Abstract
Background:
Glutathione S-transferase (GST)M1, a member of the μ class GST gene family, has been shown to be polymorphic because of a partial gene deletion. This results in a failure to express the GSTM1 gene in 50-60% of individuals. Several studies have demonstrated a possible link with the GSTM1-null genotype and susceptibility to cancer. Furthermore, a GSTM1 isoenzyme has been positively associated with protective effect against mutagenic drugs, such as alkylating agents and anthracyclines.
Objectives:
To determine whether GSTM1 polymorphisms are associated with tumour characteristics and survival in advanced breast cancer patients, and whether it may constitute a prognostic factor.
Methods:
We genotyped 92 patients receiving primary chemotherapy, which included cyclophosphamide, doxorubicine and 5-fluorouracil. The relationships between allelism at GSTM1 and clinicopathological parameters including age, menopausal status, tumour size, grade hormone receptors, involved nodes and p53 gene mutations were analysed. Of the patients with GSTM1-positive genotype, tissue samples obtained before and after treatment were available from 28 cases, allowing RNA extraction and GSTM1 expression by reverse transcription polymerase chain reaction. Relationships with clinical response to chemotherapy, and disease-free and overall survival were also evaluated. The data obtained was analysed using logistic regression to estimate the odds ratio and 95% confidence interval.
Results:
Of 92 patients, 57.6% (n = 53) were classified as heritably GSTM1-deficient, and 42.4% (n = 39) were of the GSTM1-positive genotype. There were no statistically significant relationships between GSTM1-null genotype and the clinicopathological parameters analysed. No relationship was observed between GSTM1 RNA expression and objective clinical response to chemotherapy. Objective clinical response to chemotherapy was related only to clinical tumour size (P = 0.0177) and to the absence of intraductal carcinoma (P = 0.0013). GSTM1-null genotype had no effect on disease-free or overall survival. The absence of hormone receptors (P = 0.002), the presence of a mutated p53 gene (P = 0.0098) and lack of response to primary chemotherapy (P = 0.0086) were the only factors associated with reduced disease-free or overall survival.
Conclusions:
GSTM1-null genotype alone had no effect on tumour characteristics and outcome of patients with advanced breast cancers. The lack of correlation of GSTM1 genotype with clinical tumour features, clinical response to chemotherapy and survival exclude a role for GSTM1 polymorphism as a prognostic factor in advanced breast cancer.
Keywords: advanced breast cancer, GSTM1 polymorphism, p53 gene mutations, prognosis, response to chemotherapy
Introduction
The human glutathione S-transferases (GSTs) are a multigene, isoenzyme family. Cytosolic GST isoenzymes can be classified by their substrate specificities, isoelectric points and amino acid sequence homologies into major classes termed α, μ, π and θ, which are encoded by a superfamily of genes located at different loci [1,2]. There are currently five putative α class genes encoding subunits GSTA1, GSTA2, GSTA3, GSTA4 and GSTω, whereas the GST π class contains a single gene encoding the GSTP1 protein, and the θ class consists of two genes encoding the GSTT1 and GSTT2 proteins.
The GSTM1 gene belongs to the GST μ class gene family, members of which are clustered on chromosome 1p13, and which contains five genes encoding subunits GSTM1, GSTM2, GSTM3, GSTM4 and GSTM5 [3,4]. The presence or absence of the GSTM1 gene constitutes the polymorphism, and the lack of the GSTM1 gene, which is caused by a gene deletion (the GSTM1-null genotype), affects approximately 50-60% of the population [5,6]. Homozygosity for the GSTM1-null genotype has been found to confer risk for many cancers, including those of the breast [7,8,9,10,11,12,13,14,15,16,17]. The GSTM1-null genotype was positively associated with high DNA adduct levels, suggesting it has a role in carcinogenesis [7]. Smokers with a GSTM1 deficiency had a significantly elevated risk for developing lung, laryngeal and bladder cancer [8,9]. The GSTM1-null genotype has also been associated with higher risk for environmentally related cancer, such as cancers of colon, head and neck, skin, oesophagus and stomach [9,10,11,12,13].
