting element in promoters of plant B-type cyclin genes activates M phase-specific transcription. Plant Cell 10, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubes, J., Chevalier, C., Dudits, D., Heberle-Bors, E., Inze, D., Umeda, M., and Renaudi, J.P. (2000). CDK-related protein kinases in plants. Plant Mol. Biol. 43, 607–620. [DOI] [PubMed] [Google Scholar]
- Katsuta, J., and Shibaoka, H. (1992). Inhibition by kinase inhibitors of the development and the disappearance of the preprophase band of microtubules in tobacco BY-2 cells. J. Cell Sci. 103, 397–405. [Google Scholar]
- Kumagai, A., and Dunphy, W.G. (1999). Binding of 14–3-3 proteins and nuclear export control the intracellular localization of the mitotic inducer Cdc25. Genes Dev. 13, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.R., and Liu, B. (2000). Identification of a phragmoplast-associated kinesin-related protein in higher plants. Curr. Biol. 10, 797–800. [DOI] [PubMed] [Google Scholar]
- Leiss, D., Felix, M.A., and Karsenti, E. (1992). Association of cyclin-bound p34cdc2 with subcellular structures in Xenopus eggs. J. Cell Sci. 102, 285–297. [DOI] [PubMed] [Google Scholar]
- Lloyd, C., and Hussey, P. (2001). Microtubule-associated proteins in plants: Why we need a map. Natl. Rev. Mol. Cell Biol. 2, 41–47. [DOI] [PubMed] [Google Scholar]
- Magyar, Z., et al. (1997). Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells. Plant Cell 9, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mews, M., Sek, F.J., Moore, R., Volkmann, D., Gunning, B.E.S., and John, P.C.L. (1997). Mitotic cyclin distribution during maize cell division: Implications for the sequence diversity and function of cyclins in plants. Protoplasma 200, 128–145. [Google Scholar]
- Mineyuki, Y. (1999). The preprophase band of microtubules: Its function as a cytokinetic apparatus in higher plants. Int. Rev. Cytol. 187, 1–50. [Google Scholar]
- Mineyuki, Y., Yamashita, M., and Nagahama, Y. (1991). P34cdc2 kinase homologue in the preprophase band. Protoplasma 162, 182–186. [Google Scholar]
- Mineyuki, Y., Aioi, H., Yamashita, M., and Nagahama, Y. (1996). A comparative study on stainability of preprophase bands by the PSTAIR antibody. J. Plant Res. 109, 185–192. [Google Scholar]
- Mironov, V., De Veylder, L., Van Montagu, M., and Inze, D. (1999). Cyclin-dependent kinases and cell division in plants: The nexus. Plant Cell 11, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.S., and Blobel, G. (1993). The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature 365, 661–663. [DOI] [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497. [Google Scholar]
- Nishihama, R., Ishikawa, M., Araki, S., Soyano, T., Asada, T., and Machida, Y. (2001). The NPK1 mitogen-activated protein kinase kinase kinase is a regulator of cell-plate formation in plant cytokinesis. Genes Dev. 15, 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookata, K., Hisanaga, S., Sugita, M., Okuyama, A., Murofushi, H., Kitazawa, H., Chari, S., Bulinski, J.C., and Kishimoto, T. (1997). MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells: Identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry 36, 15873–15883. [DOI] [PubMed] [Google Scholar]
- Pines, J. (1999). Four-dimensional control of the cell cycle. Natl. Cell Biol. 1, 73–79. [DOI] [PubMed] [Google Scholar]
- Planchais, S., Glab, N., Trehin, C., Perennes, C. Bureau, J.M., Meijer, L., and Bergounioux, C. (1997). Roscovitine, a novel cyclin-dependent kinase inhibitor, characterizes restriction point and G2/M transition in tobacco BY-2 cell suspension. Plant J. 12, 191–202. [DOI] [PubMed] [Google Scholar]
- Riabowol, K., Draetta, G., Brizuela, L., Vandre, D., and Beach, D. (1989). The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell 57, 393–401. [DOI] [PubMed] [Google Scholar]
- Robinson, D.N., and Spudich, J.A. (2000). Towards a molecular understanding of cytokinesis. Trends Cell. Biol. 10, 228–237. [DOI] [PubMed] [Google Scholar]