For breast cancer, the GSTM1-null genotype has been found to confer an increased risk in young post-menopausal women [14], whereas other studies did not find any association [18,19]. GST μ deletions have been reported to be associated with higher grade tumours [15], however, and to confer accumulation of epoxides, which are mutagenic [16]. GST isoenzymes catalyze the conjugation of glutathione to several electrophilic compounds, including polyaromatic hydrocarbon, which is lipophilic and stored in adipose tissues, such as those of the breast [20]. Aromatic adducts were found to be higher in women with breast cancer than in healthy control individuals [21]. Most polyaromatic compounds are metabolized to reactive epoxide intermediates by the polymorphic cytochrome p450 (CYP1AI) and detoxified by phase II enzymes, including GST. The variation in conjugation of epoxide substrate intermediates has been observed to segregate with inherited loss of the GSTM1 gene. Therefore, individuals who inherit the homozygous form of the null polymorphism in the GSTM1 gene will not be capable of conjugating and detoxifying specific substrate epoxide intermediates [16]. In addition, a wide variety of alkylating chemotherapeutic agents used in the treatment of breast cancer have been postulated to act as substrates for the GSTM1 protein products, thus reducing the effectiveness of these agents as cytotoxins [22].
In an attempt to further characterize the clinical features associated with the GSTM1-null genotype, we examined allelism at the GSTM1 locus in 92 locally advanced breast cancer patients undergoing primary chemotherapy. Allelism at the GSTM1 locus was analysed by polymerase chain reaction (PCR) in paired samples of blood and breast tissue. To determine whether levels of GSTM1 expression in patients with a positive genotype has a predictive or a prognostic value, RNA expression was measured by reverse transcription (RT)-PCR in breast tumour samples obtained before and after treatment. Results were then compared with clinicopathological factors of the patients, including characterization for p53 gene mutations [23], clinical response to chemotherapy, and disease-free and overall survival.
Materials and methods
Patients and samples
After an initial diagnostic biopsy, including characterization for p53 gene alterations, 92 women who were diagnosed with locally advanced breast carcinoma and who underwent primary chemotherapy were included in this study. The median clinical follow up was 78months (range 10-120months). Three of these patients had bilateral lesions, and in these three cases both lesions were examined. No family history for breast cancer was recorded in the 92 women. The patients received chemotherapy treatment (four or six courses, each lasting 21days) with a regimen containing cyclophosphamide, doxorubicine and 5-fluorouracil. The criteria for inclusion were as follows: inflammatory carcinomas, positive nodes and/or large (T3, T4) tumours. Clinical response to primary chemotherapy was categorized according to World Health Organization criteria and was considered as objective response (complete or partial response) or no response (stabilization or progression). In all cases neither radiotherapy nor hormone therapy were applied before chemotherapy.
Tumours were characterized before treatment by the clinical tumour size (categorized as T2, T3 or T4); the clinical nodal involvement (categorized as N0, N1 or N2); the histologic grade of Scarff, Bloom and Richardson (categorized as SBR1, SBR2 or SBR3); the hormone receptors (categorized as HR- or HR+, and considered as positive for oestrogen and/or progesterone receptors); the pathological tumour size (categorized as pT0, pT1, pT2 or pT3); and the number of involved axillary nodes (categorized as pN-, pN1+ and pN3+ for none, one or two, and three or more involved nodes, respectively).
Peripheral blood lymphocytes were obtained from each patient. Tumour samples were frozen in liquid nitrogen and stored at -80°C until analysis. Genomic DNA was extracted using proteinase, followed by phenol extraction and ethanol precipitation according to standard procedures [24].
Polymerase chain reaction method
The GSTM1-null genotype was determined by coamplifi-cation with the interferon-β gene, which served as an internal control. Primers for amplification of the GSTM1 gene corresponding to exon 4, intron 5 and exon 5 were 5' -ctgccctacttgattgatggg-3' and 5' -ctggattgtagcagatcatgc-3' (amplified product size, 271 base pairs). Primers for amplification of a part of the interferon-β gene, producing a constant 170-base pair band in all samples, were 5' -ggcacaacaggtagtaggcg-3' and 5' -gccacaggagcttctgacac-3' . Because the primers for the GSTM1 locus anneal to sites inside the coding region of the gene, the presence of the gene was determined by the presence of the band, whereas the null-genotype was determined by the lack of the band, using agarose gel electrophoresis (2%).
PCR was performed using 250ng template DNA in 10mmol/l Tris-HCl (pH8.4), 50mmol/l potassium chloride, 1.5mmol/l magnesium chloride (Bioprobe Systems, Illkirch, France), 0.2mmol/l concentrations of each deoxynucleotide triphosphate (dNTP), 500nmol/l concentrations of each primer and 2.5units of Taq DNA polymerase (Bioprobe Systems). The reaction (total volume 50 μ l) was amplified on a Omnigene thermal cycler (Hybaid Ltd, Ashford, Kent, UK). After an initial denaturation at 94°C for 3 min the reaction proceeded for 25 cycles of 50s at 94°C, 50s at 55°C and 50s at 72°C, concluded by a final extension step of 10min at 72°C. To test for contamination, negative controls (tubes containing the PCR mixture without the DNA template) were included in every run.
Reverse transcription polymerase chain reaction
Total RNA was obtained by the acid guanidine thiocyanate-phenol-chloroform extraction method [25]. Of the total RNA 1 μ g was dissolved in 20 μ l reverse transcriptase buffer (Gibco/BRL, Cergy Pontoise, France) containing 200 μ mol/l dNTP, 500 ng random hexamer and 200U avian myeloblastosis virus (AMV) reverse transcriptase (Superscript; Gibco/BRL). The reaction was incubated at 42°C for 50min and heated to 70°C for 15min.
The PCR reaction was carried out in a 50 μ l volume of PCR buffer (Promega, Madison, Wisconsin, USA) containing 200 μ mol/l dNTP, 2.5 μ l complementary DNA template, 2.5U Taq DNA polymerase, and 500nmol/l of both GSTM1 and β2- microglobulin primers. The reaction was initiated by a heat step at 95°C for 2min, and carried out for 25 cycles of denaturation at 94°C for 50s, annealing at 55°C for 50s and extension at 72°C for 50s. Blanks for each reaction were included with all samples. Of PCR product 10 μ l were analysed on a 2% agarose gel with ethidium bromide staining. Band intensities were determined with a gel doc 1000 UV system (Biorad, Ivry-Sur-Seine, France), and the ratio of GSTM1 to β2- microglobulin was calculated.
Determination of p53 mutations
The determination of p53 mutations was performed as previously described [23]. Briefly, breast tumour samples were characterized before and after treatment for p53 gene mutations by PCR single-strand confirmation polymorphism and/or direct sequencing of exons 5-9 of the p53 gene.
Hormonal receptor assay
The oestrogen and progesterone receptor levels were determined in cytosolic tumours using enzyme immunoassay methods (Abbott Laboratories, Rungis, France). The cutoff level used for oestrogen and progesterone was 20fmol/mg cytosolic proteins.
Statistical analysis
The Pearson χ2 test was used as a homogeneity test for proportion. A stepwise logistic regression model with the logoistic regression (LR)-BMDP program (University of California Press, Berkeley, California, USA) [26] was used to assess the contribution of each independent factor to the GSTM1-null genotype and the probability of response to primary chemotherapy. The log-rank test using the Kaplan-Meier method was used to study the relationship between each factor and the probability of disease-free survival (median 50months) and overall survival (median 78months). A multivariate analysis using the Cox proportional hazards model was performed to assess the contribution of each independent factor to the probability of survival. For logistic regression or Cox regression models the enter and remove limits were 0.1 and 0.15, respectively. Overall significance of each factor (P value) was given by the likelihood ratio test. Results are expressed as odds ratios (OR) and corresponding 95% confidence interval (CI), with the significance of ORs being derived from the ratio of the coefficient divided by its standard error (Wald test). Statistical analysis for RNA GSTM1 expression was performed using the SAS/STAT program (SAS Institute Inc, Cary, North Carolina, USA) by using student t-test or analysis of variance, Pearson χ2 test and log-rank test. For all tests, optimality of the selected models was verified by all-possible-subsets analyses.