- Roudier, F., Fedorova, E., Gyorgyey, J., Feher, A., Brown, S., Kondorosi, A., and Kondorosi, E. (2000). Cell cycle function of a Medicago sativa A2-type cyclin interacting with a PSTAIRE-type cyclin-dependent kinase and a retinoblastoma protein. Plant J. 23, 73–83. [DOI] [PubMed] [Google Scholar]
- Sano, T., Kuraya, Y., Amino, S., and Nagata, T. (1999). Phosphate as a limiting factor for the cell division of tobacco BY-2 cells. Plant Cell Physiol. 40, 1–8. [DOI] [PubMed] [Google Scholar]
- Shiina, N., and Tsukita, S. (1999). Mutations at phosphorylation sites of Xenopus microtubule-associated protein 4 affect its microtubule-binding ability and chromosome movement during mitosis. Mol. Biol. Cell 10, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko, A., Saleh, N., Igarashi, H., Mori, H., Hauser-Hahn, I., Jiang, C.J., Sonobe, S., Lloyd, C.W., and Hussey, P.J. (2000). A new class of microtubule-associated proteins in plants. Natl. Cell Biol. 2, 750–753. [DOI] [PubMed] [Google Scholar]
- Sorrell, D.A., Combettes, B., Chaubet-Gigot, N., Gigot, C., and Murray, J.A. (1999). Distinct cyclin D genes show mitotic accumulation or constant levels of transcripts in tobacco bright yellow-2 cells. Plant Physiol. 119, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stals, H., Bauwens, S., Traas, J., Van Montagu, M., Engler, G., and Inze, D. (1997). Plant CDC2 is not only targeted to the pre-prophase band, but also co-localizes with the spindle, phragmoplast, and chromosomes. FEBS Lett. 418, 229–234. [DOI] [PubMed] [Google Scholar]
- Stoppin, V., Lambert, A.M., and Vantard, M. (1996). Plant microtubule-associated proteins (MAPs) affect microtubule nucleation and growth at plant nuclei and mammalian centrosomes. Eur. J. Cell Biol. 69, 11–23. [PubMed] [Google Scholar]
- Surana, U., Robitsch, H., Price, C., Schuster, T., Fitch, I., Futcher, A.B., and Nasmyth, K. (1991). The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell 65, 145–161. [DOI] [PubMed] [Google Scholar]
- Tio, M., Udolph, G., Yang, X., and Chia, W. (2001). cdc2 links the Drosophila cell cycle and asymmetric division machineries. Nature 409, 1063–1067. [DOI] [PubMed] [Google Scholar]
- Tournebize, R., Popov, A., Kinoshita, K., Ashford, A.J., Rybina, S., Pozniakovsky, A., Mayer, T.U., Walczak, C.E., Karsenti, E., and Hyman, A.A. (2000). Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Natl. Cell Biol. 2, 13–19. [DOI] [PubMed] [Google Scholar]
- Vasquez, R.J., Gard, D.L., and Cassimeris, L. (1999). Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil. Cytoskeleton 43, 310–321. [DOI] [PubMed] [Google Scholar]
- Veeranna, G.J., Shetty, K.T., Takahashi, M., Grant, P., and Pant, H.C. (2000). Cdk5 and MAPK are associated with complexes of cytoskeletal proteins in rat brain. Brain Res. Mol. Brain Res. 76, 229–236. [DOI] [PubMed] [Google Scholar]
- Verde, F., Labbe, J.C., Doree, M., and Karsenti, E. (1990). Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature 343, 233–238. [DOI] [PubMed] [Google Scholar]
- Verde, F., Dogterom, M., Stelzer, E., Karsenti, E., and Leibler, S. (1992). Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J. Cell Biol. 118, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak, C.E., Vernos, I., Mitchison, T.J., Karsenti, E., and Heald, R. (1998). A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 8, 903–913. [DOI] [PubMed] [Google Scholar]
- Weinmann, P., Gossen, M., Hillen, W., Bujard, H., and Gatz, C. (1994). A chimeric transactivator allows tetracycline-responsive gene expression in whole plants. Plant J. 5, 559–569. [DOI] [PubMed] [Google Scholar]
- Wolniak, S.M., and Larsen, P.M. (1992). Changes in the metaphase transit times and the pattern of sister chromatid separation in stamen hair cells of Tradescantia after treatment with protein phosphatase inhibitors. J. Cell Sci. 102, 691–715. [DOI] [PubMed] [Google Scholar]