Results
Clinicopathological data
Patients (total 92) were characterized by two variables: age and menopausal status at diagnosis. The median age of these patients was 57 years, and status was premenopausal for 51% (n = 47) and postmenopausal for 48.9% (n = 45). All samples were composed of infiltrating ductal carcinoma with an area of intraductual carcinoma in 35 cases. Of the tumours, 33.7% (n = 31) were 2-5cm in size (T2), 27.2% (n = 25) were > 5cm (T3) and 39.1% (n = 36/92) were > 5cm with skin involvement (T4). Of tumours, 60.9% (n = 56) contained significant hormone receptors (HR+), and 39.1% (n = 36) did not (HR-). Clinical complete response was found in 19.6% (n = 18) of cases. A high histological grade (SBR3) was found in 34.8% (n = 32) of tumours. An intermediate level of pathological tumour size (pT2) was found in 42.4% (n = 39) of samples. Negative lymph nodes were found in 20.7% (n = 19) of the tumours, whereas 23.9% (n = 22) of tumours were associated with >1 involved nodes and 55.4% (n = 51/92) were associated with > 3 involved nodes. Mutations in p53 were detected in 30% (n = 28) of the cases studied.
Glutathione S-transferase M1 genotype determination
The PCR method described above allowed an internal standard controlled classification of GSTM1-deficient (GSTM1-null genotype) individuals. Of the 92 patients, 57.6% (n = 53) were classified as heritably GSTM1 deficient, and 42.4% (n = 39) were GSTM1-positive genotype. Paired samples of blood and breast tissue were analysed before treatment with primary chemotherapy from the same individual, and GSTM1 genotype was identical for the two samples (Fig. 1). Among the 39 patients with GSTM1-positive genotype, tissue samples obtained before and after treatment were available from 28 cases, allowing RNA extraction and GSTM1 expression using the RT-PCR method. Two of these patients had bilateral lesions, and measurement was determined in the two tumour localizations. Thus, total GSTM1 expression, as measured by the ratio of GSTM1 to β2- microglobulin values, were performed on 30 tumour specimens. GSTM1 RNA signal was detected in all of the tumours analysed before and after treatment. The median GSTM1 expression was 1.38 (range 0.02-23.27) in the untreated tumours, and 1.16 (range 0.01-6.56) in samples obtained after chemotherapy administration.
Figure 1.
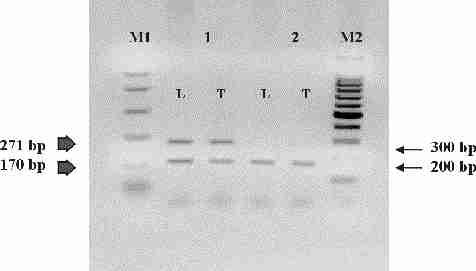
Polymerase chain reaction product of paired lymphocyte (L) and tumour (T) DNA from coamplification of glutathione S-transferase (GST)M1 (271 base pair) and interferon-β (170 base pair) genes. Lane 1 shows a homozygously present GSTM1 allele. Lane 2 shows a homozygously null-GSTM1 allele. M1 is ΦX174-HaeIII digested DNA marker. M2 is ΦX174-HinfI digested DNA marker.
Glutathione S-transferase M1 and clinicopathological characteristics of the patients
Distribution of GSTM1 genotype and its relation with clinicopathological data of the patients are shown in Table 1. There were no statistically significant associations between GSTM1-null genotype and the parameters analysed: age, menopausal and hormonal status, clinical and pathological tumour size, grade, involved nodes and p53 gene mutations. GSTM1 expression measured by RT-PCR in 30 samples (corresponding to 28 cases) before and after treatment with primary chemotherapy was also compared with the clinicopathological characteristics of the patients. None of the parameters tested were related to GSTM1 expression determined before or after treatment (data not shown).
Table 1.
Association of glutathione S-transferase (GST)M1 genotype and clinicopathological data of breast cancer patients studied
| GSTM1 | |||||
| Parameters | Variables | Present | Null | OR (95% Cl) | |
| Age (years) | >50 | 22 | 28 | ||
| >50 | 17 | 25 | 1.99 (0.531-7.44) | ||
| Status | Premenopausal | 22 | 23 | ||
| Postmenopausal | 17 | 30 | 0.59 (0.22-1.61) | ||
| Clinical tumour size | T2 | 15 | 16 | ||
| T3 + T4 | 24 | 37 | 1.7 (0.62-4.52) | ||
| Clinical nodal involvement | N0 | 14 | 11 | ||
| N1 + N2 | 25 | 42 | 3.02 (0.97-9.89) | ||
| Histologic grade | SBR1 | 8 | 6 | ||
| SBR2 + 3 | 31 | 47 | 2.4 (0.66-9.02) | ||
| Hormone receptors | HR- | 17 | 19 | ||
| HR+ | 22 | 34 | 1.04 (0.38-2.80) | ||
| Pathological tumour size | pT0 + pT1 | 19 | 26 | ||
| pT2 + pT3 | 20 | 24 | 0.8 (0.32-2.08) | ||
| Involved nodes | pN- | 10 | 9 | ||
| pN1+ pN3+ | 29 | 54 | 2.05 (0.67-6.27) | ||
| p53 | Normal | 31 | 33 | ||
| Mutated | 8 | 20 | 2.7 (0.88-7.90) | ||
CI, confidence interval; OR, odds ratio.
Relationship to clinical response to chemotherapy (Table 2) demonstrated that objective response (complete and partial responses) rate of the group with GSTM1-null genotype (75.5%) did not differ from that in those with GSTM1-positive genotype (76.9%). Thus, no significant relation was found between GSTM1 polymorphism and clinical response to chemotherapy (P = 0.8719). Also, no relation was obverved between GSTM1 RNA expression and clinical response to chemotherapy (P = 0.9524 and P = 0.5192 for before and after treatment, respectively). In contrast, clinical tumour size (P = 0.0177) and intraductal carcinoma (P = 0.0013) are strongly associated with clinical response. In multivariate analysis, the clinical tumour size (P = 0.0070, OR = 4.83, 95% CI = 1.45-16.10) and the absence of intraductal carcinoma (P = 0.0002, OR = 14.1, 95% CI = 2.52-78.50) remained the only factors linked to the clinical response.
Table 2.
Clinical response to primary chemotherapy and breast cancer outcome
| GSTM1 | |||
| Parameters | Present | Null | P |
| Objective response* | 76.9% | 75.5% | 0.8719† |
| Disease-free survival (months) | 42.00 ± 2.65 | 54.43 ± 5.67 | 0.8094‡ |
| Overall survival (months) | 74.82 ± 3.95 | 72.73 ± 4.81 | 0.9729‡ |
*Objective response was estimated as complete and partial responses. †Estimated by Pearson χ2 test. ‡Estimated by log rank test, median± standard error. GST, glutathione S-transferase.
Impact on survival of the patients
For disease-free survival, no differences were found between individuals with GSTM1-null genotype and those with positive-GSTM1 genotype (P =0.8094). Accordingly, no impact for RNA GSTM1 expression on disease-free survival (P = 0.8991 and P = 0.9096 for before and after treatment, respectively) was observed (Table 2). In contrast, the absence of hormone receptors (P = 0.0020) and the presence of p53 gene mutations (P = 0.0098) had an impact on disease-free survival. With multivariate analysis, hormone receptors status (P = 0.0002, OR=3.99, 95% CI = 1.92-8.29) and p53 gene mutations (P = 0.0138, OR = 2.36, 95% CI=1.22-4.59) remained significantly associated with metastasis recurrence risk.
No impact was also found (P = 0.9729) for GSTM1-null genotype on overall survival (Table 2), or for RT-PCR RNA expression (P = 0.1667 and P = 0.9637 for before and after treatment, respectively). Only the absence of hormone receptors (P = 0.0018), the presence of p53 gene mutations (P = 0.0071) and no response to primary chemotherapy (P = 0.0086) were associated with reduced overall survival of the patients. In multivariate analysis, hormone receptors status (P = 0.0003, OR = 5.23, 95% CI = 2.03-13.49) and p53 gene mutations (P = 0.0037, OR = 3.62, 95% CI = 1.53-8.53) were strongly related to the risk for death. Absence of clinical response to chemotherapy was less related to the overall survival (P = 0.0530, OR = 2.31, 95% CI = 1.02-5.26).
Discussion
In the present study, the frequency of GSTM1 gene deficiency among breast carcinoma patients (57%) was similar to that previously reported in this lesion [9,14,15,16,17,18,19]. Among individuals with GSTM1-positive genotype no deletion was observed in somatic tumour cells, suggesting that the deletion of GSTM1 gene was not a characteristic of breast tumour cells.
Several series have determined that GSTM1 deletions were involved in the aetiology of breast cancer [9,14,17], whereas another studies did not find any such associations [18,19]. In addition, little is known about clinical characteristics that confer GSTM1 deletion among breast cancer patients. Only one recently published study [19] reported no relation between clinicopathological parameters and GSTM1 genotype in primary breast tumours. The results obtained in the present study are consistent with these data, because no differences were noted when a variety of known prognostic factors were compared between tumours from patients with and those without GSTM1-null genotype. These factors include age, menopausal status, clinical and pathological tumour size, clinical and pathological nodal involvement, pathological grade, hormone receptors status and p53 gene mutations. These results suggest that clinical tumour features are not associated with GSTM1-null genotype, not only in primary breast cancers, but also in advanced breast cancers.
An improved survival has been observed in patients with GSTM1-null genotype and has been explained by a better response to chemotherapeutic agents related to more effective cell killing, which in turn is related to the absence of protective effect of GSTM1 allele [18]. Among the most active chemotherapeutic agents in the treatment of breast cancer are cyclophosphamide and anthracyclines. These compounds are conjugated with thiols through reactions mediated by GST. The π class of transferase is thought to be the major factor involved in the subsequent prevention of DNA alkylation [22], although there has been some suggestion that the μ class is also involved [27]. In order to determine whether the absence of GSTM1 locus favours the response to chemotherapeutic agents, and whether, on the contrary, GSTM1-positive genotype may confer a resistance to chemotherapy, we analysed GSTM1 polymorphisms in relation to clinical response to chemotherapy. The results demonstrated that the objective response rate of the group with GSTM1-null genotype did not differ from those with GSTM1-positive genotype. Thus, individuals carrying the GSTM1 locus were no more resistant to chemotherapy than those with GSTM1-null genotype. GSTM1 RNA expression levels measured in patients with positive genotype were also not associated with clinical response to chemotherapy. Among clinical parameters analysed, only clinical tumour size and the presence of intraductal carcinoma were found to influence clinical response to primary chemotherapy. The results also revealed that GSTM1-null genotype was not related to survival in advanced breast cancer patients, in contrast to the absence of hormone receptors and to the presence of p53 gene mutations, which are known to be of prognostic value in advanced breast cancer [28,29,30].
Although the group studied was not very large (n = 92), the clinical characteristics were well known and the results strongly suggest the lack of correlation of GSTM1-null genotype alone with prognostic factors, clinical reponse to chemotherapy and survival. Genotypes of other enzymes (CYP1A1, GSTT1), as well as combinations of genotypes, might be of interest with regard to cancer and carcinogen metabolism.
Taken together, these results show that advanced breast cancers arising in patients with GSTM1-null genotype have no worse clinical tumour charactaristics and outcome than those of patients without such deletions. Thus, the lack of correlation of GSTM1-null genotype with clinical tumour features, clinical response to chemotherapy and survival exclude a role for GSTM1 polymorphism as a prognostic factor in advanced breast cancer.
Acknowledgments
Acknowledgements
This work was supported by the 'Comité Départemental de Saône et Loire', 'Ligue Bourguignonne Contre le Cancer' and the 'Association Régionale pour l'Enseignement et la Recherche Scientifique/Groupe Interrégional de Recherche en Cancérologie'. We thank Mrs L Hahnel and M Arnal for their excellent technical assistance.
References
- Mannervik B, Alin P, Guthenberg C, et al. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985;82:7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B, Danielson UH. Glutathione transferases: structure and catalytic activity. CRC Crit Biochem. 1988;23:283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Vorachek WR, Xu SJ, et al. Identification of class-mu glutathione transferase genes GSTM1-GSTM5 on human chromosome 1p13. . Am J Hum Genet. 1993;53:220–233. [PMC free article] [PubMed] [Google Scholar]
- Ross VL, Board PG, Webb GC. Chromosomal mapping of the human mu class glutathione S-transferase to 1p13. Genomics. 1993;18:87–91. doi: 10.1006/geno.1993.1429. [DOI] [PubMed] [Google Scholar]
- Seidegard J, Vorachek WR, Pero RW, Pearson WR. Hereditary differences in the expression of the human glutathione transferase active on trans -stilbene oxide are due to a gene deletion. . Proc Natl Acad Sci U S A. 1988;85:7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong JL, Chang CM, Whang-Peng J, Knutsen T, Tu CPD. The human liver glutathione S-transferase gene superfamily: expression andchromosome mapping of an Hb subunit cDNA. Nucleic Acids Res. 1988;16:8541–8554. doi: 10.1093/nar/16.17.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar-Stewart V, Motulsky AG, Eaton DL, et al. The glutathione S-transferase mu polymorphism as a marker for susceptibility to lung carcinoma. Cancer Res. 1993;53:2313–2318. [PubMed] [Google Scholar]
- Lafuente A, Pujol F, Carretero P, Villa JP, Cuchi A. Human glutathione S-transferase mu (GST mu) deficiency as a a marker for susceptibility to bladder and larynx cancer among smokers. Cancer Lett. 1993;68:49–54. doi: 10.1016/0304-3835(93)90218-x. [DOI] [PubMed] [Google Scholar]
- Zhong S, Wyllie AH, Barnes D, Wolf CR, Spurr NK. Relationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancer. Carcinogenesis. 1993;14:1821–1824. doi: 10.1093/carcin/14.9.1821. [DOI] [PubMed] [Google Scholar]
- Trizna Z, Clayman GL, Spitz MR, Briggs KL, Geopfert H. Glutathione S-transferase genotypes as risk factors for head and neck cancer. Am J Surg. 1995;170:499–501. doi: 10.1016/s0002-9610(99)80339-0. [DOI] [PubMed] [Google Scholar]
- Heagerty AHM, Fitzgerald D, Smith A, et al. Glutathione S-transferase GSTM1 phenotypes and protection against cutaneous tumors. . Lancet. 1994;343:266–268. doi: 10.1016/s0140-6736(94)91115-0. [DOI] [PubMed] [Google Scholar]
- Nimura Y, Yokoyama S, Fujimori M, et al. Genotyping of the CYP1A1 and GSTM1 genes in esophageal carcinoma patients with special reference to smoking. Cancer. 1997;80:852–857. doi: 10.1002/(sici)1097-0142(19970901)80:5<852::aid-cncr4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Harada S, Misawa S, Nakamura T, et al. Detection of GST1 gene deletion by the polymerase chain reaction and its possible correlation with stomach cancer in Japanese. Hum Genet. 1992;90:62–64. doi: 10.1007/BF00210745. [DOI] [PubMed] [Google Scholar]
- Ambrosone CB, Freudenheim JL, Graham S, et al. Cytochrome P4501A1 and glutathione S-transferase (M1) genetic polymorphisms and postmenopausal breast cancer risk. Cancer Res . 1995;55:3483–3485. [PubMed] [Google Scholar]
- Murray GJ, Weaver RJ, Paterson PJ, et al. Expression of xenobiotic metabolizing enzymes in breast cancer. J Pathol. 1993;169:347–353. doi: 10.1002/path.1711690312. [DOI] [PubMed] [Google Scholar]
- Wiencke JK, Kelsey KT, Lamela RA, Toscano W. Human glutathione-S-transferase deficiency: a marker of susceptibility to induced chromosome damage. Cancer Res. 1990;50:1585–1590. [PubMed] [Google Scholar]
- Helzlsower KJ, Selmin O, Huang HY, et al. Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. J Natl Cancer Inst. 1998;90:512–518. doi: 10.1093/jnci/90.7.512. [DOI] [PubMed] [Google Scholar]
- Kelsey KT, Hankinson SE, Colditz GA, et al. Glutathione S-transferase class μ deletion polyphorphism and breast cancer: results from prevalent versus incident cases. Cancer Epidemiol Biomarkers Prev. 1997;6:511–515. [PubMed] [Google Scholar]
- Bailey LR, Roodi N, Verrier CS, et al. Breast cancer and CYP1A1, GSTM1, and GSTT1 polymorphisms: evidence of a lack of association in Caucasians and African Americans. Cancer Res. 1998;58:65–70. [PubMed] [Google Scholar]
- Peresa FP, Estabrook A, Hewer A, et al. Carcinogen-DNA adducts in human breast tissue. Cancer Epidemiol Biomarkers Prev . 1995;4:233–238. [PubMed] [Google Scholar]
- Li D, Wang M, Dhingra K, Hittelman WN. Aromatic DNA adducts in adjacent tissues of breast cancer patients: clues to breast cancer etiology. . Cancer Res. 1996;56:287–293. [PubMed] [Google Scholar]
- Fields WR, Li Y, Townsend AJ. Protection by transfected glu-tathione S-transferase isoenzymes against carcinogen-induced alkylation of cellular macromolecules in human MCF-7 cells. Carcinogenesis . 1994;15:1155–1160. doi: 10.1093/carcin/15.6.1155. [DOI] [PubMed] [Google Scholar]
- Lizard-Nacol S, Coudert B, Riedinger JM, Fargeot P, Guerrin J. p53 gene alterations are associated with a decreased responsiveness to neoadjuvant chemetherapy in human breast cancer. Int J Oncol. 1997;10:1203–1207. doi: 10.3892/ijo.10.6.1203. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: a Laboratory Manual Cold Spring Harbour, New York: Cold Spring Harbour Laboratory. 1982.
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Engelman L. Stepwise logistic regression. BMDP Statistical Software Manual. Vol 2. Edited by Dixon WJ, Brown MB, Engelman L, Jennrich RI. University of California Press: Berkeley. 1992. pp. 1013–1046.
- Albin N, Massad L, Toussain C, et al. Main drug-metabolizing enzyme systems in human breast tumors and peritumoral tissues. Cancer Res. 1993;53:3541–3546. [PubMed] [Google Scholar]
- Järvinen TAH, Holli K, Kuukasjärvi T, Isola JJ. Predictive value of topoisomerase Iiα and other prognostic factors for epiribicin chemotherapy in advanced breast cancer. Br J Cancer . 1998;77:2267–2273. doi: 10.1038/bjc.1998.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Sjögren S, Inganäs M, Norberg T, et al. The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. J Natl Cancer Inst. 1996;88:173–182. doi: 10.1093/jnci/88.3-4.173. [DOI] [PubMed] [Google Scholar]


